Ramgarhia Polytechnic - UNITS AND DIMENSIONS PHYSICS... · 2020. 4. 10. · 1 UNITS AND DIMENSIONS...
Transcript of Ramgarhia Polytechnic - UNITS AND DIMENSIONS PHYSICS... · 2020. 4. 10. · 1 UNITS AND DIMENSIONS...
-
1
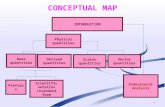
UNITS AND DIMENSIONS
PHYSICAL QUANTITIES
The quantities which can be
measured directly or indirectly
are called physical quantities.
Examples: Length, mass,
time, temperature, electric
current, speed, density, area
TYPES OF PHYSICAL
QUANTITIES
Physical quantities are of two
types:
1. Fundamental or Basic
quantities
2. Derived quantities
FUNDAMENTAL OR BASIC
QUANTITIES
Physical quantities which are
not related to each other or to
other quantities are called
fundamental or basic
quantities.
BOiqk rwSIAW
rwSIAW, ijnWH nUM is`Dy jW Ais`Dy qOr 'qy mwipAw jw skdw hY, BOiqk rwSIAW kihMdy hn[
audwhrxW: lMbweI, puMj, smW, qwpmwn, ibjleI krMt, cwl, Gxqw, KyqrPl
BOiqk rwSIAW dIAW iksmW:
BOiqk rwSIAW do qrWH dIAW huMdIAW hn:
1. mùFlIAW rwSIAW 2. ivauNqpMn rwSIAW
mu`FlIAW rwSIAW
BOiqk rwSIAW, ijnWH dw Awps iv`c jW dUjIAW rwSIAW nwl koeI sMbMD nhIN huMdw,nUM mu`FlIAW rwSIAW ikhw jWdw hY[
audwhrxW: lMbweI, puMj, smW, qwpmwn, ibjleI krMt
-
2
DERIVED QUANTITES
Physical quantities which can
be obtained from fundamental
or basic quantities by
multiplication or division are
called derived quantities.
Examples:, area, volume,
density, weight, force, power,
energy, pressure, speed
UNIT
To measure a physical
quantity, it is compared with a
standard of same kind. This
chosen standard is called unit.
TYPES OF UNITS
Units are of two types:
1. Fundamental or Basic
units
2. Derived units
FUNDAMENTAL OR BASIC
UNITS
Units which are not related to
each other or to other units
are called fundamental or
basic units.
ivauNqpMn rwSIAW
BOiqk rwSIAW, ijnWH nUM mùFlIAW rwSIAW qoN guxW jW Bwg kr ky pRwpq kIqw jw skdw hY, nUM ivauNqpMn rwSIAW ikhw jWdw hY[
audwhrxW: KyqrPl, GxPl dbwA, cwl, , Gxqw, Bwr, bl, SkqI, aUrjw
iekweI
iksy BOiqk rwSI nUM mwpx leI ies dI ausy iksm dy stYNfrf nwl qulnw kIqI jWdI hY[ies cuxy hoey stYNfrf nUM iekweI kihMdy hn[
iekweIAW dIAW iksmW
iekweIAW dIAW do iksmW huMdIAW hn:
1. mùFlIAW iekweIAW 2. ivauNqpMn iekweIAW
mu`FlIAW iekweIAW
iekweIAW, ijnWH dw Awps iv`c jW dUjIAW iekweIAW nwl koeI sMbMD
-
3
Examples: units of Length,
mass, time, temperature,
electric current,
DERIVED UNITS
Units which can be obtained
from fundamental or basic
units by multiplication or
division are called derived
units.
