Role of L3T4+ and 38+ T-Cell Subsets in Resistanceagainst
Transcript of Role of L3T4+ and 38+ T-Cell Subsets in Resistanceagainst
INFECTION AND IMMUNITY, Feb. 1991, p. 529-5360019-9567/91/020529-08$02.00/0Copyright ©3 1991, American Society for Microbiology
Role of L3T4+ and 38+ T-Cell Subsets in Resistance againstInfection with Treponema pallidum subsp. pertenue in Hamsters
HSI LIU,1t JEFFREY D. ALDER,1 BRET M. STEINER,2 JOAN STEIN-STREILEIN,3 LONY LIM,4
AND RONALD F. SCHELL' 4*
Department of Medical Microbiology and Immunology' and Wisconsin State Laboratory of Hygiene,4 University ofWisconsin, Madison, Wisconsin 53706; Centers for Disease Control, Atlanta, Georgia 303412;
and Department of Medicine, University of Miami, Miami, Florida 331013
Received 11 July 1990/Accepted 13 November 1990
The protective immunity conferred by T-cell subsets against infection with Treponema pallidum subsp.pertenue was studied. We demonstrated that hamster T cells can be separated into two subsets by monoclonalantibody (MAb) GK 1.5 (anti-L3T4) and MAb 38. Eighty-five percent of hamster thymocytes were L3T4+ and87% were 38+ cells; 84% were dual positive for MAbs anti-L3T4 and 38. In the peripheral lymph nodes,however, the L3T4+ and 38+ T cells were mutually exclusive according to two-color immunofluorescenceanalysis. The two T-cell subsets were found to be functionally distinct according to their secretion of interleukin2 (IL-2) when stimulated with concanavalin A. The L3T4+ cells secreted IL-2 and had characteristics of Thelper cells, while the 38+ cells did not secrete IL-2 and appeared to be T cytotoxic-suppressor cells. Transferof 4 x 106 helper or cytotoxic-suppressor T lymphocytes from T. pallidum subsp. pertenue-immune hamstersprotected irradiated naive hamsters against challenge with this subspecies. IL-2 production could still bedetected in the irradiated recipients 12 days after irradiation of naive recipients, although at a low level. Thissuggests that the remaining lymph node cells could support the survival and expansion of the infusedcytotoxic-suppressor T cells. No accumulation of macrophages was observed in regional lymph nodes ofimmune T-cell recipients within 10 days of infection. Instead, there was an influx of polymorphonuclearneutrophils in all animals injected with T. pallidum subsp. pertenue. This report demonstrates that hamster Tcells can be separated into two phenotypically and functionally distinct subsets and that both T-cell subsetsconfer protection against challenge with T. pallidum subsp. pertenue.
Immunity to challenge with Treponema pallidum subsp.pallidum and other virulent treponemes develops during thecourse of infection (3, 15, 22). However, the mechanism ofresistance against infection with virulent treponemes ispoorly understood. This lack of understanding of the im-mune mechanism is primarily due to lack of appropriateanimal models for studying cell-mediated immune responsesand unavailability of monoclonal antibodies (MAbs) to dis-tinguish lymphocyte subpopulations. Classically, rabbitshave been used as the experimental animals; however,inbred rabbits are not readily available for cell transferstudies. Recently, inbred hamsters have been used forstudying immune responses to infection with treponemes (3,15, 27, 28). When infected, hamsters produce chronic skinlesions and their lymph nodes increase in weight and con-tained measurable numbers of treponemes (27, 28). HamsterT and B cells can also be separated by using MAb 14-4-4s(15, 38). In addition, Witte et al. (37) have been able toseparate hamster T cells into two subsets by using MAb 38,which presumably identifies hamster cytotoxic T cells.These advances have made hamsters suitable for studyingantibody- and cell-mediated immunity against infection withtreponemes.Azadegan et al. (3) have shown that passive transfer of
immune serum protects recipients against infection with T.pallidum subsp. pertenue. Donors of immune serum, how-ever, were unable to eliminate the treponemes from their
* Corresponding author.t Present address: Department of Pathology, Harvard Medical
School and Dana-Farber Cancer Institute, Boston, MA 02115.
own system (3), suggesting that other immune componentsare also important in determining the fate of the invadingtreponemes.We have shown previously (15) that naive hamsters in-
jected subcutaneously in the hind paws with 4 x 106 T cells(Ig- Ia-) from immune hamsters are resistant to challengewith T. pallidum subsp. pertenue in the absence of detect-able antitreponemal antibodies (titer, <1:80). The popliteallymph nodes of hamsters receiving immune T cells weighedless and had significantly fewer treponemes than lymphnodes from hamsters infused with cells from nonimmunedonors. These results suggest that T cells are involved inprotective immunity to infection with T. pallidum subsp.pertenue.
In this paper we show for the first time that hamster T cellscan be separated into two phenotypically and functionallydistinct subpopulations by using MAb GK 1.5 and MAb 38.The T-cell subset recognized by MAb GK 1.5, L3T4+ cells,has the characteristics of helper T cells, whereas 38+ cellshave the characteristics of cytotoxic-suppressor T cells. Thedistributions of these T-cell subsets in the thymus and in theperipheral lymph nodes are similar to those in mice andhumans (13, 29). Specifically, in the peripheral lymphoidorgans, expression of L3T4 and 38 determinants are mutu-ally exclusive. This finding is significant, since hamsters can
now be used to study the same type of questions thatpreviously were investigated only in mice. In the presentstudy, enriched hamster T-cell subsets were obtained byflow cytometry sorting. We demonstrated that resistance tochallenge with T. pallidum subsp. pertenue was conferred byboth helper and cytotoxic-suppressor T-cell subsets.
