Review Application of Active Edible Coatings to Improve ...
Transcript of Review Application of Active Edible Coatings to Improve ...

Food Science and Technology Research, 24 (6), 949_962, 2018Copyright © 2018, Japanese Society for Food Science and Technologydoi: 10.3136/fstr.24.949
http://www.jsfst.or.jp
Review
Application of Active Edible Coatings to Improve the Shelf-life of Cheese
Niloofar Bagheripoor1, Sadegh Khoshgozaran-aBras
2, Sara sohraBvandi3*, Nasim Khorshidian
4, Amir Mohammad Mortazavian
5,6*, Neda MollaKhalili1 and Sahar Jazaeri
6
1Student Research Committee, Department of Food Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Food Science and Technology, Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran3Department of Food Technology Research, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Food Safety Research Center (Salt), School of Nutrition and Food Sciences, Semnan University of Medical Sciences, Semnan, Iran
5Food Safety Research center, Shahid Beheshti University of Medical Sciences, Tehran, Iran6Department of Food Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Received September 7, 2017 ; Accepted October 12, 2017
Milk and dairy products are staples of the human diet. Among them, cheese holds an important stance. Due to its biological and biochemically dynamic nature, cheese is a perishable product in need of innovative preservation methods. There have been an increasing trends for the application of natural-derived edible films/coatings and natural preservatives to extend its shelf-life. The present review focuses on the application of edible coatings and highlights their potential for prolonging the shelf-life up to one month, depending on the types of cheese, its antimicrobial type, and environmental conditions such as storage temperature.
Keywords: cheese, edible film/coating, shelf life, antimicrobial
*To whom correspondence should be addressed. E-mail: Sara Sohrabvandi; [email protected] Amir Mohammad Mortazavian; [email protected]
IntroductionCheese is a well-known name for a category of fermented
milk-based foodstuff, which besides microorganisms, mainly consists of casein, fat, and water. Cheese is a biochemically and biologically unstable food product (Ferrão et al., 2016; Karimi et al., 2011a; Karimi et al., 2011b). It is exposed to a variety of chemical and microbial deteriorations during processing and storage.
The surfaces of cheeses are prone to contamination by microorganisms due to their high water activity and suitable acidity conditions (Proulx et al., 2015) and one of the most favorable strategies to prolong cheese shelf life and enhance its quality is packaging. The packaging process exerts some basic functions including minimizing chemical, biochemical,
physical and microbiological deterioration and increasing the handling and marketing of food products (Khoshgozaran et al., 2012). However, synthetic packaging films have induced serious ecological problems due to their non-biodegradability. In this context, edible films and coatings have gained remarkable attention in recent years because of their advantages compared to conventional packaging. Edible films and coatings are produced exclusively from natural and renewable sources and if not consumed along with the food, degrade more easily than polymeric packaging. In some cases they can improve the sensory properties of foods through carrying flavoring or colorant agents. Furthermore, they can be used as vehicles to deliver antioxidant or antimicrobial agents and control the release of these substances is possible (Bourtoom, 2008;

N. Bagheripoor et al.950
Mohammadi et al., 2016). On the other hand, edible films have lower tensile strength than common plastic films, while their elongation-at-break varies largely. Some edible films have elongation values comparable to those of common plastic films. Some edible films and coatings show superior oxygen barrier properties. Water permeability of edible films and coatings is generally higher than common plastic films except lipid-based edible films (Han, 2014). Although permeability and mechanical properties of edible films and coatings are generally weaker than conventional ones and complete substitution of the latter by edible films/coatings is not suitable due to the imperfections, but development of edible films/coatings is growing and partial replacement can be recommended because of reduction in using synthetic materials (Falguera et al., 2011; Han and Krochta, 2001).
Many of the recently published studies have been directed towards cheese safety and extending its shelf life. Edible coatings, which add antimicrobials on to the surface of cheeses, are gaining a great deal of attention since they contain a high level of active ingredients in necessary targeted areas (Dos Santos Pires et al., 2008; Fajardo et al., 2010; Kristo et al., 2008; Ollé Resa et al., 2014).
Edible coating has been used to prolong the shelf life of cheeses for several years. They are mainly prepared by edible biopolymers and food-grade additives. The film-forming agents can be polysaccharides (either carbohydrates or gums), proteins, lipids, or a mixture of them (Gennadios et al., 1997). Glycerin, propylene glycol, sorbitol, sucrose, polyethylene glycol, corn syrup and water can also be added as plasticizers to change the physical properties or other functionality of the edible films (Guilbert et al., 1995; Kester 1986; Krochta 1997; Lieberman and Gilbert, 1973). Other ingredients including antioxidants, antimicrobials, nutrients, nutraceuticals, pharmaceuticals, flavors, colors as functional additives have also been used to extend the shelf life of cheeses.
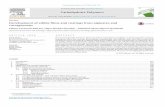
The mechanisms by which edible films/coatings increase
the shelf-life of cheeses are not only based on their antimicrobial activities, but also dependent on their barrier properties against deteriorative environmental parameters (Figure 1).
Among the recently introduced innovations such as irradiation, high-pressure processing, and electric field technology for foodstuff preservation, employment of antimicrobial compounds still occupies the most promising position. However, health concerns surrounding chemical preservatives has provoked multiple studies to replace chemicals with naturally-derived antimicrobials that are comparable to, if not better than, synthetic ones (Olasupo et al., 2003; Stevens et al., 1992). Naturally-oriented antimicrobials may be applied at the initial stages of cheese-making or on the f ina l p roduct by dus t ing , spraying , or immers ing . Antimicrobials may be also applied upon the packaging films or incorporated into the synthetic polymer substances utilized for cheese packaging, wherein they come in close contact with the cheese surface (Han, 2003; Vermeiren et al., 1999). Studies have shown that incorporation of antimicrobial agents into edible films/coatings is more effective than the direct incorporation of antimicrobial components to food products. Therefore, cheese scientists have indicated an increasing tendency to promote cheese safety and shelf-life using the antimicrobials incorporated into edible films/coatings.
Considering these facts, the objective of the present paper was to review the application of natural antimicrobial incorporated edible films/coatings to extend the shelf-life of cheese, which has been rarely explored. Some applications of edible films/coatings in cheese are summarized in Table1.
Some Natural Antimicrobials for Cheese Preservation through Coating
Different antimicrobial agents are used widely in foods. To use an antimicrobial agent in food packaging, it is necessary to follow the guidelines and regulation of country which they are
Fig. 1. The illustration of barrier properties of edible films and coatings to affect the cheese shelf-life.

Active Coatings for Improvement of Cheese Shelf-life 951
Tabl
e 1.
Th
e pu
blic
atio
ns o
n ap
plic
atio
n of
edi
ble
film
s and
coa
tings
in c
hees
es
Che
ese
type
Stor
age
tem
pera
ture
Film
or c
oatin
g co
mpo
nent
s ( %
)O
ptim
al fi
lm o
r coa
ting
( %)
Con
sequ
ence
s of a
pplic
atio
nR
efer
ence
s
Moz
zare
lla4 ℃
- Inn
ovat
ive
gel:
mix
ture
of p
olys
acch
arid
es
(aga
rose
and
gel
lan
as ty
pica
l com
pone
nts)
Inno
vativ
e ge
lSh
elf-
life
of c
hees
e w
as p
rolo
nged
to 2
0 da
ys.
(Lau
rienz
o et
al.,
200
6)
Kas
har
6 ℃
- Aci
d ca
sein
(7.5
)/ gl
ycer
ol (2
.5)/C
aCl 2
(0.0
125)
- Aci
d ca
sein
(7.5
)/ gl
ycer
ol (2
.5)/
CaC
l 2 (0
.012
5)/n
atam
ycin
(0.0
7 %
)
Aci
d ca
sein
(7.5
)/ gl
ycer
ol
(2.5
)/ C
aCl 2
(0.0
125)
/nat
amyc
in
(0.0
7 %
)
Nat
amyc
in-in
corp
orat
ed c
asei
n co
atin
g w
as a
ble
to su
ppre
ss m
ould
gro
wth
on
the
surf
ace
of c
hees
e fo
r abo
ut o
ne m
onth
w
ithou
t any
adv
erse
qua
lity
effe
cts.
(Yild
irim
et a
l., 2
006)
Moz
zare
lla15 ℃
- Act
ive
gel:
sodi
um a
lgin
ate
and
lem
on
extra
ct a
t thr
ee le
vels
of 5
00, 1
000
and
1500
pp
m
Act
ive
gel (
effe
ctiv
e co
ncen
tratio
n of
lem
on e
xtra
ct w
as n
ot
men
tione
d)
Slig
ht in
crea
se in
the
shel
f life
of c
hees
e w
as re
porte
d, c
a 1
day.
(Con
te e
t al.,
200
7)
Moz
zare
lla10 ℃
- Chi
tosa
n (3
%)/
glyc
erol
(25
%) w
ith p
H 4
.4- C
hito
san
(3 %
)/ gl
ycer
ol (2
5 %
) with
pH
5.2
- Chi
tosa
n (3
%)/
glyc
erol
(25
%)/
lyso
zym
e (6
0 %
) with
pH
4.4
- Chi
tosa
n (3
%)/
glyc
erol
(25
%)/
lyso
zym
e (6
0 %
) with
pH
5.2
. in
3 ap
plic
atio
n fo
rms o
f fil
m, c
oatin
g an
d la
min
ated
film
Film
of c
hito
san
(3 %
)/ gl
ycer
ol
(25
%)/
lyso
zym
e (6
0 %
) with
pH
4.