Examples: units of, area,
volume, density, weight, force,
power, energy, pressure,
speed
Area = length x breadth
= mx m = m2
m2 is derived unit of area
Volume = length x breadth x
height
= m x m x m = m3
m3 is derived unit of volume
Speed = distance / time
= m/s =ms-1
ms-1 is derived unit of speed
nhIN huMdw nUM mu`FlIAW iekweIAW ikhw jWdw hY[audwhrxW: lMbweI, puMj, smW, qwpmwn, ibjleI krMt dIAW iekweIAW
ivauNqpMn iekweIAW
iekweIAW, ijnWH nUM mùFlIAW iekweIAW qoN guxW jW Bwg kr ky pRwpq kIqw jw skdw hY, nUM ivauNqpMn iekweIAW ikhw jWdw hY[
audwhrxW: KyqrPl, GxPl ,dbwA, cwl, , Gxqw, Bwr, bl, SkqI, aUrjw dIAW iekweIAW
KyqrPl = lMbweI x cOVweI
= mItr x mItr = mItr2
mItr2 KyqrPl dI ivauNqpMn iekweI hY[
GxPl = lMbweI x cOVweI x aucweI = mItr x mItr x mItr = mItr3
mItr3 GxPl dI ivauNqpMn iekweI hY[
cwl = dUrI / smW
= mItr/ sYikMf
mItr/ sYikMf cwl dI ivauNqpMn iekweI hY
-
4
MEASUREMENT OF
PHYSICAL QUANTITY
Measurement of physical
quantity (Q) involves two
steps:
a) Selection of unit (u)
b) Numerical value (n)
Q = n u
In system I, Q= n1 u1
In system II, Q = n2 u2
Hence, n1 u1 = n2 u2
Numerical value of a
quantity is inversely
proportional to its unit.
n α 1/u
CHARACTERISTICS OF
UNITS
1. It should be well-defined.
2. It should be of suitable
size.
3. It should be easily
reproducible.
4. It should be easily
accessible.
5. It should not change with
time.
6. It should not change with
place.
[BOiqk rwSI dw mwp
BOiqk rwSI (Q) dw mwp iv`c do stYp huMdy hn: a) iekweI dI cox (u) b) numYrIkl mùl (n)
Q = n u
pRxwlI 1 iv`c, Q= n1 u1
pRxwlI 2 iv`c, Q = n2 u2
ies leI, n1 u1 = n2 u2
iksy BOiqk rwSI dw numYrIkl mùl ies dI iekweI dy ault AnupwqI huMdw hY[ n α 1/u
iekweIAW dIAW ivSySqwvW 1. ieh cMgI qrWH pRIBwiSq hoxw
cwhIdw hY[ 2. ieh Xog Awkwr dw hoxw
cwhIdw hY[ 3. ies nUM sOiKAW hI
punrauqpwdnXog hoxw cwhIdw hY[
4. ieh sOiKAW hI phuMcXog hoxw cwhIdw hY[
5. ieh smyN nwl qbdIl nhIN hoxw cwhIdw[
-
5
7. It should not be
perishable.
8. It should not be affected
by physical conditions
like temperature,
pressure, humidity etc.
9. It should be accepted all
over the world.
SYSTEMS OF UNITS
There are four systems of
units-
1. C.G.S. System: In this
system, the unit of
length is centimeter, the
unit of mass is gram and
the unit of time is
second.
2. F.P.S. System: In this
system, the unit of
length is foot, the unit of
mass is pound and the
unit of time is second.
3. M.K.S. System: In this
system, the unit of
length is meter, the unit
of mass is kilogram and
the unit of time is
second.
6. ieh jgwH nwl qbdIl nhIN hoxw cwhIdw[
7. ieh ^qmXog nhIN hoxw cwhIdw[
8. ies 'qy BOiqk hwlqW ijvyN qwpmwn, dbwA, is`l Awid dw pRBwv nhIN hoxw cwhIdw[
9. ieh pUrI dunIAW iv`c svIkwrq hoxw cwhIdw hY[
iekweIAW dIAW pRxwlIAW
iekweIAW dIAW cwr pRxwlIAW huMdIAW hn-
1.sI.jI.AYs.pRxwlI:ies pRxwlI iv`c lMbweI dI iekweI sYNtImItr, puMj dI iekweI grwm Aqy smyN dI iekweI sYikMf huMdI hY[
2. AYP.pI.AYs.pRxwlI:ies pRxwlI iv`c lMbweI dI iekweI Pu`t, puMj dI iekweI pONf Aqy smyN dI iekweI sYikMf huMdI hY[
3. AYm.ky.AYs.pRxwlI:ies pRxwlI iv`c lMbweI dI iekweI mItr, puMj dI iekweI iklogrwm Aqy smyN dI iekweI sYikMf huMdI hY[
-
6
3. S.I.(System
International de Units):
In this system, there are
seven basic units and two
supplementary units.