529
Vol. 59, No. 2
530 LIU ET AL.
MATERIALS AND METHODS
Animals. Male and female inbred LSH/Ss LAK hamsters,6 to 9 weeks old, were obtained from Charles River BreedingLaboratories, Inc. (Wilmington, Mass.). Hamsters weighing80 to 100 g were housed three or four per cage at an ambienttemperature of 21°C.Organism. T. pallidum subsp. pertenue (Haiti B) was
originally isolated from a lesion on the lower abdomen of an11-year-old boy with typical generalized frambesiform yaws(35). The strain has been maintained by passage in hamsters(28). The inguinal lymph nodes of hamsters were removedaseptically 5 to 6 weeks after intradermal injection of 106viable treponemes into the inguinal region. The lymph nodeswere teased apart in RPMI 1640 medium (GIBCO, LongIsland, N.Y.) containing 5% fetal bovine serum (HycloneLaboratories, Inc., Logan, Utah) and forced through sterile60-mesh stainless-steel wire mesh filters. After centrifuga-tion at 270 x g for 3 min to remove cellular debris, thenumber of treponemes in the supernatant was determined bydark-field microscopy (18). The suspension of treponemeswas dispensed into vials at a concentration of 5 x 106/ml andstored at -70°C until used.Antibody reagents. Hybridoma cell lines 14-4-4s and GK
1.5 producing murine anti-Ia MAb 14-4-4s and MAb anti-L3T4 were provided by Colleen Hayes (University of Wis-consin, Madison). MAb GK 1.5 has a specificity for themouse L3T4a molecule and was originally described byDialynas et al. (6). MAb 14-4-4s recognizes the Ia.7 speci-ficity that is present on I-Ek in the mouse and is specific forhamster B cells as previously described (15, 21). The hybrid-oma cell lines 14-4-4s and GK 1.5 were grown in RPMI 1640medium containing 10% fetal bovine serum or Dulbecco'sminimum essential medium containing 15% fetal bovineserum, respectively. Five days after cultivation, the super-natant was centrifuged at 270 x g, collected, dispensed in5-ml aliquots, and stored at -20°C. The aliquots werethawed on the day of experimentation and used at a finaldilution of 1:10. MAb 38 (hamster cytotoxic-suppressorT-cell marker, immunoglobulin G2a) ascitic fluid was main-tained at -70°C, thawed, and used at a 1:50 dilution (37). Allantibody reagents were titrated to optimal concentrationsbefore experimentation. Mab 38 was also labeled with biotin(10, 32). Briefly, 2 mg of antibody solution was incubatedwith 0.25 mg of biotin-HO-succinimide (Sigma, St. Louis,Mo.) in 100 ,ul of dimethyl sulfoxide (Sigma) and stirred for2 h. The solution was then dialyzed overnight against Dul-becco's phosphate-buffered saline (DPBS; Sigma), with sev-eral changes of buffer. The resulting solution was sterilizedby filtration (pore size, 0.45 ,um; Corning Glass Works,Corning, N.Y.) and stored at -70°C until use. Avidin-fluorescein isothiocyanate (FITC) and streptavidin-phyco-erythrin conjugates were purchased from Becton-DickinsonImmunocytometry Systems (Mountain View, Calif.).
Preparation of thymus and lymph node cells. Single-cellsuspensions of thymus and lymph node cells were preparedby teasing apart the thymus or lymph nodes with forceps andgently pressing them through a stainless-steel 60-meshscreen into RPMI 1640 medium containing 5% fetal bovineserum. The cells were then washed twice with RPMI 1640medium and suspended to 2 x 107 cells per ml in DPBS.
T-cell enrichment. MAb 14-4-4s was added to suspensionsof 2 x 106 lymph node cells per ml at a final dilution of 1:10and maintained at 4°C for 60 min. Cells were washed twiceby centrifugation at 270 x g for 10 min in DPBS andresuspended to 2 x 106/ml, and a 1:1 mixture of baby rabbit
and guinea pig complements was added at a final concentra-tion of 1:10. The complement was absorbed with hamsterspleen cells. The suspension of cells was incubated at 37°Cfor 60 min and washed, and the cells were resuspended.Controls for complement treatment included cells incubatedwith a mock antibody plus complement or cells incubatedwith complement only. This process does not result insignificant loss of cells or change in viability. Viability wasdetermined by eosin dye exclusion.Flow cytometry analysis. In one-color analysis, 2 x 106
enriched lymph node T cells were incubated with FITC-conjugated MAb 38 (1:50) for 30 min, washed twice withDPBS, fixed with 2% formaldehyde, and stored at 4°C in thedark until analyzed. In two-color analysis, 2 x 106 cells wereincubated with MAb GK 1.5 hybridoma ascitic fluid (1:50).MOPC-195 myeloma ascitic fluid, which contained immuno-globulin of the G2b isotype at 3 mglml of protein, was usedas a control antibody (Litton Bionetics, Inc., Kensington,Md.) at a 1:50 dilution. This concentration is similar to thatof MAb GK 1.5 ascitic fluid. After 30 min of incubation at4°C, the cells were washed twice with DPBS and incubatedwith fluorescein-conjugated goat anti-rat immunoglobulin(Organon Teknika-Cappel Corp., West Chester, Pa.) at afinal dilution of 1:20 for 30 min at 4°C. Biotinylated MAb 38was added, the cells were washed twice with DPBS, andthen phycoerythrin-conjugated streptavidin (Becton Dickin-son) was added. Surface immunofluorescence of 10,000 cellswas determined with a Coulter Epics C cell sorter (CoulterImmunology, Hialeah, Fla.). For sorting, cells were stainedand suspended in RPMI 1640 medium containing 5% fetalbovine serum and 5 mM HEPES (N-2-hydroxyethylpipera-zine-N'-2-ethanesulfonic acid) buffer solution. The cellswere sorted at 2,500 to 3,000 cells per s. The purity of cellswas 92 to 95% according to postsorting analysis. Eachanalysis was repeated at least three times, and similar resultswere obtained. The study of macrophages and polymorpho-nuclear neutrophils (PMNs) was performed by forward-angle and 90°-angle light scatter analyses as previouslydescribed (5).