4
Chi
tosa
n-ly
sozy
me
com
posi
te fi
lms
sign
ifica
ntly
redu
ced
the
grow
th o
f E.
coli,
P. fl
uore
scen
s, L.
mon
ocyt
ogen
es a
nd
mol
d w
ith le
sser
ant
imic
robi
al e
ffect
on
yeas
t.
(Dua
n et
al.,
200
7)
Fior
di L
atte
10 ℃
- Sod
ium
alg
inat
e (8
%)/
lyso
zym
e (0
.25
mg/
mL)
and
ED
TA (5
0 m
M)
- Sod
ium
alg
inat
e (8
%)/
lyso
zym
e (0
.25
mg/
mL)
and
ED
TA (5
0 m
M)
with
MA
P (3
0 %
CO
2, 5
% O
2, 65
% N
2)
Sodi
um a
lgin
ate
(8 %
)/ly
sozy
me
(0.2
5 m
g/m
L) a
nd
EDTA
(50
mM
) with
MA
P (3
0 %
C
O2,
5 %
O2,
65 %
N2)
Incr
easi
ng th
e sh
elf l
ife o
f che
ese
to m
ore
than
3 d
ays.
(Con
te e
t al.,
200
9)
Reg
iona
l Sal
oio
4 ℃
- Chi
tosa
n (0
.5 &
1.5
%)/g
lyce
rol (
0.5
&
2.0
%)/
sorb
itol (
0.5
& 2
.0 %
)/ co
rn o
il (0
.5 %
)- G
alac
tom
anna
n (0
.5 &
1.5
%)/g
lyce
rol (
0.5
& 2
.0 %
)/ so
rbito
l (0.
5 &
2.0
%)/
corn
oil
(0.5
%)
- Aga
r (0.
5 &
1.5
%)/g
lyce
rol (
0.5
& 2
.0 %
)/ so
rbito
l (0.
5 &
2.0
%)/
corn
oil
(0.5
%)
gala
ctom
anna
n (1
.5 %
)/gly
cero
l (2
.0 %
)/ co
rn o
il (0
.5 %
)C
oate
d ch
eese
had
low
er g
as tr
ansf
er ra
tes
and
decr
ease
of t
he w
eigh
t los
s (ca
. 8-f
old
less
) with
con
trolle
d m
old
grow
th o
n th
e su
rfac
e.
(Cer
quei
ra e
t al.,
200
9)
Fior
di L
atte
4 ℃
- Alg
inat
e (8
%)/
CaC
l 2 (5
%) w
ith M
AP
(30
%
CO
2, 5
% O
2, 65
% N
2)- A
lgin
ate
(8 %
)/ C
aCl 2
(5 %
)/ ly
sozy
me
(0.2
5 m
g m
L_ 1 )/ N
a 2-E
DTA
(50
mM
) with
MA
P- C
hito
san
(0.0
12 %
)/ A
lgin
ate
(8 %
)/ C
aCl 2
(5 %
)/ w
ith M
AP
- Chi
tosa
n (0
.012
%)/
Alg
inat
e (8
%)/
CaC
l 2 (5
%)/
lyso
zym
e (0
.25
mg
mL_ 1 )/
Na 2
-ED
TA
(50
mM
) with
MA
P
Chi
tosa
n (0
.012
%)/
Alg
inat
e (8
%)/
CaC
l 2 (5
%)/
lyso
zym
e (0
.25
mg
mL_ 1 )/
Na 2
-ED
TA (5
0 m
M) w
ith M
AP
(30
% C
O2,
5 %
O
2, 65
% N
2)
Sign
ifica
nt sh
elf l
ife p
rolo
ngat
ion
to 5
da
ys.
(Del
Nob
ile e
t al.,
200
9)

N. Bagheripoor et al.952
Reg
iona
l Sal
oio
4 ℃
- Chi
tosa
n (0
.5 %
)/ gl
ycer
ol (0
.5 %
)/ Tw
een
80 (0
.2 %
)- C
hito
san
(0.5
%)/
glyc
erol
(0.5
%)/
Twee
n 80
(0.2
%)/
nata
myc
in (0
.5 m
g m
L_ 1 )
Chi
tosa
n (0
.5 %
)/ gl
ycer
ol (0
.5 %
)/ Tw
een
80 (0
.2 %
)/ na
tam
ycin
(0.5
m
g m
L_ 1 )
Chi
tosa
n-ba
sed
coat
ing/
film
s can
be
used
as
car
rier f
or n
atam
ycin
to e
xten
d ch
eese
sh
elf-
life.
(Faj
ardo
et a
l., 2
010)
Ric
otta
4 ℃
- Gal
acto
man
nan
(0.5
%)/
glyc
erol
(1.5
%)
- Gal
acto
man
nan
(0.5
%)/
glyc
erol
(1.5
%)/
nisi
n (5
0 IU
g_ 1 )
Gal
acto
man
nan
(0.5
%)/
glyc
erol
(1
.5 %
)/ ni
sin
(50
IU g
_ 1 )G
alac
tom
anna
n-ba
sed
film
s con
tain
ing
nisi
n ca
n be
use
d to
redu
ce L
. m
onoc
ytog
enes
pos
t-con
tam
inat
ion
on
chee
se.
(Mar
tins e
t al.,
201
0)
Reg
iona
l Sal
oio
4 ℃
or 2
0 ℃
- Chi
tosa
n (0
.5 %
)/ gl
ycer
ol o
r sor
bito
l (2.
0 %
)- G
alac
tom
anna
n (1
.5 %
)/ gl
ycer
ol (2
.0 %
)/ co
rn o
il (0
.5 %
)
Gal
acto
man
nan
(1.5
%)/
glyc
erol
(2
.0 %
)/ co
rn o
il (0
.5 %
)C
oatin
g ca
n co
nsid
erab
ly im
prov
e th
e re
duct
ion
of w
ater
loss
and
dec
reas
ed
the
colo
r cha
nge
and
mic
robi
al c
ount
s;
ther
efor
e, it
can
be
used
to im
prov
e th
e ch
eese
shel
f life
.
(Cer
quei
ra e
t al.,
201
0)
Ric
otta
4 ℃
- Chi
tosa
n (0
.8 %
)/ w
hey
(2.4
%) w
ith M
AP
(40
% C
O2,
60 %
N2)
Chi
tosa
n (0
.8 %
)/ w
hey
(2.4
%)
with
MA
P (4
0 %
CO
2, 60
% N
2)C
hito
san/
whe
y ed
ible
film
s red
uced
m
icro
bial
gro
wth
and
del
ayed
the
deve
lopm
ent o
f und
esira
ble
acid
ity
with
out e
xerti
ng a
ny a
dver
se e
ffect
s on
chee
se se
nsor
ial p
rope
rties
und
er M
AP
cond
ition
.
(Di P
ierr
o et
al.,
201
1)
Min
i red
Bab
ybel
4 ℃
- Sod
ium
cas
eina
te (6
% w
/v)/
sorb
itol (
25 %
)/ ni
sin
(100
0 IU
cm
-2 )So
dium
cas
eina
te (6
% w
/v)/
sorb
itol (
25 %
)/ ni
sin
(100
0 IU
/cm
2 )
Nis
in-in
corp
orat
ed so
dium
cas
eina
te fi
lms
wer
e pr
omis
ing
to e
xten
d th
e ch
eese
shel
f lif
e an
d po
ssib
ly to
enh
ance
the
mic
robi
al
safe
ty o
f che
eses
.
(Cao
-Hoa
ng e
t al.,
201
0)

Active Coatings for Improvement of Cheese Shelf-life 953
Tabl
e 2.
D
efin
ition
and
act
ion-
mod
e of
ant
imic
robi
als a
pplie
d to
pro
long
the
shel
f life
of t
he c
hees
e
Nam
eD
efini
tion
Mod
e of
act
ion
Ref
eren
ces
Lyso
zym
eA
lytic
enz
yme
also
kno
wn
as m
uram
idas
e or
N
-ace
tylm
uram
ide
glyc
anhy
drol
ase,
are
gly
cosi
de
hydr
olys
es, e
nzym
es (E
C3.
2.1.
17),
occu
rrin
g in
va
riety
of n
atur
al fo
odst
uff s
uch
as m
ilk a
nd e
ggs.
- Lys
ozym
e bi
nds a
nd h
ydro
lyze
s the
1, 4
-bet
a-lin
kage
s bet
wee
n N
-ace
tylm
uram
ic a
cid
and
N-a
cety
l-D-g
luco
sam
ine
grou
ps o
ccur
ring
in a
pe
ptid
ogly
can
laye
r in
cellu
lar w
alls
of b
acte
ria a
nd b
etw
een
N-a
cety
l-D-
gluc
osam
ine
resi
dues
in c
hito
dext
rins.
(Con
te e
t al.,
200
6; C
rapi
si e
t al
., 19
94; P
eter
s et a
l., 1
989)
Nis
inA
nat
ural
pol
ypep
tide
prod
uced
by
certa
in st
rain
s of
the
food
-gra
de la
ctic
aci
d ba
cter
ium
(Lac
toco
ccus
la
ctis
ssp.