Basic Units:
1. metre for length
2. kilogram for mass
3. second for time
4. kelvin for temperature
5. ampere for electric
current
6. candela for luminous
intensity
7. mole for amount of
substance
Supplementary Units:
1. radian for plane
angle.
2. steradian for solid
angle
4. AYs. AweI.:ies pRxwlI iv`c s`q byisk Aqy do pUrk iekweIAW huMdIAW hn[
byisk iekweIAW: 1. lMbweI leI mItr 2. puMj leI iklogrwm 3. smyN leI sYikMf 4. qwpmwn leI
kYlivn 5. ibjleI kurMt leI
AYmpIAr 6. pRkwSmwn qIbrqw
leI kYNfylw 7. pdwrQ dI mwqrw
leI mol
splImYNtrI iekweIAW:
1. smql kox leI ryfIAn
2. Tos kox leI strfIAn
-
7
ADVANTAGES OF SI
1. It is a rational System
of units: In this system
only one unit is used for
one physical quantity.
For example, newton for
all types of forces, joule
for all types of energies.
2. It is coherent system of
units: In this system all
the derived units are
obtained by multiplying
or dividing basic units,
without involving any
numerical factor.
3. It is closely related to
C.G.S. system of units.
4. It is metric system of
units. In this system
power of 10 is used.
AYs AweI dy Pwiedy
1. ieh iekweIAW dI qrksMgq pRxwlI hY[ies pRxwlI iv`c ie`k rwSI leI ie`ko iekweI dI vrqoN huMdI hY[audwrhx dy qOr 'qy ,bl dIAW swrIAW iksmW leI inaUtn, swrIAW aUrjw dIAW iksmW leI jUL iekweI [
2. ieh iekweIAW dI susMgq(AnukUl) pRxwlI hY[ies pRxwlI iv`c swrIAW ivauNqpMn iekweIAW byisk iekweIAW nUM guxW jW Bwg kr ky pRwpq kIqw jWdw hY, ibnWH koeI numYrkIl PYktr Swiml kIqy[
3. ieh pRxwlI iekweIAW dI sI.jI.AYs. pRxwlI nwl nyVlw sMbMD r`KdI hY[
4. ieh iekweIAW dI mIitRk pRxwlI hY[ies pRxwlI iv`c 10 dI pwvr dI vrqoN huMdI hY[
-
8
TABLE 1
Basic Quantity
C.G.S. SYSTEM
M.K.S. SYSTEM
F.P.S. SYSTEM
Length centimetre metre Foot
Mass gram kilogram Pound
Time second second second
TABLE 2
TABLE 3
S.No.
Quantity Unit Symbol
1 Plane Angle radian rad
2 Solid angle steradian sr
S.No.