Passive transfer of protection. Thirty hamsters were in-fected intradermally in the inguinal region with 5 x 105viable T. pallidum subsp. pertenue treponemes. At 10 to 16weeks after infection, the hamsters were treated with peni-cillin (4,000 U) to terminate infection (27). Pooled immunelymph node cells were obtained from these hamsters 10 daysafter treatment with penicillin. No treponemes were ob-served in the suspensions of immune cells. Normal lymphnode T cells were also obtained from hamsters 10 days afterpenicillin treatment. Lymph node T cells or T-cell subsets (4x 106 cells per hamster) were injected subcutaneously intothe right hind paw. All hamsters were then challengedintradermally by injecting 5 x 105 viable T. pallidum subsp.pertenue treponemes in the popliteal region. Hamsters weresacrificed 3 weeks after infection. Although inbred hamsterswere used in this investigation, all recipients of cells wereirradiated as a means of creating a more suitable environ-ment for the proliferation of the transferred cells. Whole-body gamma irradiation (500 rads) was delivered with acobalt-60 irradiator (Picker Co., Cleveland, Ohio). Hamsterssurvived this level of radiation without reconstitution ofbone marrow cells. The cell transfer study presented wasrepeated three times with similar results.
Assessment of protection. The lymph node weight and thenumber of treponemes in the lymph node were employed asparameters for evaluating the response of hamsters passivelyimmunized against treponemal infection. The weight of the
INFECT. IMMUN.
L3T4+- AND 38+-MEDIATED RESISTANCE TO T. PALLIDUM
A1
ii)-.I S ----
3 k,100%
B2 1
C
2 1
4 6% 66%
2
4
mAb GK 1.5 (anti-L3T4)Relative Green Fluorescence (Log)
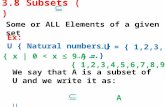
FIG. 1. Two-color flow cytometry analysis of the expression of L3T4 and 38 on thymus and lymph node cells. Control cells (A) were
stained with MOPC-195 (immunoglobulin G2b), FITC-conjugated goat anti-rat immunoglobulin (1:20), and avidin-phycoerythrin. Thymocytes(B) or T-cell-enriched lymph node cells (C) were incubated with MAb GK 1.5 ascitic fluid (1:50) for 30 min and then with FITC-conjugatedgoat anti-rat immunoglobulin (1:20). Biotinylated MAb 38 (1:50) was then added for an additional 30 min, the cells were washed, andavidin-phycoerythrin (1:10) was added. The cells were washed with DPBS and resuspended in DPBS for flow cytometry analysis. Both x andy axes are in log fluorescence. Experiments were repeated three times, and similar results were seen.
popliteal lymph nodes was determined with a Mettler H32balance. The number of treponemes per lymph node was
determined according to a modification of the proceduredescribed by Miller (18). In brief, duplicate slides of eachhomogenized lymph node were prepared, and 100 fields per
slide were examined for treponemes by dark-field micros-copy. Data are presented as the mean number of treponemesper lymph node.
Mitogen studies. Single-cell suspensions of lymph nodecells were diluted to 5 x 106 cells per ml in RPMI 1640medium supplemented with 10% heat-inactivated fetal bo-vine serum, 2 mM L-glutamine, 2 x 10-5 M 2-mercaptoeth-anol, 100 U of penicillin per ml, and 100 ,ug of streptomycin(Hazelton, Denver, Pa.) per ml. Suspensions of 0.1 ml ofmedium containing 2 x 105 lymph node cells were culturedin flat-bottomed 96-well microtiter plates (Costar, Cam-bridge, Mass.) at a final volume of 0.2 ml per well. Conca-navalin A (ConA; Sigma) was prepared according to themanufacturer's instructions, diluted in RPMI 1640 mediumcontaining 5% fetal bovine serum, and added to cell culturesat 2 ,ug/ml. Cultures were incubated in triplicate at 37°C for48 h in a humid atmosphere of 5% CO2 and 95% air. Eighthours before being harvested, each culture was pulsed with1 ,uCi of [3H]thymidine (specificity, 6.7 ,uCi/mol; New En-gland Nuclear, Boston, Mass.) per ml. Cultures were har-vested with a multiple-cell harvester (Whittaker, M. A.Bioproducts, Inc., Walkersville, Md.) onto fiberglass filterpaper. The filter paper disks were placed in Biosafe II liquidscintillation fluid (Research Products International Corp.,Mount Prospect, Ill.) and counted in a Beckman scintillationcounter. Results were expressed as counts per minutestandard error.