Lac
tis) d
urin
g fe
rmen
tatio
n.
- Firs
tly, n
isin
com
es in
a c
ompl
ex re
actio
n w
ith L
ipid
II (a
pre
curs
or c
ompo
nent
in
the
proc
ess o
f cre
atio
n of
bac
teria
l cel
l wal
ls).
Con
sequ
ently
, the
yie
lded
ni
sin–
lipid
II c
ompl
ex c
reat
es th
e po
res w
ithin
the
cyto
plas
mic
mem
bran
e, b
eing
fo
llow
ed b
y ef
flux
of v
ital c
ellu
lar s
ubst
ance
s, w
hich
in tu
rn c
ause
s pre
vent
ba
cter
ia fr
om g
row
th o
r eve
n be
ing
hydr
olyz
ed.
(Del
ves-
brou
ghto
n, 2
005)
Nat
amyc
inA
lso
know
n as
pim
aric
in b
elon
ging
to p
olye
thyl
ene
antib
iotic
s, is
a n
atur
al a
ntifu
ngal
age
nt, w
hich
is
prod
uced
by
the
Stre
ptom
yces
nat
alen
sis a
nd re
late
d
spec
ies,
durin
g su
bmer
ged
aero
bic
ferm
enta
tion.
- Nat
amyc
in, t
o pr
even
t the
gro
wth
of f
ungi
, bin
ds th
e m
embr
ane
ster
ols,
pres
ent
excl
usiv
ely
in th
e fu
ngi p
lasm
a m
embr
anes
, whi
ch re
sults
in d
isto
rting
the
sele
ctiv
ity o
f the
mem
bran
e pe
rmea
bilit
y.
(Ham
ilton
-Mill
er, 1
974)
Chi
tosa
nA
nat
ural
cat
ioni
c hy
drop
hilic
pol
ysac
char
ide
[pol
y-α-
(1-4
)-am
ino-
2deo
xy-D
-glu
cose
], ob
tain
ed b
y al
kalin
e
N-d
eace
tyla
tion
of c
hitin
.
- Chi
tosa
n al
ters
the
cell
perm
eabi
lity
caus
ed b
y th
e in
tera
ctio
n be
twee
n
posi
tivel
y ch
arge
d ch
itosa
n m
olec
ules
and
the
nega
tivel
y ch
arge
d m
icro
bial
cel
l
mem
bran
es, a
nd/o
r pre
vent
s the
mic
roor
gani
sms f
rom
gro
wth
thro
ugh
chel
latin
g
the
nece
ssar
y nu
trien
ts a
nd sp
ore
elem
ents
as w
ell a
s tra
ce m
etal
s, an
d/or
inte
rfer
es w
ith m
RN
A a
nd p
rote
in sy
nthe
sis a
s it b
inds
with
DN
A, w
hich
is a
s a
cons
eque
nce
of it
s pen
etra
tion
tow
ard
the
mic
roor
gani
sms n
ucle
i
(Cue
ro e
t al.,
199
1; L
iu e
t
al.,
2004
; Rab
ea e
t al.,
200
3;
Shah
idi e
t al.,
199
9)
Lem
on e
xtra
ctA
nat
ural
com
poun
d ex
tract
ed fr
om c
itrus
frui
ts- N
ot m
entio
ned.
(Bel
letti
et a
l., 2
004)
EDTA
A p
olya
min
o ca
rbox
ylic
aci
d an
d a
colo
urle
ss, w
ater
-
solu
ble
solid
.
- ED
TA a
cts a
s che
latin
g ag
ent,
bind
ing
met
al c
atio
ns; i
t des
tabi
lizes
the
lipop
olys
acch
arid
e la
yer o
f out
er m
embr
ane
of G
ram
-neg
ativ
e ba
cter
ia, w
hich
in
turn
mak
es th
em se
nsiti
ve to
com
mon
ant
imic
robi
als s
uch
as le
mon
ext
ract
.
(Hel
ande
r et a
l., 1
997)

N. Bagheripoor et al.954
going to use (Ahvenainen, 2003). The applied active components to prolong the shelf-life of coated cheese are listed in Table 2.
Natural antimicrobial agents are preferable in foods edible packaging, not only because their regulation process is easier but also because consumers prefer them compared to other antimicrobial agents (Han, 2003). Regarding these facts, some natural antimicrobial agents which are mostly used in edible packaging for cheese are discussed in the next section.
Lysozyme To inhibit the growth of Clostridia, which is capable of fermenting the lactate and producing gas in cheese, the application dosage of 25 mg/g cheese for lysozyme has been suggested (Crapisi et al., 1994). In general, Gram-positive bacteria are reported as sensitive microorganisms to lysozyme attacks (Conte et al., 2006). However, Gram-negative bacteria are less sensitive, since their outer membrane is mainly composed of lipopolysaccharide which acts as a shield to protect the action site on the peptidoglycan present in the bacterial cell wall. For Gram-negative bacteria, a non-enzymatic mode of action has been proposed, in which the bacterial membrane can be disrupted by the positive charge of lysozyme or can be opened up by substances such as chitosan. As a result, lysozyme can get access to the action site situated on the peptidoglycan and hydrolyzes it, which causes cell lysis (Song et al., 2002). The application of lysozyme to extend the shelf life of dairy products, especially cheese, has been examined in different studies (Lucera et al., 2014). The effect of different immobilization methods on the functionality of lysozyme have also been investigated (Lian et al., 2012). The result indicated that covalently bounded enzymes maintained 80 % of their initial activity.
Nisin Nisin is a hydrophobic, cationic, toxicologically safe, and small polypeptide (3.5 kDa), which has been recognized as a natural antibacterial preservative by FAO/WHO. The FDA has categorized it as “generally recognized as safe (GRAS)” and can be applied in food products such as cheese (Nguyen et al., 2008). Acidic conditions increase the antimicrobial activity of nisin, since it has been reported that it is more stable at low pH (Rollema et al., 1995). Nisin is also claimed to have high antimicrobial activity against Gram-positive bacteria such as Listeria monocytogenes and Clostridium botulinum and is especially efficient against spores (Ko et al., 2001). It has been illustrated that Gram-negative bacteria can be sensitized by pre-treatments involving exposure to sub-lethal heat, osmotic shock, freezing, and agents such as EDTA, which are effective in destroying the outer membrane integrity, and in turn nisin is permitted to access cytoplasmic membrane (Delves-Broughton, 2005). The incorporation of nisin as an antimicrobial agent in different edible coating bases of cheese have been investigated in different studies (Coma et al., 2001; Jalilzadeh et al., 2015; Ramos et al., 2012). The results of nisin addition to galactomannan films indicated that nisin addition was effective in retarding the microbial growth
significantly. The physical and mechanical properties of the films were also affected by nisin addition in a way that the oxygen permeability decreased and CO2 permeability, opacity, tensile strength increased (Coma et al., 2001).
Natamycin Natamycin has been introduced as a fungicide agent that is more efficient in demolishing fungi at low concentrations in comparison with other well-known fungicides such as sorbates and propionates (Hamilton-Miller, 1974). It has been characterized as insoluble in water as well as most of other food-grade solvents mainly because of its heavy molecular weight (666 g. mol
_1) and its conjugated double-bond structure (Shahani, 1973). Therefore, it is applicable for the surface treatment of cheese (with an aqueous solution containing 200‒300 ppm of natamycin), wherein it does not negatively affect the flavor and cheese microflora as Penicillium does (De Ruig and Berg, 1985). Despite the available proofs for the non-toxicity of natamycin, even at high levels of ingestion, its usage as a food preservative has been limited to 0.3 mg. kg
_1 (as daily maximum allowable amount) according to joint FAO/WHO Expert Committee on Food Additives (Delves-broughton, 2005). In addition, the maximal amount of natamycin must not exceed 1 mg. dm
_2 and its maximal concentration must not be detected further than 5 mg. kg
_1 at 2 mm depth in the finished products (de Oliveira et al., 2007).
Chitosan Chitosan has been indicated to offer considerable antimicrobial activities in the case of wide range of microorganisms including bacteria, yeasts, molds, and even viruses (Coma et al., 2002; Rhoades and Roller, 2000). Multiple factors have been identified to be effective in the biological activity of chitosan including substitution, derivatization and deacetylation degree, molecular weight, length and position of a substituent in glucosamine units of chitosan, target microorganism, and last but not the least, pH of chitosan solution (Chirkov, 2002; Devlieghere et al., 2004; Rabea et al., 2003; Zheng and Zhu, 2003). At acidic pH, lower than that of chitosan pKa (around 6.1 to 6.7 for chitosan with 90 % deacetylation), the NH2 groups located on the glucosamine residues of chitosan are protonated and come in interaction with the negatively charged microbial cell membranes, which leads to the damaged cell membrane permeability of microorganisms (Tsai and Su, 1999).
EDTA (ethylenediaminetetraacetic acid) EDTA is a well-known chelating agent, which has been widely employed to control oxidation and other metal ion-catalyzed deteriorative reactions in food products. This chelating agent is also recognized as an effective agent that can act synergistically with other antimicrobials such as lysozyme, especially against Gram-negative bacteria (Branen and Davidson, 2004; Gill and Holley, 2000). Multiple types of EDTA salts have been applied in food products; Na2-EDTA, for instance, is the most common and has been approved to be used in canned carbonated soft drinks, canned cooked vegetables, potato salad, and other foods

Active Coatings for Improvement of Cheese Shelf-life 955
as well as aqueous multivitamins preparation (Regulations, 1998) within the range of 35 to 800 ppm (Heimbach et al., 2000). Moreover, EDTAʼs NOAEL value (no observed adverse lethal effect level) is 500 mg. kg
_1. day_1 and it is worth
mentioning that it does not accumulate in biological tissues and more importantly, is not carcinogenic (SIAM, 2001).