Quantity Unit Symbol
1 Length metre m
2 Mass kilogram kg
3 Time second s
4 Temperature kelvin K
5 Electric Current ampere A
6 Luminous Intensity
candela cd
7 Amount of substance
mole mol
-
9
MULTIPLES SUBMULTIPLES
PREFIX SYMBOL VALUE
deci d 10-1
centi c 10-2
milli m 10-3
micro µ 10-6
nano n 10-9
pico p 10-12
femto f 10-15
atto a 10-18
SOME OTHER UNITS
1. Micron (micrometre)= 10-6 m
2. Angstrom (Å) = 10-10 m ( It is unit of length convenient on
the atomic scale
3. Fermi (F) = 10-15 m
4. Astronomical unit (AU) = 1.496 x 1011m
5. Light year = 9.46 x 1015m
6. Parsec = 3.08 x 1016m
PREFIX SYMBOL VALUE
Deca da 101
Hector h 102
Kilo k 103
Mega M 106
Giga G 109
Tera T 1012
Peeta P 1015
Exa E 1018
-
10
IMPORTANT CONVERSIONS
1kg = 1000 g
1m = 100 cm
1 cm = 10 mm
1m = 1000 mm
1 inch = 2.54 cm
1 foot =12 inch = 30.48 cm
1 pound = 453 g
1 day = 24 h = 86400 s
1 year = 365 days= 31536000 s
1 quintal = 100 kg
1 ton = 10 quintal = 1000 kg
-
11
DIMENSIONS OF A
PHYSICAL QUANTITY
Dimensions of a physical
quantity are the powers to
which the basic units must be
raised to obtain the derived
units of that quantity.
If [M], [L] and [T] denote
mass, length, time, then
Area = length x breadth
= [L] x[L]
= [L2]
= [M0L2T0]
Area has zero dimensions in
mass, 2 dimensions in length,
0 dimensions in time.
Volume = length x breadth x
height
=[L] x[L]x [L]
= [L3]
= [M0L3T0]
BOiqk rwSIAW dy AwXwm
iksy BOiqk rwSI dy AwXwm auh Gwq hn, ijnWH 'qy mùFlIAW iekweIAW nUM auBwirAw jwvy qW jo aus rwSI dIAW ivauNqpMn iekweIAW pRwpq ho skx[
jy [M], [L] and [T] puMj, lMbweI Aqy smyN nUM drswaux qW
KyqrPl = lMbweI x cOVweI
= [L] x[L]
= [L2]
= [M0L2T0]
KyqrPl iv`c puMj dy 0, lMbweI dy 2, smyN dy 0 AwXwm huMdy hn[
GxPl = lMbweI x cOVweI x aucweI
=[L] x[L]x [L]
= [L3]
=[M0L3T0]
-
12
Volume has zero dimensions
in mass, 3 dimensions in
length, 0 dimensions in time.
Speed = Distance / Time
= [L]/ [T]
= [LT-1]
Speed has 1 dimension in
length, -1 dimension in time.
DIMENSIONAL FORMULA
Dimensional formula of a
physical quantity is defined as
the expression which shows
which basic unit enters into
the derived units of that
quantity and with what
powers.
Dimensional formula for area
is [M0L2T0]. This shows that
length enters in the derived
units of area with power of 2.
DIMENSIONL EQUATION
Dimensional equation of a
physical quantity is defined as
the equation obtained by
GxPl iv`c puMj dy 0 ,lMbweI dy 3, smyN dy 0 AwXwm huMdy hn[
cwl = dUrI / smW
= [L]/ [T]
= [LT-1]=[M0L1T-1]
cwl iv`c puMj dy 0 , lMbweI dy 1, smyN dy -1 AwXwm huMdy hn[
AwXwm sUqr
iksy BOiqk rwSI dw AwXwm sUqr auh hY jo drswauuNdw hY ik aus rwSI dIAW ivauNqpMn iekweIAW iv`c ikhVI mu`FlI iekweI AwauNdI hY Aqy iks Gwq nwl[
KyqrPl dw AwXwm sUqr hY [M0L2T0]. ieh drswauNdw hY ik KyqrPl dIAW ivauNqpMn iekweIAW iv`c lMbweI 2 dI Gwq nwl AwauNdI hY[
AwXwm smIkrn
iksy BOiqk rwSI dI smIkrn auh smIkrn hY, jo rwSI nUM ies dy AwXwm sUqr dy brwbr krn nwl pRwpq huMdI hY, nUM AwXwm smIkrn
-
13
equating quantity with its
dimensional formula .
