MCAS Guide Pages 7-8 DNA Structure RNA DNA & RNA activities.
Report exp 6 and 7 (DNA and RNA)
-
Upload
kevin-balda -
Category
Education
-
view
21.472 -
download
3
Transcript of Report exp 6 and 7 (DNA and RNA)

Experiment #6NUCLEIC ACID - RNA
Group 7BALDA
SANTOSTALAG
VILLACORTA

Objectives1. To be able to isolate RNA from yeast2. To be able to get the percentage by mass of
RNA from yeast3. To be able to identify products of hydrolysis
of RNA 4. To perform tests for identification of products
of RNA hydrolysis

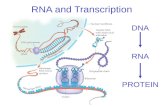
IntroductionRNA (ribonucleic acid) \
• Genetic material of certain viruses• Made up of a single strand of nucleotides• Directs the middle steps of protein production• Polymer of Purine and Pyrimidine
ribonucleotides linked through 3’-5’ phosphodiester bridges
• With Uracil and D-ribose

Experiment Proper
6.2 Hydrolysis of RNAMaterials• Mortar and pestle• Erlenmeyer Flask• 0.2% NaOH• 10% NaOH• 0.5%CuSO4

Hydrolysis
• Chemical process involving the addition of water causing a molecule to cleave into two parts
Nucleoprotein Nucleic Acids
ProteinNucleotides
Phosphate
D-ribosePyrimidine
Purine

Experiment Proper
6.3 Identification TestsMaterials• NaOH• CuSO4
• 10% NH4OH
• 2% AgNO3
• Benedict’s Reagent• 0.2M Ammonium Molybdate

Identification Tests
Biuret Test-test to detect the presence of peptide bonds Principle: Formation of purple colored product due to the reduction of Cu2+ to Cu+ made by the detected reducing sugar
Reagents: NaOH and CuSO4

Identification Tests
10% AgNO3 and 10% NH4OH-test to detect the presence of Purine Bases Principle: Hydrolysis of N-β-glucosidic bonds between purine bases and ribose or deoxyribose results in a release of purine bases(A and G) caused by NH4OH. Ag+ precipitate caused the formation of foamy gelatinous substance.
Reagents: AgNO3 and NH4OH

Identification Tests
Benedicts Test-test to detect the presence Reducing Sugars • Principle: Cu2+ react with reducing sugars = precipitate of
cuprous oxide (Cu2O) produces a change in the Benedict’s reagent from blue to green or reddish-orange, depending on amount of reducing sugar. The cyclic sugar is reduced to linear aldehyde.
• Green = small amn’t of reducing sugars• Red-orange = abundance of reducing sugarsReagents: NaOH, CuSO4 and tartaric acid

Identification Tests
Ammonium Molybdate Test-test for Inorganic Phosphate Principle: hydrolysis of pyrophosphate to phosphate forming yellow ppt.
Reagents: (NH4)6 Mo7O24 · 4H2O and H2SO4

Experiment Proper
Test ResultBiuret’s Test Purple Sol’n
10% NH4OH & 10% AgNO3
Brown Sol’n
Benedict’s Test Green Sol’nAmmonium Molybdate
Brown Sol’n

Test
Experiment Proper
Biuret’s Test10% NH4OH &
10% AgNO3Benedict’s Test
Ammonium Molybdate

Ideal Results
Test ResultBiuret’s Test Purple Sol’n
10% NH4OH & 10% AgNO3
Gelatinous White Precipitate
Benedict’s Test Green – Orange Sol’nAmmonium Molybdate
Yellow Precipitate

Comparison Test Result Result
Biuret’s Test Purple Sol’n Purple Sol’n
10% NH4OH & 10% AgNO3 Brown Sol’n
Gelatinous White
Precipitate
Benedict’s Test Green Sol’n Green – Orange Sol’n
Ammonium Molybdate Brown Sol’n Yellow
Precipitate

PLQ’s1. What products of hydrolysis did the yeast
RNA yield? Nucleobases (A, G, U and C) Sugar (D-ribose) Phosphate

PLQ’s2. Cite qualitative tests to characterize the products of RNA hydrolysis. Are these specific for RNA?(explain)
Bial’s Test- Test for pentoses.- specimen is heated with sol’n of orcinol, HCl, and FeCl3- pentoses : dehydrated to furfural = reacts with orcinol
to form a green product

PLQ’s Wheeler and Johnson's test
- Treatment of Cytosine and Uracil w/ bromine water yields dialuric acid = green coloration
- Addition of Ba(OH)2 = purple.