Properties of Edible Films/coatings for Cheese Coating Physical properties Thickness The effectiveness of an edible packaging is
determined by the coating material properties and execution method (Zhong et al., 2014). Film thickness is a key factor in the ratio of gas and moisture transmission which contributes to the appearance and taste of products (Embuscado and Huber, 2009). In a study conducted by Zhong et al. (2014), it was revealed that the thickness of the edible film was different based on the coating method (dipping, enrobing, spraying and electrostatic spraying) which ranged from 30.6 to 83.3 µm. Di Pierro et al. (2011) indicated an increase in the thickness of chitosan film as a result of 2.4 % milk whey protein addition, which could be attributed to the increase in the total weight of film-forming solutions. Martins et al. (2010) reported that the incorporation of nisin (50 IU. g
_1) caused increased thickness in galactomannan-based films, which was rationalized as the formation of larger clusters in the film matrix due to the hydrophobic interactions desirable for nisin molecules in the film-forming solution (Ko et al., 2001).
Optical properties Despite the fact that both opacity and color of films have a direct impact on the appearance of coated food products and consumer acceptability, films should have appropriate barrier properties against light exposure in order to prevent undesirable light-induced reactions such as oxidation of lipids, resulting in an off-flavor (Yang and Paulson, 2000). As expected, components contained in film-preparing solutions possess a significant role in opacity and color parameters of edible films. Martins et al. (2010) observed that incorporation of nisin (at 50 IU. g
_1) influenced film transparency and increased its opacity, whereas nisin-free galactomannan films were transparent and yellowish, which can be related to the dispersion of nisin and glycerol within the galactomannan matrixes. Fajardo et al. (2010) illustrated that chitosan-based films containing natamycin at the level of 0.5 mg. mL
_1 cause an increase in their opacity compared to the films without natamycin. Yang and Paulson (2000) demonstrated that quantity of lipid in film-forming preparations had an impact on film opacity as the opacity of gellan films. Cerqueira et al. (2009) indicated that the films of both galactomannan and agar containing sorbitol and corn oil at the same level (0.5 %) had higher values of opacity, while with regard to chitosan-based films; high opacity belonged to the oil-free ones. However, Martins et al. (2010) showed that galactomannan films experienced no significant alterations in their color parameters as a result of nisin incorporation at the level of 50 IU. g
_1.
Sivarooban et al. (2008) also reported similar data as nisin was incorporated into soy protein-based films.
Mechanical properties Proper mechanical properties, such as tensile strength (TS) indicating the threshold tensile stress of film and elongation at break (E, in %) as the maximum change in the length of a film before being broken are necessary for a film to strengthen film resistance against external tensions and to retain its integrity over the whole stages of processing, application, shipment, and storage up to being delivered to final consumers (Srinivasa et al., 2007). In addition, since the mechanical characteristics of films are in tight relationship with their chemical structures and are influenced by laboratory circumstances such as humidity and temperature factors, environmental conditions should be controlled when mechanical tests are being conducted (Martins et al., 2010).
Martins et al. (2010) indicated that incorporation of nisin (at 50 IU. g
_1) into the film-forming solution of galactomannan (0.5 %) resulted in a significant increase in tensile strength of the yielded film. They demonstrated that the possible network formation between nisin and galactomannan molecules could be the probable explanation for the observed increase. However, Sivarooban et al. (2008) demonstrated that the incorporation of nisin into soy protein-based films led to a decrease in tensile strength. Martins et al. (2010) also pointed out that the elongation at break values of galactomannan films was significantly increased as a result of nisin addition (at the level of 50 IU. g
_1), which was consistent with the results obtained by Pranoto et al. (2005) for nisin-incorporated chitosan films at the same level of nisin addition.
Chemical propertiesWettability Wettability or surface hydrophobicity of the
coatings is defined as the contact angles of a liquid over a solid. It is crucial for the applied coating to be well-spread over the food surface and leave no uncoated areas (Karbowiak et al., 2006). Wettability feature is influenced by the polarity compatibility of the coating-forming preparations and that of apolar surface of cheese (Cerqueira et al., 2009). In a study conducted by Martins et al. (2010), wettability of the yielded coatings decreased as galactomannan concentration increased in coat-forming solutions (from 0.5 to 1.5 %) and the oil concentration decreased to 0 %. Also, it was shown that higher values of wettability belonged to the coatings which contained glycerol at the level of 1.5 % as a plasticizer, which principally influenced texture, structure, and surface properties of the coatings (Casariego et al., 2008). The same results were obtained by Cerqueira et al. (2009), who observed that oil-free chitosan films presented better values of wettability (at 0.5 % level of chitosan). In addition, they reported that, in order to yield chitosan films with suitable wettability, the presence of Tween 80 at the level of 0.2 % was unavoidable. This observation could be attributed to the high ratio between the Tween 80 and chitosan concentrations, as Tween 80 reduces the superficial tension of the liquid and thereby enhances the

N. Bagheripoor et al.956
compatibility between the film-forming solution and the cheese surface. Moreover, the presence of oil accompanying Tween 80 in the solution, will result in the formation of a micelle structure, wherein chitosan and oil are interlinked by the hydrophilic and hydrophobic proportions of the Tween 80 molecules and Tween 80 molecules in turn occupy the micelles and could not present any contribution to decreasing wettability values (Cerqueira et al., 2009).
Migration or release of antimicrobial compounds In general, migration of antimicrobial agents is a diffusion process and should follow the guidelines governed by the European parliament and council law as stated by the “Legislation for migration of components from the packaging film to the product”. The document outlines antimicrobials migration is not allowed to exceed 60 mg of packaging material per kg of foodstuff (Parliament, 2004). In order to sustain a critical surface concentration of the antimicrobial agent on foodstuff, a slow release is preferable, which is influenced by the water activity of release medium, thickness, and solubility of films/coatings as well as storage temperature (de Oliveira et al., 2007; Hanušová et al., 2010; Vargas et al., 2009).
Fajardo et al. (2010) were the only investigators who studied the migration of the antimicrobial agent from edible films to cheese surface. In order to monitor the release behavior, they put the chitosan films in contact with phosphate buffer saline (PBS) solution and cheese surface at 4 ℃. As expected, the diffusion coefficient of natamycin within the film was 3.60 × 10
_10 ± 0.26 × 10_10 cm2. s
_1, which was higher than that of natamycin released to PBS solution. Results of the natamycin migration from chitosan films to Saloio cheese presented a higher XF value (deviation of the transport mechanism from the ideal Fickian behavior) of 0.79 ± 0.06 in comparison with that of PBS solution (0.36 ± 0.02); the significant reduction in water activity of release medium could be the possible explanation (Siepmann and Siepmann, 2008). Despite slow release from chitosan films to the surface of the cheese samples (1.29 ± 0.35 × 10
_12 cm2. s_1), Fajardo et al.
(2010) obtained the release values of up to 80 % for the initial natamycin concentration released after 23 days of storage; hence, they established that their film preparation is an efficient method and can be exploited as a potential active packaging material for cheese types.
Permeability propertiesWater vapor permeability (WVP) WVP is generally
defined as the efficiency of a film as a barrier for water vapor diffusion, which mainly occurs through the hydrophilic portion of films. It is particularly essential for foodstuff films/coatings, since one of the main targets of food packaging is to manage water loss, resulting in the weight loss of foodstuff and/or water condensation. Water vapor permeability is affected as a funct ion of mult iple variables , such as preparat ion methodology, thickness, biopolymer water content, porosity degree, and composition (especially hydrophilic-hydrophobic
ratio) of films (Bifani et al., 2007; Hernández-Muñoz et al., 2004).
Regarding the incorporation of antimicrobial agents, Martins et al. (2010) demonstrated that the incorporation of nisin at the level of 50 IU. g
_1 affected the WVP of galactomannan-based films (0.5 %) and it was reduced without a statistically significant difference. The authors explained their observation as being correlated with nisinʼs smaller molecular size or its lower quantity in the film matrix. Kristo et al. (2008) obtained no provoked changes in the values of WVP of sodium caseinate films as a result of nisin incorporation. However, they observed an improvement in WVP property, which could be related to the presence of hydrophobic-type physical obstacles (Sebti et al., 2002). Fajardo et al. (2010) reported that natamycin incorporation at 50 mg. mL
_1 into chitosan films increased the WVP values, which was not statistically significant.