Dimensional equation for area
is
Area = [M0L2T0]
ikhw jWdw hY[
KyqrPl dI AwXwm smIkrn hY
KyqrPl = [M0L2T0]
-
14
LIST OF DIMENSIONAL
FORMULAE
1. Area = length x breadth
= [L] x[L]
= [L2]
= [M0L2T0]
2. Volume=length x breadth
x height
=[L] x[L]x [L]
= [L3]
=[M0L3T0]
3. Density = mass/ volume
=[M]/ [L3]
=[ML-3]
=[M1L-3T0]
4. Speed = Distance / Time
= [L]/ [T]
= [LT-1]
= [M0 L1T-1]
5. Velocity=displacement/ti
me
= [L]/ [T]
AwXwm sUqrW dI sUcI
1. KyqrPl = lMbweI x cOVweI = [L] x[L]
= [L2]
= [M0L2T0]
2. GxPl = lMbweI x cOVweI x aucweI = [L] x[L]x [L]
= [L3]
=[M0L3T0]
3. Gxqw = puMj / GxPl
=[M]/ [L3]
=[ML-3]
=[M1L-3T0]
4. cwl = dUrI /smW = [L]/ [T]
= [LT-1]
= [M0 L1T-1]
5. vyg = ivsQwpn / smW = [L]/ [T]
= [LT-1]
-
15
= [M0 L1T-1]
6. Acceleration=velocity/time
= [LT-1]/ [T]
= [LT-2]
= [M0 L1T-2]
7. Momentum=mass x
velocity
= [M] X [LT-1]
=[M LT-1]
=[M1 L1T-1]
8. Force = mass x
acceleration
= [M] X [LT-2]
=[M LT-2]
=[M1 L1T-2]
9. Work = force x distance
= [M1 L1T-2] x[L]
= [M1 L2T-2]
10. Power = work / time
= [M1 L2T-2]/ [T]
= [M1 L2T-3]
11. Energy = work
= [M1 L2T-2]
12. Pressure = Force / Area
= [M1 L1T-2]/[L2]
= [M0 L1T-1]
6. pRvyg = vyg / smW = [LT-1]/ [T]
= [LT-2]
= [M0 L1T-2]
7. sMvyg = puMj x vyg
= [M] X [LT-1]
=[M LT-1]
=[M1 L1T-1]
8. bl = puMj x pRvyg
= [M] X [LT-2]
=[M LT-2]
=[M1 L1T-2]
9. kMm = bl x dUrI = [M1 L1T-2] x[L]
= [M1 L2T-2]
10. SkqI = kMm /smW = [M1 L2T-2]/ [T]
= [M1 L2T-3]
11. aUrjw = kMm = [M1 L2T-2]
12. dbwA = bl / KyqrPl
= [M1 L1T-2]/[L2]
=[M1 L-1T-2]
-
16
=[M1 L-1T-2]
13. Surface Tension =
Force / length
= [M1 L1T-2]/[L]
=[M1 L0T-2]
14. Gravitational
Constant(G)
F = G m1 xm2 / d2
G= F d2 / m1 x m2
= [M1L1T-2][L2]/ [M]x[M]
= [M-1 L3T-2]
15. Coefficient of
viscosity (η)
F = η Av /d
η = F d / A v
= [M1L1T-2]x[L]/ [L2] x [LT-1]
= [M1L-1T-1]
16. Angle = arc /radius
= [L] /[L]
= [L0]
= [M0L0T0]
17. Angular
Displacement = angle
covered = [M0L0T0]
13. sqHw qxwA = bl / lMbweI = [M1 L1T-2]/[L]
= [M1 L0T-2]
14. grUqvI siQr AMk (G) F = G m1 xm2 / d2
G= F d2 / m1 x m2
=[M1L1T-2][L2]/
[M]x[M]
= [M-1 L3T-2]
15. icpicpwiht dw guxWk (η)
F = η Av /d
η = F d / A v
=[M1L1T-2]x[L]/ [L2] x [LT-1]
= [M1L-1T-1]
16. kox = cwp / ArD ivAws = [L] /[L]
= [L0]
= [M0L0T0]
17. koxI ivsQwpn = kox= [M0L0T0]
-