IL-2 assay. Interleukin 2 (IL-2) production was assayed bythe ability of the culture supernatant from ConA-stimulatedlymphocytes to support the growth of an IL-2-dependent cellline, CTLL-2. Murine recombinant IL-2 (rIL-2) was a giftfrom E. I. Du Pont de Nemours Co., Glenolden Laboratory(Glenolden, Pa.). Briefly, 100 ,u of culture supernatant was
added to 96-well flat-bottomed culture plates at a 1:2 dilu-tion. Four thousand CTLL-2 cells suspended in 100 RI ofRPMI 1640 medium were then added to each well. Thecultures were incubated for 24 h at 37°C in an atmosphere of5% CO2. Eight hours before termination of the cultures, 1pRCi of [3H]thymidine per ml was added to each well. Thecultures were harvested with a cell harvester (Whittaker,M. A. Bioproducts) onto fiberglass filter papers. Tritiated-thymidine incorporation was determined by a Beckmanscintillation counter. Results in counts per minutes werethen converted to units per milliliter on the basis of one-halfthe maximum proliferative response of the positive controlculture equaling 1 U (8, 9).
Statistical analysis. Data were analyzed by the analysis ofvariance procedure. When a significant F ratio indicatedreliable mean differences, the Student t test was used toexamine pairs of means. The alpha level was set at 0.05before the experiments were started.
RESULTS
Distribution of L3T4+ and 38+ T cells in the thymus andperipheral lymph nodes. Hamster thymocytes and enrichedlymph node T cells were labeled with MAb GK 1.5 (anti-L3T4) and MAb 38 by using two-color immunofluorescencestaining. Of the thymocytes, 85 and 87% were L3T4+ and38+ cells, respectively. Among the thymocytes, 84% were
dual positive for L3T4 and 38 (Fig. 1B, quadrant 2), whereasin the peripheral lymph node, the expressions of L3T4 and38 determinants were mutually exclusive (Fig. 1C). Weconcluded from these analyses that the distribution of thetwo T-cell subsets in the hamster is similar to that observedin mice (29). Thus, in the thymus, dual-positive cells pre-dominate, whereas in peripheral lymph nodes, the twosubsets are mutually exclusive.ConA responsiveness and IL-2 secretion by L3T4+ and 38+
cells. The functions of the two T-cell subsets were compared.Lymph node cells were enriched for L3T4+ cells by treating
A-,
O0
Oa
E
m._
27% 1%
. .
O.. ~. f .,~,.-:u_ _
VOL. 59, 1991 531
532 LIU ET AL.
T
Medium Con A
II
UIU
L3T4+ CELLS
WHOLE CELLS38+ CELILS
rIL-2 rIL-2+Con A
TREATMENTSFIG. 2. ConA response of enriched L3T4 and 38 cells. L3T4+ cells were obtained by treating lymph node cells with MAbs 14-4-4s and 38
and complement. 38+ cells were obtained by treating lymph node cells with MAbs 14-4-4s and GK 1.5 and complement. The enriched cellswere cultured at 2 x 105 cells per well in the presence or absence of 2 jig of ConA and/or 4,000 U of murine rIL-2 per ml for 24 h. The cultureswere pulsed with 1 ,uCi of [3H]thymidine per ml 8 h before termination. SE, Standard error of the mean.
them with MAbs 14-4-4s and 38 and complement. Likewise,38+ cells were obtained by treating lymph node cells withMAbs 14-4-4s and GK 1.5 and complement. The enrichedcells were then stimulated with ConA in the presence orabsence of rIL-2. L3T4+ cells responded to ConA stimula-tion without the addition of exogenous factors. 38+ cells,however, required the addition of rIL-2 to respond to ConAstimulation (Fig. 2). Unfractionated whole-lymph-node cellsresponded to ConA in the absence or presence of rIL-2. Inaddition, examination of the supernatants of ConA-treatedL3T4+ and 38+ cells revealed that L3T4+ cells secreted IL-2(5.4 U/ml) when stimulated with ConA, whereas 38+ cellswere unable to secrete IL-2 (<1 U/ml) when stimulated withConA. Unfractionated whole-lymph-node cells responded toConA stimulation by secreting 40 U of IL-2 per ml. Similarresults were obtained when this experiment was performedby labeling enriched lymph node T cells with MAb 38-FITC,collecting both 38+ and 38- (L3T4+) cells by flow cytometrysorting, stimulating the T-cell subsets with ConA in vitro,and then testing the culture supernatant with the IL-2-dependent cell line (data not shown). These results suggestthat the two T-cell subsets differ both phenotypically andfunctionally.
Protection conferred by T-cell subsets against infection withT. pallidum subsp. pertenue. We next examined the ability ofthe L3T4+ (Th) and 38+ (Tc) cells to confer protectionagainst infection with T. pallidum subsp. pertenue. Lymphnode cells from immune and normal hamsters were enrichedfor T cells by treatment with MAb 14-4-4s and complement.Immune T-cell subsets, 38+ and 38- cells (4 x 106 cells per
animal), obtained by flow cytometry sorting with fluores-cence labeled MAb 38, were infused into naive irradiatedrecipient hamsters which were then challenged with T.pallidum subsp. pertenue. As shown in Table 1, a significantdecrease (P < 0.05) was observed in the lymph node weightand number of treponemes in recipients of either 4 x 106immune 38- (L3T4+, Th) or immune 38+ (Tc) cells. Theseresults suggest that both T-cell subsets are capable of
conferring protection against challenge with T. pallidumsubsp. pertenue. These experiments were repeated threetimes with similar results.
IL-2 production by irradiated hamster lymph node cells.Since L3T4+ cells but not 38+ cells produce IL-2, there wasa possibility that the transferred 38+ cells would not functionappropriately without helper factors from the environmentof the recipients. The ability of lymph node cells fromirradiated hamsters to support the survival and growth of thetransferred 38+ cells was examined by assaying IL-2 pro-duction in irradiated hamsters for 12 days. The resultsindicate that the amount of IL-2 produced from lymph nodecells of irradiated hamsters is lower than that from lymphnode cells of nonirradiated hamsters (Fig. 3A). The resultsalso suggest that the number of viable cells decreasedsignificantly within 1 day after irradiation and remained at alow level even 12 days later (Fig. 3B).