Oxygen permeability (O2P) Films with an appropriate oxygen barrier characteristics are able to present a great contribution to food quality and shelf-life promotion. In general, polysaccharide-based films are well-known as good oxygen barriers according to the available reports on their firmly packed and arranged network structure (Cerqueira et al., 2010; Vargas et al., 2009). Cerqueira et al. (2009) showed that the increase of galactomannan and agar concentrations to 1.5 % contributed to the decrease in the oxygen permeability of films. They also indicated that higher concentrations of glycerol increased O2P of films. However, the films from galactomannan solution presented a lower value of O2P with glycerol at the concentration of 2 %. In the latter case, the effect of the galactomannan concentration apparently restricted the effect of glycerol concentration, while reduction in glycerol level from 2 to 0 % in the films made from agar solutions was followed by a decline in their O2P values. In fact, gas molecules diffusion into the film matrix was enhanced as a function of the plasticizer, which reduced the intermolecular attractions occurring within polymeric chains (Kester, 1986). However, it is also reported that the incorporation of plasticizer declines the quantity of present cracks and pores, which in turn results in the modification of dispersion and reduction of gaseous diffusion (Garcia et al., 2000). In the case of chitosan-based films, the samples with higher concentrations of plasticizer (glycerol at 2 %) had statistically improved O2P (Cerqueira et al., 2009). However, the authors observed that the replacement of glycerol with sorbitol (at the same level) stimulated an increase in the O2P value of the film samples. This difference could be presumably attributed to different molecular sizes and hygroscopicity between sorbitol and glycerol (Hong and Krochta, 2003). Fajardo et al . (2010) observed that incorporation of natamycin (0.5 mg. mL
_1) into chitosan-based films resulted in an increased value of O2P, which can be related to solving more sites formed for the oxygen molecules that leads to the increased mobility of oxygen molecules within

Active Coatings for Improvement of Cheese Shelf-life 957
the polymer and, as a result, the degree of oxygen permeability. However, Martins et al. (2010) found that nisin addition at 50 IU. g
_1 to galactomannan films could reduce the O2P of films compared with the nisin-free ones, which can be explained by the small size of nisin molecules; in consequence, filling the small empty spaces in the polymer matrix and probably oxygen diffusion was surpassed. Di Pierro et al. (2011) reported a significant decline in O2P values of chitosan-based films when whey protein (2.4 %) was incorporated.
Carbon dioxide permeability (CO2P) Permeability of packaging films to CO2 is a considerable feature, since the manipulation of film permeability to carbon dioxide could present an affirmative impact on the efficiency of antimicrobial components against spoilage microorganisms (Martins et al., 2010). The possible mechanism of CO2 action includes the modifications in the portion of short-chain fatty acids in the cell membrane and consequent ly af fects the membrane permeability (Gandhi and Chikindas, 2007). In general, permeability of CO2 is more influenced by alterations in the film composition. Cerqueira et al. (2009) showed the increase of the polysaccharide concentrations (galactomannan, agar and chitosan) provoked a decrease of CO2P, wherein the highest CO2P belonged to the films consisting of polysaccharides at the concentration of 0.5 %. Martins et al. (2010) indicated that nisin incorporation (at 50 IU g
_1) into galactomannan solution (0.5 %) affected film permeability to carbon dioxide and significantly increased CO2P compared to the nisin-free film samples. They attributed this observation to the interactions between CO2 non-polar molecules and apolar nisin molecules. Moreover, in the study performed by Fajardo et al. (2010), it was shown that the incorporation of natamycin at the level of 0.5 mg. mL
_1 into chitosan-based films (with chitosan at 0.5 %) caused an increase in the filmsʼ CO2 permeability, which can be attributed to the better solubility of CO2 in the films with natamycin and/or a heterogeneous structure formed because of natamycin larger size and bulky shape crystals, resulting in the creation of holes in the film matrix. However, the combination of milk whey protein at the level of 2.4 % with chitosan as film-forming solution considerably decreased CO2 permeability (Di Pierro et al., 2011).
Characteristics of Edible-coated CheesesPhysicochemical properties pH value pH parameter is considered as a crucial marker
of an optimal cheese-making process, since the acidity of products is one of the interior factor which affects the product flavor and also inhibits the growth of pathogens with the influence of enzymes activities as well as mineral balance (Corradini, 1995). For the pH evolution of the coated cheeses over storage period, most works have reported a slight increase; however, no significant difference has been found between all treatments (neither coated nor uncoated) (Fajardo et al., 2010; Gammariello et al., 2008; Laurienzo et al., 2006; Martins et
al., 2010; Yildirim et al., 2006). The non-significant increases reported for pH value could be justified by one or more of the following: the liberation of alkaline components during the growth of proteolytic microorganisms (Martins et al., 2010), increase in cheese buffering capacity as a result of peptide bond cleavage and creation of new groups (Yildirim et al., 2006) and mass exchange between the cheese and coating (Del Nobile et al., 2009). There are also other works which illustrate a non-significant slight reduction in the pH value of samples over the storage time (Conte et al., 2007; Di Pierro et al., 2011) due to the lactic acid synthesis by lactic acid bacteria and substances from proteolysis and lipolysis such as acidic amino acids and free fatty acids (Dermiki et al., 2008; Whitley et al., 2000) as well as CO2 being dissolved if modified atmosphere is applied (Khoshgozaran et al., 2012).
Moisture and weight loss In fact, cheese moisture variation depends on kinetics, wherein water molecules infuse through the coating matrix which, in turn, depends on time, temperature, and barrier properties of coating materials (Marzo et al., 2006) as well as coating compositions. There are documents in which researchers have indicated the efficiency of coatings in reducing and slowing down the moisture and weight loss of the coated cheese over the storage time (Cerqueira et al., 2010; Fajardo et al., 2010; Martins et al., 2010). These findings present economic advantages for food suppliers. For instance, Martins et al. (2010) reported a significant difference of moisture content between coated Ricotta cheeses containing nisin and samples without nisin at the end of the storage period. They demonstrated that their observed variations could be related to the fact that nisin could play a role, because of its hydrophobic property, as a moisture barrier that retards the infusion of water vapor molecules within film/coating matrix. It was also demonstrated that hydrocolloid-based coatings are suitable to preserve moisture of the food during storage and transportation. κ-carrageenan, alginates and gellan are recommended to be very beneficial and of commercial importance to the dairy industry in terms of cheese weight maintenance (Williams and Phillips, 2014). It was demonstrated that coating of goat’s milk cheese with edible chitosan-essential oil films twice or thrice reduced the weight loss due to the increase of total solid content and thickness of the films (Cano Embuena et al., 2016).
Microbiological analysesBacteria In ready-to-eat foods such as cheese, “zero
tolerance” for L. monocytogenes has been necessitated by USFDA (Duan et al., 2007). In the case of cheese, some observations have been provided that L. monocytogenes incubated in some cheese types survives but fails to grow during storage. The observed failure of L. monocytogenes to multiply on cheese samples has been explained by the direct competition of lactic acid bacteria for available substrate and the inhibiting action from their produced bacteriocin (Coppola et al., 1995; Lillevang, 2004). Despite this natural inhibitory

N. Bagheripoor et al.958
effect of lac t ic acid bacter ia present in cheese , L. monocytogenes is a pathogen that cannot be taken for granted, since the growth of L. monocytogenes even at refrigeration temperatures is expected (Neetoo et al., 2008). Martins et al. (2010) reported that the galactomannan coating of Ricotta cheese by plain galactomannan had a slight antimicrobial effect on inoculated L. monocytogenes; however, nisin-added coating inhibited the growth of L. monocytogenes during the storage time (7 days at 4 ℃). Efficiency of nisin in restricting the L. monocytogenes in terms of multiplication might be attributed to its amphipathic property, as cheese contains a high level of fat, which could be desirable for interactions and bonds with nisin molecules because of its hydrophobic nature. Therefore, nisin is well-absorbed on the cheese surface (Rollema et al., 1995). On the other hand, nisinʼs amphipathic feature enhances i t s adso rp t ion on to the hydroph i l i c su r f ace o f L. monocytogenes, resulting in the formation of pores in the bacterial membrane and the cells lysis is achieved (Cha et al., 2003; Dykes and Withers, 1999). Duan et al. (2007) indicated that in comparison with plain chitosan coating for Ricotta cheese , more an t imic rob ia l e f f i c i ency towards L. monocytogenes was observed (at 10 ℃) due to the addition of 60 % lysozyme in chitosan film-forming solutions, which was in line with the sensitivity of Gram-positive bacteria to lysozyme. In addition, they investigated the effect of pH adjustment on the antimicrobial capability of the applied coatings and highlighted that pH adjustment (from 5.2 to 4.4) significantly affected the antimicrobial activity of chitosan-lysozyme composite coating applications on L. monocytogenes, which can be related to the facilitated antilisterial effectiveness of chitosan under acidic conditions. Cao-Hoang et al. (2010) reported reduction in Listeria innocua counts in surface-inoculated semi-hard mini-red Babybel cheese samples, which were coated with sodium caseinate and stored at 4 ℃, as compared to the control samples; with regard to in-depth inoculated cheese samples, antimicrobial efficiency was found to be dependent on distance from surface in contact with the active films to the cheese matrix.