PLQ’s Murexide Test
- Murexide (NH4C8H4N5O6, or C8H5N5O6.NH3) or ammonium purpurate [MX] = ammonium salt of purpuric acid.
- Test for presence of uric acid = white, odorless, tasteless crystalline substance from purine degradation
- Positive : yellow residue- A & G : purines = 2-ringed crystalline organic base
– a uric acid; heterocyclic aromatic org. compound composed of pyrimidine ring fused w/ an imidazole ring

PLQ’s3. What are differences of RNA and DNA?
DNA RNA
Based on Function
Responsible for the storage & transmission of the genetic
materialInvolve in the manufacture of
proteins
Based on sugar composition 2-deoxy-D-ribose D-ribose
Based on Pyrimidine base
compositionWith Thymine With Uracil
Based on Structure Double Helix Made up of a single strand

Experiment #7NUCLEIC ACID - DNA
Group 7BALDA
SANTOSTALAG
VILLACORTA

Objectives1. To be able to identify products of hydrolysis
of DNA2. To perform tests for identification of products
of DNA hydrolysis

IntroductionDNA (deoxyribonucleic acid) \
• Carries the Genetic Code of Life• Made up of a double strand of nucleotides in
anti-parallel direction• With Thymine and 2-deoxy-D-ribose• Widely studied for medical diagnosis and
production of therapeutic proteins

Experiment Proper
7.1 Hydrolysis of DNAMaterials• 10% NH4OH• 2% AgNO3• 5g NaCl• Ammonium
Molybdate• Benedict’s Reagent
• 0.2% NaOH• 10% NaOH• 0.5%CuSO4
• Filter paper• Wire Loop

Hydrolysis
• Chemical process involving the addition of water causing a molecule to cleave into two parts
Nucleoprotein Nucleic Acids
ProteinNucleotides
Phosphate
2-deoxy D-ribosePyrimidine
Purine

Experiment Proper
Materials• NaOH• CuSO4
• 10% NH4OH
• 2% AgNO3
• Benedict’s Reagent• 0.2M Ammonium Molybdate

Identification Tests
Biuret Test-test to detect the presence of peptide bonds Principle: Formation of purple colored product due to the reduction of Cu2+ to Cu+ made by the detected reducing sugar
Reagents: NaOH and CuSO4

Identification Tests
10% AgNO3 and 10% NH4OH-test to detect the presence of Purine Bases Principle: Hydrolysis of N- β -glucosidic bonds between purine bases and ribose or deoxyribose results in a release of purine bases(A and G) caused by NH4OH. Ag+ precipitate caused the formation of foamy gelatinous substance.
Reagents: AgNO3 and NH4OH

Identification Tests
Benedicts Test-test to detect the presence Reducing Sugars • Principle: Cu2+ react with reducing sugars = precipitate of
cuprous oxide (Cu2O) produces a change in the Benedict’s reagent from blue to green or reddish-orange, depending on amount of reducing sugar. The cyclic sugar is reduced to linear aldehyde.
• Green = small amn’t of reducing sugars• Red-orange = abundance of reducing sugarsReagents: NaOH, CuSO4 and tartaric acid

Identification Tests
Ammonium Molybdate Test-test for Inorganic Phosphate Principle: hydrolysis of pyrophosphate to phosphate forming yellow ppt.
Reagents: (NH4)6 Mo7O24 · 4H2O and H2SO4

Results
Test ResultBiuret’s Test Light Blue Sol’n
10% NH4OH & 10% AgNO3
Clear w/ very little white ppt.
Benedict’s Test Blue Sol’nAmmonium Molybdate
White Sol’n

Test
Experiment Proper
Biuret’s Test10% NH4OH &
10% AgNO3Benedict’s Test
Ammonium Molybdate

Ideal Results
Test ResultBiuret’s Test Purple Sol’n
10% NH4OH & 10% AgNO3
Gelatinous White Precipitate
Benedict’s Test Green – Orange Sol’nAmmonium Molybdate
Yellow Precipitate

Comparison Test Result Ideal Result
Biuret’s Test Light Blue Sol’n
Purple Sol’n
10% NH4OH & 10% AgNO3
Clear w/ very little white
ppt.
Gelatinous White
Precipitate
Benedict’s Test Blue Sol’n Green – Orange Sol’n
Ammonium Molybdate
White Sol’n Yellow Precipitate

PLQ’s1. What are the reagents used to extract DNA and RNA?
RNA : NaOH and C2H5OH(ethanol) with Conc. HCl DNA : NaCl, ethanol and detergent

PLQ’s2. Did you have the same results of qualitative tests performed in experiment 6? (Explain)
No, but reality check, the results in both experiments should be identical for they yield the same hydrolysate and the tests will yield the same products except for the Benedict’s test because the sugar that it will detect is 2-deoxy-D-ribose where in experiment 6, the sugar is D-ribose.

PLQ’s3. What tests specifically detect ribose and 2-deoxy ribose? Discuss the principle.
Based from the experiment, it is the Benedict’s Test.
-Cu2+ react with reducing sugars = precipitate of cuprous oxide (Cu2O) produces a change in the Benedict’s reagent from blue to green or reddish-orange, depending on amount of reducing sugar. The cyclic sugar is reduced to linear aldehyde.

PLQ’s
Dische Diphenylamine Test- used to detect the presence of DNA. A positive test for DNA
is indicated by a blue color change.- The reaction depends on the conversion of the pentose to
ω-hydroxylaevulinic aldehyde which then reacts with diphenylamine to give a blue colored complex. The intensity of the blue color is proportional to the concentration of DNA. Dische reagent does not react with the ribose sugar in RNA and does not form a blue-colored complex
But…

PLQ’s Bial’s Test
- Test for pentoses.- specimen is heated with sol’n of orcinol, HCl,
and FeCl3- pentoses : dehydrated to furfural = reacts with
orcinol to form a green product

PLQ’s4. Can DNA be isolated from beef? Discuss the process briefly.
Yes!breakage of cell -> membrane lipid removal -> protein removal -> RNA removal -> precipitation of DNA