17
18. Angular velocity =
angular displacement /
time
= [L0] /[T]
= [L0T-1]
= [M0L0T-1]
19. Angular
Acceleration = angular
velocity / time
= [L0T-1] /[T]
= [L0T-2]
= [M0L0T-2]
20. Torque = Force x ┴
distance
= [M1 L1T-2] x[L]
= [M1 L2T-2]
21. Angular momentum
= momentum x ┴
distance
= [M1 L1T-1] x [L]
= [M1 L2T-1]
22. Time Period = time
for one vibration
= [T]
= [M0L0T1]
18. koxI vyg = koxI ivsQwpn / smW = [L0] /[T]
= [L0T-1]
= [M0L0T-1]
19. koxI pRvyg = koxI vyg / smW = [L0T-1] /[T]
= [L0T-2]
= [M0L0T-2]
20. tOrk = bl x ┴ dUrI = [M1 L1T-2] x[L]
= [M1 L2T-2]
21. koxI sMvyg = sMvyg x ┴ dUrI = [M1 L1T-1] x [L]
= [M1 L2T-1]
22. AwivRqI kwl = [T]
= [M0L0T1]
-
18
23. Frequency = 1 /
Time period
= 1/ [T]
= [T-1]
= [M0L0T-1]
24. Planck's Constant
(h)
Energy, E = h ν
h = E / v
= [M1 L2T-2] / [T-1]
=[M1 L2T-1]
25. Impulse = Force x
time
= [M1 L1T-2] x [T]
= [M1 L1T-1]
26. Stress = Force /
area = [M1 L1T-2]/[L2]
=[M1 L-1T-2]
27. Strain = Change in
dimensions (length,
volume) / original
dimensions
= [L]/[L] = [L0]
= [M0L0T0]
23. AwivRqI = 1 / AwivRqI kwl
= 1/ [T]
= [T-1]
= [M0L0T-1]
24. plYNk siQr AMk(h) aUrjw , E = h ν
h = E / v
= [M1 L2T-2] / [T-1]
=[M1 L2T-1]
25. Awvyg = bl x smW
= [M1 L1T-2] x [T]
= [M1 L1T-1]
26. qxwA = bl /KyqrPl
= [M1 L1T-2]/[L2]
=[M1 L-1T-2]
27. stryn = AwXwmW iv`c qbdIlI/ Asl AwXwm = [L]/[L] = [L0]
= [M0L0T0]
-
19
28. Modulus of
Elasticity
= Stress /Strain =[M1 L-1T-2]/ [M0L0T0]
=[M1 L-1T-2]
29. Moment of Inertia =
mass x (┴ distance )2
= [M] x [L]
= [ML2]
= [ML2T0]
28. Lckqw dw mwfUls = strYs / stryn =[M1 L-1T-2]/ [M0L0T0]
=[M1 L-1T-2]
29. jVHqw dw mumYNt = puMj x (┴ dUrI )2 = [M] x [L]
= [ML2]
= [ML2T0]
-
20
DIMENSIONAL QUANTITIES
The quantities which have
certain dimensions are called
dimensional quantities.
Examples: Length, mass,
time, area, volume, density,
speed, force, power
DIMENSIONLESS
QUANTITIES
The quantities which have no
dimensions are called
dimensionless quantities.
Examples: Angle, strain,
DIMENSIONAL CONTANTS
The constants which have
certain dimensions are called
dimensional constants.
Examples: Gravitational
constant, Planck's constant,
Coefficient of viscosity
DIMENSIONLESS
CONSTANTS
The constants which have no
dimensions are called
dimensionless constants.