Ollé Resa et al. (2016) implied that incorporation of natamycin and nisin to starch edible films inhibited the growth of psychrotrophic bacteria present in the Port Salut cheese stored at refrigeration temperature. Duan et al. (2007) indicated that the incorporation of 60 % lysozyme into chitosan films and coatings presented a greater antimicrobial effect against inoculated Pseudomonas fluorescens in Mozzarella cheese stored at 10 ℃, compared to the application of chitosan alone. It was also reported that all chitosan-lysozyme blend coating treatments significantly reduced the growth of E. coli during the entire storage time. The observed inhibitory effect of chitosan could be related to the electrostatic interaction between ‒NH3+ groups of chitosan acetate and phosphoryl groups of phospholipid components of E. coli membranes, which in turn destroys cellular membrane of bacteria and
results in the release of cellular contents (Liu et al., 2004) and low pH (below 5.5) (Nielsen, 2004). However, the hurdle strategy has been suggested to inhibit the growth of coliforms and Pseudomonas. Del Nobile et al. (2009) indicated that chitosan coating plus modified atmosphere packaging (30 % CO2, 5 % O2, and 65 % N2) was the only efficient combination for suppressing both Pseudomonas and coliforms from being multiplied on Fior di Latte cheese at 4 ℃. Conte et al. (2009) concluded the same finding with the active coating consisting of lysozyme (0.25 mg/mL) and EDTA (50 mM), which was accompanied by modified atmosphere packaging (with the same gaseous composition) for Fior di Latte cheese stored at 10 ℃. Conte et al. (2009) illustrated that none of the packaging strategies of active coating and coating plus modified atmosphere packaging of Fior di Latte cheese stored at 10 ℃influenced the micro-flora load of cheese (cocci and rod lactic acid bacteria). Inefficiency of the coating on these useful dairy bacteria is a good advertising tool for being “synthetic preservative-free and rich in viable lactic acid bacteria” (Coppola et al., 1995). However, Del Nobile et al. (2009) reported that the cell load of lactic acid bacteria in MAP-packaged Fior di Latte cheese samples coated with chitosan preparation decreased over the storage time, especially for coccoid-shaped. Di Pierro et al. (2011) observed an increase in lactic acid bacteria count of all Ricotta cheeses; however, in chitosan/whey coated samples, it did not reach the approved microbiological limit acceptability, 7 log cfu/g by ICMSF
(ICMSF, 1986), in comparison with the uncoated samples on the 30th day of storage at 4 ℃.
Mold and yeast Conte et al. (2009) detected no molds on Fior di Latte cheeses during the storage period stored at 10 ℃(applying active coating followed by modified atmosphere packaging (30 % CO2, 5 % O2, and 65 % N2). Duan et al. (2007) reported the complete inhibition of mold growth in the Mozzarella cheese packaged with chitosan films. However, they indicated that pH adjustment and lysozyme addition (60 %) did not significantly affect the antimicrobial effectiveness of chitosan packaging neither on considered mold nor on the yeast. Yildirim et al. (2006) also illustrated the failure of casein-based coatings in inhibiting the mold growth on Kashar cheese and visual evaluation of the casein-coated cheese samples highlighted that the cheese surfaces were almost completely covered by mold colonies just after one week of ripening. Incorporation of natamycin retarded visible mold growth for about one month.
Duan et al. (2007) indicated that chitosan films and coatings did not present an effective inhibitory action on inoculated yeast (Candida inconspicua) onto the surface of Mozzarella cheese. However, the inhibitory effect of chitosan on yeast, other than C. inconspicua, has been confirmed (Devlieghere et al., 2004; Kisko et al., 2005; Roller and Covill, 1999). Application of three coatings consisting of chitosan and essential oils in cheeses prepared from goatʼs milk showed a

Active Coatings for Improvement of Cheese Shelf-life 959
delaying or inhibitory effect toward Penicillium and Mucor (Cano Embuena et al., 2016).
Sensorial analysis Similar to other types of preservation methods, coating is expected to maintain the cheese as fresh as possible without presenting any adverse effects, especially the coatings incorporated with active components that have been reported by Conte et al. (2007) (applying lemon extract on Mozzarella cheese) and Del Nobile et al. (2009) (applying lysozyme and EDTA on Fior di Latte cheese) as having either negligible or no significant adverse influence on the sensorial characteristics of coated samples. Also, these authors have found no significant differences between the sensorial properties of all treatments. Conte et al. (2009) reported that there were no significant differences between all the treatments with respect to the sensorial properties of Fior di Latte cheese stored at 10 ℃ (chitosan coated plus MAP (30 % CO2, 5 % O2, and 65 % N2). This observation was confirmed by Del Nobile et al. (2009) for the same cheese type which was coated and MA-packaged with chitosan (0.012 %)/ alginate (8 %)/ CaCl2 (5 %)/ lysozyme (0.25 mg mL
_1)/ Na2-EDTA (50 mM) with MAP (30 % CO2, 5 % O2, 65 % N2) stored at 4 ℃ and by Di Pierro et al. (2011) for Ricotta cheese coated and MA-packaged by chitosan (0.8 %)/ whey (2.4 %) with MAP (40 % CO2, 60 % N2) stored at 4 ℃. However, Laurienzo et al. (2006) reported that the gel-coated Mozzarella cheese (stored at 4 ℃), when compared with the gel-free samples, was soft and elastic, and when cut, the typical milk drop was observed with the s l ight ly aromatic odor of f resh cheese. Kampf and Nussinovitch (2000) illustrated that the coated semi-hard and dry white brined cheese by the hydrocolloids of κ-carrageenan, alginate and gellan, stored at 4 ℃, was softer, glosser and less brittle than the non-coated cheese. They also showed that the samples coated with the same gum solutions presented no off-flavors and no bitter taste as a result of CaCl2 application as a cross-linking agent. Cerqueira et al. (2010) indicated that hardness of galactomannan-coated Saloio cheese stored at 4 ℃ was not comparable with the uncoated samples and had lower hardness values. Moreover, Yildirim et al. (2006) demonstrated that Kashar cheese, coated with casein received the highest surface appearance score and concluded that casein-coating did not adversely affect any of the sensorial features of the samples.
ConclusionsAs shown in the present review, the innovative naturally-
derived coatings, especially the ones carrying natural antimicrobials, were confirmed to be able to prolong the shelf-life of considered cheese if are tailor-made besides leaving no adverse effects on sensorial properties. Barrier properties of edible coatings against deteriorative environmental factors must not be taken for granted; they include light, to prevent light-induced deteriorations such as lipid oxidation, oxygen and carbon dioxide to create a slightly modified atmosphere to
inhibit oxygen-induced deteriorative reactions such as fat oxidation and growth of aerobic microorganisms, and water vapor transmission to prevent cheese weight loss which is economically considerable. Eventually, due to the simplicity of application and the advantages offered by edible coatings for extending the cheese shelf-life, a good argument can be made for edible coatings in cheese manufacturing industry while meeting the expectations from both sides of customers and producers in terms of food safety.
Acknowledgments This study is related to the Grant number 10124 (part 5-1, articles published in journal with IF higher than 2) in Shahid Beheshti University of Medical Sciences.
ReferencesAhvenainen, R. (2003). Active and intelligent packaging. In “ Novel
Food Packaging Techniques”, ed. by R. Ahvenainen. Woodhead Publishing Ltd, Cambridge, pp. 5‒21.
Belletti, N., Ndagijimana, M., Sisto, C., Guerzoni, M.E., Lanciotti, R., and Gardini, F. (2004). Evaluation of the antimicrobial activity of citrus essences on Saccharomyces cerevisiae. J. Agr. Food Chem., 52, 6932‒6938.
Bifani, V., Ramírez, C., Ihl, M., Rubilar, M., García, A., and Zaritzky, N. (2007). Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymethylcellulose-based edible films. LWT-Food Sci. Technol., 40, 1473‒1481.
Bourtoom, T. (2008). Edible films and coatings: characteristics and properties. Int. Food Res. J., 15, 237‒248.
Branen, J.K. and Davidson, P.M. (2004). Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol., 90, 63‒74.
Cano, Embuena, A.I., Cháfer, Nácher, M., Chiralt Boix, A., Molina Pons, M., Borrás Llopis, M., Martínez, B., Carmen, M., and González Martínez, C. (2016). Quality of goat’s milk cheese as affected by coating with edible chitosan-essential oil films. Int. J. Dairy Technol., 70, 68‒76.
Cao-Hoang, L., Chaine, A., Grégoire, L., and Waché Y. (2010). Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiol., 27, 940‒944.
Casariego, A., Souza, B.W.S, Vicente, A.A., Teixeira, J.A., Cruz, L., and Díaz R. (2008). Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocolloid., 22, 1452‒1459.
Cerqueira, M.A., Lima, A.M., Souza, B.W.S., Teixeira, J.A., Moreira, R.A., and Vicente, A.A. (2009). Functional polysaccharides as edible coatings for cheese. J. Agr. Food Chem., 57, 1456‒1462.
Cerqueira, M.A., Sousa-Gallagher, M.J., Macedo, I., Rodriguez-Aguilera, R., Souza, B.W., Teixeira, J.A., and Vicente, A.A. (2010). Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of “Regional” cheese. J. Food Eng., 97, 87‒94.

N. Bagheripoor et al.960
Cha, D.S., Cooksey, K., Chinnan, M.S., and Park H.J. (2003). Release of nisin from various heat-pressed and cast films. LWT-Food Sci. Technol., 36, 209‒213.
Chirkov S. (2002). The antiviral activity of chitosan (review). Appl. Biochem. Micro., 38, 1‒8.
Coma, V., Martial-Gros, A., Garreau, S., Copinet, A., Salin, F., and Deschamps, A. (2002). Edible antimicrobial films based on chitosan matrix. J. Food Sci., 67, 1162‒1169.