Accumulation of phagocytic cells in lymph nodes of ham-sters receiving immune T cells. The ability of immune T cellsto recruit phagocytic cells into the infected lymph nodes was
TABLE 1. Ability of hamster T-cell subsets to confer protectionagainst challenge with T. pallidum subsp. pertenuea
Lymph node (mean ± SE)Type of cellstransferred No. of treponemes
UnfractionatedNormal T 3.5 ± 0.8 222 ± 42Immune T 1.8 ± 0.3b 21 ± 15b
FractionatedImmune 38- 2.3 0.6b 23 ± gbImmune 38+ 1.5 0.3b 13 ± 4b
None 4.8 ± 0.3 125 ± 32
aFour animals per group. Each hamster received 4 x 106 immune or normalcells, and all hamsters were sacrificed 21 days after infection.
b P < 0.05, determined by analysis of variation followed by Student's t test.
z0F-
C0.-
PS0
_ _4
40000 -
30000 -
20000-
10000 -
0
INFECT. IMMUN.
L3T4+- AND 38+-MEDIATED RESISTANCE TO T. PALLIDUM
z
r.4
04
FIG.cells fiobtaine
examisters ii
ing fo
cytomlymphin the(macr4B). Iineutrcpallidiand EFig. 4cytomthe lyi
5). Wthe lyin thewithin
Thi:ratedsets, I
30 stimulation and secrete IL-2. 38+ T cells, however, do not-U-NORMAL 4L-2 A. produce IL-2 and require exogenous IL-2 to respond to
25 - IRRADIATED IL-2 ConA stimulation. The tissue distribution of the two T-cellsubsets is similar in hamsters, mice, and humans (13, 29).
20 The hamster thymus contained predominantly dual-positivecells and small numbers of single-positive cells. In the
15 peripheral lymph nodes, however, L3T4+ and 38+ cells weremutually exclusive. The difference in IL-2 secretion between
10 the hamster T-cell subsets is also identical to that in themouse (31). In addition, Witte et al. (37) showed that 38-
5 (L3T4+) cells mediate delayed-type hypersensitivity re-sponses and 38+ cells mediate cytotoxicity against virally
o-___________________________________ infected targets. Therefore, L3T4+ cells and 38+ cells rep-0 2 4 6 8 10 12 14 resent helper and cytotoxic-suppressor T cells, respectively,in the hamster. We further demonstrated that MAb GK 1.5
DAYS POST-IRRADIATION (anti-L3T4) blocks the antigen- and mitogen-specific prolif-erative responses of hamster T cells (14a). This implies thatMAb GK 1.5 recognizes the CD4 molecule in the hamster,which further strengthens our conclusion that MAb GK 1.5recognizes hamster helper T cells. It is not clear, however,
108 whether the molecule recognized by MAb 38 is the CD8-U-- NORMAL B. homolog in the hamster.-0I tRADIA1MD T-cell-mediated protection against T. pallidum subsp.
pertenue infection resembles protection mechanisms againstmany bacterial, viral, and parasitic infections, since bothhelper-inducer and cytotoxic-suppressor T cells can conferlo 7 protection (14, 20, 33, 34). The mechanisms by which L3T4+
0 hand 38+ cells confer protection against treponemal infectionare not clear; however, several mechanisms could be pro-posed. First, L3T4+ T cells might secrete lymphokineswhich could amplify immune responses such as recruitingmore helper or cytotoxic T cells or macrophages into the
6l area of active infection. Second, L3T4+cells might enhance
10 *4 the phagocytic and/or treponemicidal activity of macro-0 2 4 6 8 10 12 14 phages. Third, the immune T cells and T-cell subsets might
DAYS POST-IRRADIATION kill treponemes directly or through the secretion of lymphok-3. (A) IL-2 production by 106 ConA-stimulated lymph node ines.
rom irradiated and normal hamsters. (B) Number of cells The first explanation is not supported by the present4d from irradiated and normal lymph nodes (LN). findings in that a significant influx of lymphocytes into the
area of infection was not seen early on. Furthermore, theinflux of cells observed later might be due to the accumula-tion of treponemes in the lymph node and might be irrelevant
ned by taking lymph node cells from recipient ham- to the protection that is observed before 21 days of infection.nfected with T. pallidum subsp. pertenue and perform- The second explanation needs to be elaborated. An accumu-erward- and 90-angle light scatter analysis by flow lation of macrophages has been observed in rabbit testesLetry. Figure 4A and B illustrate the bitmap of a normal infected with treponemes prior to resolution of orchitis (16).node preparation (lymphocyte population is indicated Phagocytosis by macrophages in vivo and in vitro has beenwindow in Fig. 4A) and a resident macrophage control demonstrated by several groups (1, 11, 17, 30). It has alsocophage population is indicated in the window in Flg. been suggested that the appearance of T cells before the
icontrast, flow cytometry analysis showed an influx of accumulation of macrophages is directly related to the;phils in the lymph nodes of all groups injected with T disappearance of the treponemes (16). However, no signifi-um subsp. pertenue 1 day after infection (Fig. 4C, D, cant accumulation of macrophages in hamsters infused with). Neutrophils have a distinct distribution (compare immune T cells was noted. Thus, a role for immune T cellsXC, D, and E with Fig. 4B. Using the same flow or T-cell subsets in recruiting macrophages in response toLetry analysis, we showed that the number of PMNs in treponemal challenge at the early time of immune T-cellmph nodes declined slightly 5 days after infection (Fig. transfer may be ruled out. Immune T cells and T-cell subsetse did not observe an accumulation of macrophages in probably use other mechanisms to eliminate treponemes.mph nodes of hamsters infused with immune T cells or A third possible mechanism for the protection of hamsterslymph nodes of hamsters that received normal T cells against challenge with T. pallidum subsp. pertenue by T cells
i 10 days after cell transfer. and T-cell subsets is based on the fact that PMNs andmacrophages were incapable of phagocytizing treponemes
DISCUSSION until the treponemes were killed by immune T cells. Directcytotoxicity by T cells has been demonstrated in some
s is the first report that hamster T cells can be sepa- bacterial infections (25). Podwinska (24) showed that ainto two phenotypically and functionally distinct sub- lymphotoxinlike lymphokine could kill treponemes in vitro.L3T4+ and 38+ cells. L3T4+ T cells respond to ConA Therefore, it is possible that immune T cells use an unknown