Examples: 1,2,3...... π, e,
AwXwmI rwSIAW
rwSIAW ijnHW dy koeI nw koeI AwXwm huMdy hn, nUM AwXwmI rwSIAW ikhw jWdw hY[ audwhrxW: lMbweI, puMj, smW, KyqrPl, GxPl, Gxqw, cwl, bl, SkqI AwXwmhIx rwSIAW rwSIAW ijnHW dy koeI AwXwm nhIN huMdy, nUM AwXwmhIx rwSIAW ikhw jWdw hY[ audwhrxW: kox, stryn AwXwmI siQr AMk siQr AMk ijnHW dy koeI nw koeI AwXwm huMdy hn, nUM AwXwmI siQr AMk ikhw jWdw hY[ audwhrxW: gurUqvI siQr AMk, plYNk siQr AMk, AwXwmhIx siQr AMk
siQr AMk ijnHW dy koeI AwXwm nhIN huMdy, nUM AwXwmhIx siQr AMk ikhw jWdw hY[
audwhrxW:
1,2,3...... π, e,
-
21
PAIRS OF PHYSICAL
QUANTITIES HAVING SAME
DIMENSIONS
1. Speed and Velocity
2. Frequency and Angular
velocity
3. Work, Energy, Torque
4. Pressure , Stress,
Modulus of Elasticity
5. Impulse and Momentum
6. Angular Momentum and
Planck's constant
BOiqk rwSIAW dy joVy ijnWH dy AwXwm ie`ko ijhy hn
1. cwl Aqy vyg 2. AwivRqI Aqy koxI vyg 3. kMm, aUrjw , tOrk 4. dbwA, strY`s, lckqw
dw mwfUls 5. Awvyg Aqy sMvyg 6. koxI sMvyg Aqy plYNk
siQr AMk
-
22
PRINCIPLE OF
HOMOGENEITY OF
DIMENSIONS
It states that dimensions of
each term in a physical
relation must be the same.
Explanation:
v = u + a t
Dimensions of v = [M0 L1T-1]
Dimensions of u = [M0 L1T-1]
Dimensions of a = [M0 L1T-2]
Dimensions of t = [M0 L0T1]
Dimensions of a t = [M0 L1T-2]
x [M0 L0T1] = [M0 L1T-1]
Hence, the dimensions of
each term in the physical
relation are the same.
AwXwmW dI iekswrqw dw isDWq
iksy vI BOiqk smIkrn iv`c hryk trm dy AwXwm ie`ko ijhy hoxy cwhIdy hn[ ivAwiKAw: v = u + a t
v dy AwXwm = [M0 L1T-1]
u dy AwXwm = [M0 L1T-1]
a dy AwXwm = [M0 L1T-2]
t dy AwXwm = [M0 L0T1]
a t dy AwXwm = [M0 L1T-2] x [M0 L0T1] = [M0 L1T-1]
ies qrWH , BOiqk smIkrn iv`c hryk trm dy AwXwm ie`ko ijhy hn[
-
23
Q. If x = at +bt2 + c, where x
is distance and t is time,
what are dimensions of
constants a, b and c.
Ans. Given, x = at +bt2 + c
According to principle of
homogeneity of dimensions,
dimensions of each term must
be the same.
Dimensions of x = Dimensions
of at
[x] = [at]
[a] = [x] /[t] = [L] /[T] = [LT-1]
Dimensions of x = Dimensions
of bt2
[x] = [bt2]
[b] = [x] /[t2] = [L] /[T2] = [LT-2]
Dimensions of x = Dimensions
of c
[x] = [c]
[c = [x] = [L]
Q. If v = at +bt2 , where v is
velocity and t is time , what
are dimensions of
constants a and b.
pR. jy x = at +bt2 + c, ij`Qy x dUrI Aqy t smW hY, siQr AMkW a, b Aqy c dy AwXwm kI hn?
h`l . id`qw hY, x = at +bt2 + c
AwXwm dI iekswrqw dy inXm Anuswr hryk trm dy AwXwm ie`ko ijhy hoxy cwhIdy hn[
x dy AwXwm = at dy AwXwm
[x] = [at]
[a] = [x] /[t] = [L] /[T] = [LT-1]
x dy AwXwm = bt2 dy AwXwm
[x] = [bt2]
[b] = [x] /[t2] = [L] /[T2] = [LT-2]
x dy AwXwm = c dy AwXwm
[x] = [c]
[c = [x] = [L]
pR. jy v = at +bt2, ij`Qy v vyg Aqy t smW hY, siQr AMkW a Aqy b dy AwXwm kI hn?