Coma, V., Sebti, I., Pardon, P., Deschamps, A., and Pichavant, F.H. (2001). Antimicrobial edible packaging based on cellulosic ethers, fatty acids, and nisin incorporation to inhibit Listeria innocua and Staphylococcus aureus. J. Food Protect., 64, 470‒475.
Conte, A., Gammariello, D., Di Giulio, S., Attanasio, M., and Del Nobile, M.A. (2009). Active coating and modified-atmosphere packaging to extend the shelf life of Fior di Latte cheese. J. Dairy Sci., 92, 887‒894.
Conte, A., Scrocco, C., Sinigaglia, M., and Del Nobile, M.A. (2007). Innovative active packaging systems to prolong the shelf life of Mozzarella cheese. J. Dairy Sci., 90, 2126‒2131.
Conte, A., Sinigaglia, M., and Del Nobile M.A. (2006). Antimicrobial effectiveness of lysozyme immobilized on polyvinylalcohol- based film against Alicyclobacillus acidoterrestris. J. Food Protect., 69, 861‒865.
Coppola, R., Sorrentino, E., Cinquanta, L., Rossi, F., Iorizzo, M., and Grazia L. (1995). Shelf-life of Mozzarella cheese samples packaged without liquid and stored at different temperatures. Ital. J. Food Sci., 7, 351‒359.
Corradini, C. (1995). I componenti minerali. In “ Chimica e Tecnologia del Latte”. ed. by C. Corradini. Tecniche Nuove, Italy, pp. 63–67.
Crapisi, A., Lante, A., Pasini, G., and Spettoli, P. (1994). Enhanced microbial cell lysis by the use of lysozyme immobilized on different carriers. Process Biochem., 28, 17‒21.
Cuero, R., Osuji, G., and Washington, A. (1991). N-carboxymethylchitosan inhibition of aflatoxin production: role of zinc. Biotechnol. Lett., 13, 441‒444.
De Oliveira, T.M., de Fátima Ferreira Soares, N., Pereira, R.M., and de Freitas Fraga, K. (2007). Development and evaluation of antimicrobial natamycin-incorporated film in gorgonzola cheese conservation. Packag. Technol. Sci., 20, 147‒153.
De Ruig, W. and Berg, G. (1985). Influence of the fungicides sorbate and natamycin in cheese coatings on the quality of the cheese. Neth. Milk Dairy J., 39, 165‒172.
Del Nobile, M.A., Gammariello, D., Conte, A., and Attanasio, M. (2008). A combination of chitosan, coating and modified atmosphere packaging for prolonging Fior di latte cheese shelf life. Carbohyd. Polym., 78, 151‒156.
Delves-broughton, J. (2005). Nisin as a food preservative. Food Aust., 57, 525‒527.
Dermiki, M., Ntzimani, A., Badeka, A., Savvaidis, I.N., and Kontominas, M.G. (2008). Shelf-life extension and quality attributes of the whey cheese “Myzithra Kalathaki” using modified atmosphere packaging. LWT - Food Sci. Technol., 41, 284‒294.
Di Pierro, P., Sorrentino, A., Mariniello, L., Giosafatto, C.V.L., and
Porta, R. (2011). Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT - Food Sci. Technol., 44, 2324‒2327.
Dos Santos Pires, A.C., De Fátima Ferreira Soares, N., De Andrade, N.J., Da Silva, L.H.M., Camilloto, G.P., and Bernardes, P.C. (2008). Development and evaluation of active packaging for sliced mozzarella preservation. Packag. Technol. Sci., 21, 375‒383.
Duan, J., Park, S.I., Daeschel, M., and Zhao, Y. (2007). Antimicrobial chitosan-lysozyme (CL) films and coatings for enhancing microbial safety of mozzarella cheese. J. Food Sci., 72, 355‒362.
Dykes, G.A. and Withers, K.M. (1999). Sub-lethal damage of Listeria monocytogenes after long-term chilled storage at 4 ℃. Lett. Appl. Microbiol., 28, 45‒48.
Embuscado, M.E. and Huber, K.C. (2009). “Edible films and coatings for food applications”. Springer.
Fajardo, P., Martins, J.T., Fuciños, C., Pastrana, L., Teixeira, J.A., and Vicente, A.A. (2010). Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng., 101, 349‒356.
Falguera, V., Quintero, J.P., Jiménez, A., Muñoz, J.A., and Ibarz, A. (2011). Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci. Tech., 22, 292‒303.
Ferrão, L.L., Silva, E.B., Silva, H.L.A., Silva, R., Mollakhalili, N., Granato, D., Freitas, M.Q., Silva, M.C., Raices, R.S.L., Padilha, M.C., Zacarchenco, P.B., Barbosa, M.I.M.J., Mortazavian, A.M., and Cruz, A.G. (2016). Strategies to develop healthier processed cheeses: Reduction of sodium and fat contents and use of prebiotics. Food Res. Int., 86, 93‒102.
Gammariello, D., Di Giulio, S., Conte, A., and Del Nobile, M.A. (2008). Effects of natural compounds on microbial safety and sensory quality of Fior di Latte cheese, a typical Italian cheese. J. Dairy Sci., 91, 4138‒4146
Gandhi, M. and Chikindas, M.L. (2007). Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol., 113, 1‒15.
Garcia, M., Martino, M., and Zaritzky, N. (2000). Lipid addition to improve barrier properties of edible starch-based films and coatings. J. Food Sci., 65, 941‒944.
Gennadios, A., Hanna, M.A., and Kurth, L.B. (1997). Application of edible coatings on meats, poultry and seafoods: A review. LWT- Food Sci. Technol., 30, 337‒350.
Gill, A.O. and Holley, R.A. (2000). Inhibition of bacterial growth on ham and bologna by lysozyme, nisin and EDTA. Food Res. Int., 33, 83‒90.
Guilbert, S., Gontard, N., and Cuq, B. (1995). Technology and applications of edible protective films. Packag. Technol. Sci., 8, 339‒346
Hamilton-Miller, J.M.T. (1974). Fungal Sterols and the Mode of Action of the Polyene Antibiotics. Adv. Appl. Microbiol., 17, 109‒134.
Han, J.H. (2003). Antimicrobial food packaging. In “Novel food packaging techniques”, ed. by R Ahvenainen. Woodhead Publishing, Cambridge, pp. 50‒60.
Han, J.H. (2014). Edible films and coatings. In “Innovatives in food

Active Coatings for Improvement of Cheese Shelf-life 961
packaging”, ed. by J.H Han. Academic Press, pp. 224‒231. Han, J.H. and Krochta, J.M. (2001). Physical properties and oil
absorption of whey protein coated paper. J. Food Sci., 66, 294‒299.Hanušová, K., Šťastná, M., Votavová, L., Klaudisová, K., Dobiáš, J.,
Voldřich, M., and Marek, M. (2010). Polymer films releasing nisin and/or natamycin from polyvinyldichloride lacquer coating: nisin and natamycin migration, efficiency in cheese packaging. J. Food Eng., 99, 491‒496.
Heimbach, J., Rieth, S., Mohamedshah, F., Slesinski, R., Samuel-Fernando, P., Sheehan, T., Dickmann, R., and Borzelleca, J. (2000). Safety assessment of iron EDTA [sodium iron (Fe3+) ethylenediaminetetraacetic acid]: Summary of toxicological, fortification and exposure data. Food Chem. Toxicol., 38, 99‒111.
Helander, I.M., Von Wright, A., and Mattila-Sandholm, T.M. (1997). Potential of lactic acid bacteria and novel antimicrobials against gram-negative bacteria. Trends Food Sci. Technol., 8, 146‒150.
Hernández-Muñoz, P., López-Rubio, A., Del-Valle, V., Almenar, E., and Gavara, R. (2004). Mechanical and Water Barrier Properties of Glutenin Films Influenced by Storage Time. J. Agr. Food Chem., 52, 79‒83.
Hong, S.I. and Krochta, J.M. (2003). Oxygen barrier properties of whey protein isolate coatings on polypropylene films. J. Food Sci., 68, 224‒228.
ICMSF. (1986) Sampling for microbiological analysis: Principles and scientific applications (2nd ed.). Toronto, Canada: International Commission on Microbiological Specifications for Foods.
Jalilzadeh, A., Tunçtürk, Y., and Hesari, J. (2015). Extension shelf life of cheese: A review. Int. J .Dairy Sci., 10, 44‒60.
Kampf, N. and Nussinovitch, A. (2000). Hydrocolloid coating of cheeses. Food Hydrocolloid., 14, 531‒537.
Karbowiak, T., Debeaufort, F., and Voilley, A. (2006). Importance of surface tension characterization for food, pharmaceutical and packaging products: A review. Crit. Rev.Food Sci., 46, 391‒407.
Karimi, R., Mortazavian, A.M., and Amiri-Rigi, A. (2011). Selective enumeration of probiotic microorganisms in cheese. Food Microbiol., 29, 1‒9.
Karimi, R., Mortazavian, A.M., and Cruz, A.G. (2011). Viability of probiotics in cheese during production and storage. Dairy Sci. Technol., 91, 283‒308.
Kester, J.J. and Fennema, O.R. (1986). Edible films and coatings: a review. Food Technol., 40, 47‒59.
Khoshgozaran, S., Azizi, M.H., and Bagheripoor-Fallah, N. (2012). Evaluating the effect of modified atmosphere packaging on cheese characteristics: A review. Dairy Sci. Technol., 92, 1‒24.