VOL. 59, 1991 533
534 LIU ET AL.
A. Normal Lymph Node
.~v aI
:
.-
90 DEGREE ANGLELIGHT SCATTER (LOG)
C. Normal T cells
adF-'F-4
0.
uz
biP.izra
D. Immune T cells E. No Cells
Lymphocytes90 DEGREE ANGLE LIGHT SCATTER (LOG)
FIG. 4. Forward- and 90°-angle light scatter analysis of lymph node cells by flow cytometry. Animals for panels C through E received 500rads of irradiation and also were challenged with 5 x 105 T. pallidum subsp. pertenue treponemes in the popliteal lymph node region. x axisrepresents log green fluorescence, and y axis represents linear forward light scatter.
mechanism to directly kill treponemes, while PMNs phago-cytize and remove the dead spirochetes. An alternativeexplanation is that immune T cells enhance the ability ofPMNs to phagocytize and kill the invading treponemes.Interferon secreted by immune T lymphocytes has beenshown to enhance Fc receptor expression and respiratoryburst by PMNs (4, 23). Future experiments are required toclarify the interactions between immune T cells, trepo-nemes, and PMNs.
It is not clear why macrophages did not accumulate in thelymph nodes of hamsters infused with immune T cells. Thisphenomenon, however, may explain the inability of recipi-ents of immune T cells to eliminate T. pallidum subsp.pertenue completely from the infected nodes. Macrophagesaccumulated after 5 days of infection might not be able toeliminate the invading treponemes, since the treponemesmight have become coated with host proteins and thusescaped immune surveillance (2, 7). It is generally believedthat PMNs are not able to phagocytize T. pallidum. Our
findings that PMNs accumulated in the regional lymph nodesin all groups injected with T. pallidum subsp. pertenuewithin the first 24 h is supported by Musher et al. (19). Theseinvestigators observed an influx of PMNs within 24 h at thesite where T. pallidum was injected. Furthermore, Musher etal. (19) demonstrated in vitro that the incoming PMNs areable to ingest and destroy the treponemes. The phagocytosistakes place within 5 min of incubation; degranulation andloss of treponemal integrity were observed after 4 h ofincubation. They were not able to explain, however, theinability of PMNs to eradicate the invading treponemes.Wecke et al. (36) showed an increased number of T. pallidumtreponemes inside PMNs after a high dose of penicillin. Theysuggested that the motility of T. pallidum prevents phagocy-tosis. Thus, these observations support the previous expla-nation that immune T cells or T-cell subsets kill T. pallidumsubsp. pertenue directly. The results further suggest that therole of macrophages and neutrophils might be limited tophagocytosis and removal of dead treponemes.
B. Peritoneal Macrophagesad
c;
u:=:
EP i
:b- r
PMNs
.3 -.2
4~~~~~~~~~~~1:0
0 11%, a L:.r -. 0 --ay eo60
ril
INFECT. IMMUN.
L.;,",,.5
L
L3T4+- AND 38+-MEDIATED RESISTANCE TO T. PALLIDUM
20
15 -
10 -
z
2
5-
I I I I I
0 2 4 6 8 10DAYS AFTER INFECTION
FIG. 5. Flow cytometry analysis of percentage of PMNs fromlymph nodes of hamsters which had been irradiated, infused withcells, and infected with T. pallidum subsp. pertenue. NT, Recipientsof normal T cells; IT, recipients of immune T cells; no cell, animalsthat did not receive any cell transfer. Each point represents themean value from three animals. The experiments were repeatedthree times, and similar results were observed.
It was suspected that 38+ cells might not function appro-
priately in irradiated recipient hamsters because of a lack ofIL-2-producing cells in irradiated animals. The results shownin Fig. 3A indicate that lymph node cells in hamstersirradiated with 500 rads produce as much IL-2 as cells fromnormal nonirradiated animals. The results should be inter-preted with caution, however, because the number of viablecells from irradiated lymph nodes was adjusted to the sameconcentration as that of the normal lymph nodes in order toevaluate IL-2 production in ConA-stimulated cultures. It isclear from Fig. 3B that the total number of live lymphocytesdeclined significantly in irradiated lymph nodes. Sprent et al.(31), however, showed that cytotoxic cells do not requirehelp from IL-2-producing cells to function in vivo.