Kisko, G., Sharp, R., and Roller, S. (2005). Chitosan inactivates spoilage yeasts but enhances survival of Escherichia coli O157:H7 in apple juice. J. Appl. Microbiol., 98, 872‒880.
Ko S., Janes M.E., Hettiarachchy N.S., Johnson M.G., Physical and chemical properties of edible films containing nisin and their action against Listeria monocytogenes. J. Food Sci., 2001, 66, 1006‒1011.
Kristo, E., Koutsoumanis, K.P., and Biliaderis, C.G. (2008) Thermal, mechanical and water vapor barrier properties of sodium caseinate films containing antimicrobials and their inhibitory action on Listeria
monocytogenes. Food Hydrocolloid., 22, 373‒386. Krochta, J.M. and De Mulder-Johnston, C. (1997). Edible and
biodegradable polymer films: challenges and opportunities. Food Technol., 51, 61–74.
Laurienzo, P., Malinconico, M., Pizzano, R., Manzo, C., Piciocchi, N., Sorrentino, A., and Volpe M.G. (2006). Natural polysaccharide-based gels for dairy food preservation. J. Dairy Sci., 89, 2856‒2864.
Lian, Z.X., Ma, Z.S., Wei, J., and Liu, H. (2012). Preparation and characterization of immobilized lysozyme and evaluation of its application in edible coatings. Process Biochem., 47, 201‒208.
Lieberman, E. and Gilbert, S. (1973). Gas permeation of collagen films as affected by cross-linkage, moisture, and plasticizer content. J. Polymer Sci. Polymer Symposia., 41, 33‒43.
Lillevang S. (2004). Cheese production: quality control and sanitation. In “Handbook of food and beverage fermentation technology”, ed. by Y.H., Hui, L., Meunier-Goddik, A.S., Hansen, J., Josephsen, W.K., Nip, P.S., Stanfield, and F., Toldra. Marcel Dekker, New York, pp. 343‒352.
Liu, H., Du, Y., Wang, X., and Sun, L. (2004). Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol., 95, 147‒155.
Lucera, A., Mastromatteo, M., Conte, A., Zambrini, A.V., Faccia, M., and Del Nobile, M.A. (2014). Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packaging and Shelf Life, 1, 25‒29.
Martins, J.T., Cerqueira, M.A., Souza, B.W.S., Carmo Avides, M.D.O., and Vicente, A.A. (2010). Shelf life extension of ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against listeria monocytogenes. J. Agr. Food Chem., 58, 1884‒1891.
Marzo, S.D., Di Monaco, R., Cavella, S., Romano, R., Borriello, I., and Masi, P. (2006). Correlation between sensory and instrumental properties of Canestrato Pugliese slices packed in biodegradable films. Trends Food Sci. Technol., 17, 169‒176.
Mohammadi, R., Mohammadifar, M.A., Mortazavian, A.M., Rouhi, M., Ghasemi, J.B., and Delshadian, Z. (2016). Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem., 190, 186–193.
Neetoo, H., Ye, M., Chen, H., Joerger, R.D., Hicks, D.T., and Hoover, D.G. (2008). Use of nisin-coated plastic films to control Listeria monocytogenes on vacuum-packaged cold-smoked salmon. Int. J. Food Microbiol., 122, 8‒15.
Nguyen, V.T., Gidley, M.J., and Dykes, G.A. (2008). Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol., 25, 471‒478.
Nielsen, E.W. (2004). Principles of cheese production: quality control and sanitation. In “Handbook of food and beverage fermentation technology”, ed. by Y.H., Hui, L., Meunier-Goddik, A.S., Hansen, J., Josephsen, W.K., Nip, P.S., Stanfield, and F., Toldra. Marcel Dekker, New York, pp. 219‒230.
Olasupo, N.A., Fitzgerald, D.J., Gasson, M.J., and Narbad, A. (2003). Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl.

N. Bagheripoor et al.962
Microbiol., 37, 448‒451. Ollé Resa, C.P., Gerschenson, L.N., and Jagus, R.J. (2016). Starch
edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian Port Salut cheese. Food Control, 59, 737‒742.
Ollé Resa, C.P., Jagus, R.J., and Gerschenson, L.N. (2014). Effect of natamycin, nisin and glycerol on the physicochemical properties, roughness and hydrophobicity of tapioca starch edible films. Mater. Sci. Eng. C. Mater. Biol. Appl., 40, 281‒287.
Parliament E-ECa. (2004) Regulation (EC) 1935/2004 of the European Parliment and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing directives 80/590/EEC. Available at: http://europa.eu.int/eurlex/lex/LexUriServ/site/en/oj/2004/1_338/1_33820041113en0004001.
Pranoto, Y., Rakshit, S.K., and Salokhe, V.M. (2005a). Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT - Food Sci. Technol., 38, 859‒865
Pranoto, Y., Salokhe, V.M., and Rakshit, S.K. (2005b). Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int., 38, 267‒272.
Proulx, J., Hsu, L.C., Miller, B.M., Sullivan, G., Paradis, K., and Moraru, C.I. (2015). Pulsed-light inactivation of pathogenic and spoilage bacteria on cheese surface. J. Dairy Sci., 98, 5890‒5898.
Rabea, E.I., Badawy, M.E.T., Stevens, C.V., Smagghe, G., and Steurbaut, W. (2003). Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules, 4, 1457‒1465.
Ramos, Ó.L., Pereira, J., Silva, S.I., Fernandes, J.C., Franco, M., Lopes-da-Silva, J., Pintado, M., and Malcata, F.X. (2012). Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci., 95, 6282‒6292.
Regulations C-CoF. (1998) Food and drugs. Washington, DC, USA: US Government Printing Office.
Rhoades, J. and Roller, S. (2000). Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol., 66, 80‒86.
Rollema, H.S., Kuipers, O.P., Both, P., De Vos, W.M., and Siezen, R.J. (1995). Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol., 61, 2873‒2878.
Roller, S. and Covill, N. (1999). The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol., 47, 67‒77.
Sebti, I., Ham-Pichavant, F., and Coma, V. (2002). Edible bioactive fatty acid-cellulosic derivative composites used in food-packaging applications. J. Agr. Food Chem., 50, 4290‒4294.
Shahani, K.M., Bullerman, L.B., Barnhart, H.M., and Hartung, T.E. (1973). Effect of an antifungal, pimaricin, upon the retardation of food spoilage and incidence of toxins. Proc. 1st International Congress of Bacteriology, 2, 41.
Shahidi F., Arachchi, J.K.V., and Jeon Y.J. (1999). Food applications
of chitin and chitosans. Trends Food Sci. Technol., 10, 37‒51.SIAM. (2001) SIDS initial assessment profile. CAS No. 60-00-4. EDTA.
Available at: http://www.jetoc.or.sp/HP-SIDSpdffiles/60-00-4.pdfwww.jetoc.or.sp/HP-SIDSpdffiles/60-00-4.pdf S.
Siepmann, J. and Siepmann, F. (2008). Mathematical modeling of drug delivery. Int. J. Pharm., 364, 328‒343.
Sivarooban, T., Hettiarachchy, N.S., and Johnson M.G. (2008). Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int., 41, 781‒785.
Sofos, J.N., Beuchat, L.R., Davidson, P., and Johnson E.A. (1998). Naturally occurring antimicrobials in food. Regul. Toxicol. Pharmacol., 28, 71‒72.
Song, Y., Babiker, E.E., Usui, M., Song, Y., Babiker, E.E., Usui, M., Saito, A., and Kato, A. (2002). Emulsifying properties and bactericidal action of chitosan–lysozyme conjugates. Food Res. Int., 35, 459‒466.
Srinivasa, P.C., Ramesh, M.N., and Tharanathan, R.N. (2007). Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocoll., 21, 1113‒1122.
Stevens, K.A., Sheldon, B.W., Klapes, N.A., and Klaenhammer, T.R. (1992). Effect of treatment conditions on nisin inactivation of gram-negative bacteria. J. Food Prot., 55, 763‒766.
Tsai, G.J. and Su, W.H. (1999). Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot., 62, 239‒243.
Vargas, M., Albors, A., Chiralt, A., and González-Martínez, C. (2009). Characterization of chitosan-oleic acid composite films. Food Hydrocoll., 23, 536‒547
Vermeiren, L., Devlieghere, F., Van Beest, M., De Kruijf, N., and Debevere, J. (1999). Developments in the active packaging of foods. Trends Food Sci. Technol., 10, 77‒86.
Whitley, E.J., Muir, D., and Waites, W.M. (2000). The growth of Listeria monocytogenes in cheese packed under a modified atmosphere. J. Appl. Microbiol., 88, 52‒57.
Williams, P.A. and Phillips G.O. (2014). “Gums and stabilisers for the food industry: the changing face of food manufacture: the role of hydrocolloids”. Cambridge. Royal Society of Chemistry.
Yang, L. and Paulson, A.T. (2000). Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int., 33, 571‒578.
Yildirim, M., Güleç, F., Bayram, M., and Yildirim Z. (2006). Properties of Kashar cheese coated with casein as a carrier of natamycin. Ital. J. Food Sci., 18, 127‒138.
Zheng, L.Y. and Zhu, J.F. (2003). Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym., 54, 527‒530.
Zhong, Y., Cavender, G., and Zhao, Y. (2014). Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT - Food Sci. Technol., 56, 1‒8.