Recently, -y5F T cells have been shown to be activated andpresumably involved in the protective immune response
against Mycobacterium tuberculosis (12). These -yb+ CD4-CD8- T cells have not been described in the hamster, nor isit known if they are involved in protection against infectionwith T. pallidum subsp. pertenue. It is possible that theCD4- CD8- T cells exist in the 38- population and mediateprotective immunity. Cross-contamination of 38+ T cellswith the 38- T-cell population and vice versa might haveoccurred. However, only 3 to 7% cross-contamination wasdetected, which is approximately 3 x 105 cells. Previously,we showed that 4 x 106 cells are required to confer protec-tion (15). This small number of contaminating cells may nothave contributed to the protection observed. We cannotexclude cooperation between the T-cell subsets.
Finally, treponemes have been shown to acquire hostproteins (2, 7), which might protect the treponemes fromimmune recognition. In addition, Radolf et al. (26) showedthat treponemes possess few surface protein antigens, whichmight contribute to poor antigenicity. These factors couldexplain the survival of treponemes in the presence oftreponemicidal antibody, immune T cells, and macrophages.We have been able to separate hamster T cells into two
phenotypically and functionally distinct subsets, L3T4+ (Thelper) and 38+ (T cytotoxic-suppressor) cells. For the firsttime, it has been demonstrated that both T-cell subsets canconfer protective immunity against infection with T. palli-dum subsp. pertenue. We were unable to demonstrate re-
cruitment of macrophages into the lymph nodes of hamstersthat had been infused with immune T cells. This suggeststhat other protective mechanisms may be used by the T cellsor T-cell subsets. Additional experiments are needed todetermine why the host cannot eradicate treponemes com-pletely despite the presence of both cell- and antibody-mediated imnmune responses. The availability of an inbredhamster model and monoclonal reagents provides a uniqueopportunity to gain insight into the mechanisms of cell-mediated immunity against treponemal infection.
ACKNOWLEDGMENTS
We thank Audrey Prieve for flow cytometry analysis and sortingand Jean Hawkins for preparation of the manuscript.
This work was supported by funds from the World HealthOrganization and by Public Health Service grant AI-22199 from theNational Institute of Allergy and Infectious Diseases.
REFERENCES1. Alder, J. D., N. Daugherty, 0. N. Harris, H. Liu, B. M. Steiner,
and R. F. Schell. 1989. Phagocytosis of Treponema pallidumpertenue by hamster macrophages on membrane filters. J.Infect. Dis. 160:289-297.
2. Alderete, J. F., and J. B. Baseman. 1979. Surface-associatedhost proteins on virulent Treponema pallidum. Infect. Imnmun.26:1048-1056.
3. Azadegan, A. A., R. F. Schell, B. M. Steiner, J. E. Coe, and J. K.Chan. 1986. Effect of immune serum and its immunoglobulinfractions on hamsters challenged with Treponema pallidum ssp.pertenue. J. Infect. Dis. 153:1007-1013.
4. Berton, G., L. Zent, M. A. Cassatella, and F. Rossi. 1986.Gamma interferon is able to enhance the oxidative metabolismof human neutrophils. Biochem. Biophys. Res. Commun. 138:1276-1282.
5. Bjerknes, R., C.-F. Bass0e, H. Sjursen, 0. D. Laerum, and C. 0.Solberg. 1989. Flow cytometry for the study of phagocytefunction. Rev. Infect. Dis. 11:16-33.
6. Dialynas, D. P., Z. S. Quan, K. A. Wall, A. Pierres, J. Quintans,M. R. Loken, M. Pierres, and F. W. Fitch. 1983. Characteriza-tion of the murine T cell surface molecule, designated L3T4,identified by monoclonal antibody GK 1.5: similarity of L3T4 tothe human Leu-3/T4 molecule. J. Immunol. 131:2445-2451.
7. Fitzgerald T. J., J. N. Miller, L. A. Repesh, M. Rice, and A.Urquhart. 1986. Binding of glycosaminoglycans to the surface ofTreponema pallidum and subsequent effects on complementinteractions between antigen and antibody. Genitourin. Med.61:13-20.
8. Gillis, S., M. M. Ferm, W. Ou, and K. Smith. 1978. T cellgrowth factor: parameters of production and a quantitativemicro assay for activity. J. Immunol. 120:2027-2032.
9. Gillis, S., and K. A. Smith. 1977. Long term culture of tumorspecific cytotoxic cells. Nature (London) 268:154-156.
10. Guesdon, J. L., T. Ternynck, and S. Avrameas. 1979. The use ofavidin-biotin interaction in immunoenzymatic techniques. J.Histochem. Cytochem. 27:1131-1139.
11. Hardy, P. H., Jr., D. J. Graham, E. E. Nell, and A. M.Dannenberg, Jr. 1979. Macrophages in immunity to syphilis:suppressive effect of concurrent infection with Mycobacteriumbovis BCG on the development of syphilitic lesions and growthof Treponema pallidum in tuberculin-positive rabbits. Infect.Immun. 26:751-763.
12. Janis, E. M., S. H. E. Kaufmann, R. H. Schwartz, and D. M.Pardoll. 1989. Activation of -yb T cells in the primary immuneresponse to Mycobacterium tuberculosis. Science 244:713-716.
13. Lanier, L. L., E. G. Engleman, P. Gatenby, G. F. Babcock, N. L,Warner, and L. A. Herzenberg. 1983. Correlation of functionalproperties of human lymphoid cells subsets and surface markerphenotypes using multiparameter analysis and flow cytometry.Immunol. Rev. 74:143-160.
14. Larsen, H. S., M.-F. Feng, D. W. Horohov, N. Moore, and B. IXRouse. 1984. Role of T-lymphocyte subsets in recovery fromh
cl NT
= rr-a- NO CELL
VOL. 59, 1991 535
536 LIU ET AL.
herpes simplex virus infection. J. Virol. 50:56-59.14a.Liu, H., and R. F. Schell. 1990. Abstr. Am. Soc. Biochem. Mol.
Biol.-Am. Assoc. Immunol. Joint Meet., abstr. 213, p. A1731.15. Liu, H., B. M. Steiner, J. D. Alder, D. K. Baertschy, and R. F.
Schell. 1990. Immune T cells sorted by flow cytometry conferprotection against infection with Treponema pallidum subsp.pertenue in hamsters. Infect. Immun. 58:1685-1690.
16. Lukehart, S. A., S. A. Baker-Zander, R. M. C. Lloyd, and S.Sell. 1980. Characterization of lymphocyte responsiveness inearly experimental syphilis. II. Nature of cellular infiltration andTreponema pallidum distribution in testicular lesions. J. Immu-nol. 124:461-467.
17. Lukehart, S. A., and J. N. Miller. 1978. Demonstration of the invitro phagocytosis of Treponema pallidum by rabbit peritonealmacrophages. J. Immun. 121:2014-2023.
18. Miller, J. N. 1971. Spirochetes in body fluids and tissues.Charles C Thomas, Publisher, Springfield, Ill.
19. Musher, D. M., M. Hague-Park, F. Gyorkey, D. C. Anderson,and R. E. Baughn. 1983. The interaction between Treponemapallidum and human polymorphonuclear leukocytes. J. Infect.Dis. 147:77-86.
20. Orme, I. M. 1988. Characteristics and specificity of acquiredimmunologic memory to Mycobacterium tuberculosis infection.J. Immunol. 140:3589-3593.
21. Ozato, K., N. Mayer, and D. H. Saches. 1980. Hybridoma celllines secreting monoclonal antibodies to mouse H-2 and Iaantigens. J. Immunol. 124:533-540.
22. Pavia, C. S., J. D. Folds, and J. B. Baseman. 1978. Cell-mediatedimmunity during syphilis. A review. Br. J. Vener. Dis. 54:144-150.
23. Perussia, B., E. T. Dayton, R. Lazarus, V. Fanning, and G.Trinchiere. 1983. Immune interferon induces the receptor formonomeric IgGl on human monocytic and myeloid cells. J.Exp. Med. 158:1092-1113.
24. Podwinska, J. 1987. Identification of cells producing anti-trepo-nemal lymphotoxin (ATL). Arch. Immunol. Ther. Exp. 35:63-70.
25. Powderly, W. G., J. R. Schreiber, G. B. Pier, and R. B.Markham. 1988. T cell countersuppressor cells in the generationof T cell immunity to Pseudomonas aeruginosa. J. Immunol.140:2746-2752.
26. Radolf, J. D., M. V. Norgard, and W. W. Schulz. 1989. Outermembrane ultrastructure explains the limited antigenicity ofvirulent Treponema pallidum. Proc. Natl. Acad. Sci. USA
86:2051-2055.27. Schell, R. F., A. A. Azadegan, S. G. Nitskansky, and J. L.
LeFrock. 1982. Acquired resistance of hamsters to challengewith homologous and heterologous virulent treponemes. Infect.Immun. 37:617-621.
28. Schell, R. F., J. L. LeFrock, J. P. Babu, and J. K. Chan. 1979.Use of CB hamsters in the study of Treponema pertenue. Br. J.Vener. Dis. 55:316-319.
29. Scollay, R., P. Bartlett, and K. Shortman. 1984. T cell develop-ment in the adult murine thymus: changes in the expression ofthe surface antigens Ly2, L3T4 and B2A2 during developmentfrom early precursor cells to emigrants. Immunol. Rev. 82:80-103.
30. Sell, S., S. A. Baker-Zander, and H. C. Powell. 1982. Experi-mental syphilitic orchitis in rabbits: ultrastructural appearanceof Treponema pallidum during phagocytosis and dissociation bymacrophages in vivo Lab. Invest. 46:355-364.
31. Sprent, J., M. Schaefer, D. Lo, and R. Korngold. 1986. Func-tions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo.Immunol. Rev. 91:195-218.
32. Stahli, C., V. Miggiano, J. Stocker, T. Staehelin, P. Haring, andB. Takacs. 1983. Distinction of epitopes by monoclonal antibod-ies. Methods Enzymol. 92:242-253.
33. Stern, J. J., M. J. Oca, B. Y. Rubin, S. L. Anderson, and H. W.Murray. 1988. Role of L3T4+ and Lyt2+ cells in experimentalvisceral leishmaniasis. J. Immunol. 140:3971-3977.
34. Suzuki, Y., and J. S. Remington. 1988. Dual regulation ofresistance against Toxoplasma gondii infection by Lyt2+ andLytl+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946.
35. Turner, T. B., and D. H. Hollander. 1957. Biology of thetreponematoses. WHO monograph series no. 35, p. 270. WorldHealth Organization, Geneva.
36. Wecke, J., J. Bartunek, and G. Stuttgen. 1976. Treponemapallidum in early syphilitic lesions in humans during high-dosage penicillin therapy: an electron microscopical study.Arch. Dermatol. Res. 257:1-15.
37. Witte, P. L., J. Stein-Streilein, and J. W. Streilein. 1985.Description of phenotypically distinct T-lymphocyte subsetswhich mediate helper/DTH and cytotoxic functions in theSyrian hamster. J. Immunol. 134:2908-2915.
38. Witte, P. L., and J. W. Streilein. 1983. Monoclonal antibodies tohamster class II MHC molecules distinguish T and B cells. J.Immunol. 130:2282-2286.
INFECT. IMMUN.