-
24
Ans. Given, v = at +bt2
According to principle of
homogeneity of dimensions,
dimensions of each term must
be the same.
Dimensions of v = Dimensions
of at
[v] = [at]
[a] = [v] /[t] = [LT-1] /[T] = [LT-2]
Dimensions of v = Dimensions
of bt2
[v] = [bt2]
[b] = [v] /[T2] = [LT-1] /[T2] =
[LT-3]
Q. The Vander Waal gas equation
is (P+ a/V2)(V-b) = RT. Find the
dimensions of 'a' and 'b' by using
dimensional analysis
Sol. Given equation is
(P+ a/V2)(V-b) = RT
According to principle of
homogeneity of dimensions,
dimensions of each term must
be the same.
Dimensions of P =
Dimensions of a/V2
h`l . id`qw hY, v = at +bt2
AwXwm dI iekswrqw dy inXm Anuswr hryk trm dy AwXwm ie`ko ijhy hoxy cwhIdy hn[
v dy AwXwm = at dy AwXwm
[v] = [at]
[a] = [v] /[t] = [LT-1] /[T] = [LT-2]
v dy AwXwm = bt2 dy AwXwm
[v] = [bt2]
[b] = [v] /[T2] = [LT-1]/ [T2]
= [LT-3]
pR. (P+ a/V2)(V-b) = RT vWfr vwl gYs smIkrn hY[ AwXwmI ivSlySx dI vrqoN nwl 'a" Aqy 'b' dy AwXwm pqw kro [
h`l : id`qI smIkrn hY :
(P+ a/V2)(V-b) = RT
AwXwm dI iekswrqw dy inXm Anuswr hryk trm dy AwXwm ie`ko ijhy hoxy cwhIdy hn[
P dy AwXwm = a/V2 dy AwXwm
-
25
[a/V2] = [P]
[a] = [P V2] = [ M1L-1T-2][L3]2
= [ M1L5T-2]
Dimensions of b = Dimensions of
V
[b] =[V] = [ L3]
[a/V2] = [P]
[a] = [P V2] = [ M1L-1T-2][L3]2
= [M1L5T-2]
b dy AwXwm = V dy AwXwm
[b] =[V] = [ L3]
-
26
APPLICATIONS OF
DIMENSIONAL EQUATIONS
1. Checking the
correctness of a physical
relation
2. Conversion of units from
one system of units into
another system
3. Derivation of equations
1. Checking the
correctness of a
physical relation
The method of dimensions
can be used to check the
correctness of a physical
relation or formula. For this
principle of homogeneity of
dimensions is used. If the
dimensions of each term in
the given relation are the
same, relation is correct. If not
same, the relation is not
correct.
AwXwmI smIkrnW dy aupXog
1. iksy BOiqk rwSI dI shIkrqw jWc krnw
2. iekweIAW dI ie`k pRxwlI qoN dUjI pRxwlI 'c qbdIl krnw
3. smIkrnW dw ivauNqpMn krnw
1. iksy BOiqk rwSI dI shIkrqw jWc krnw
AwXwmW dy FMg nUM iksy BOiqk smIkrn jW PwrmUly dI shI hox dI jWc leI vriqAw jw skdw hY[ies mksd leI AwXwmW dI iekswrqw dy inXm dI vrqoN kIqI jWdI hY[jy kr id`qI hoeI smIkrn jW PwrmUly iv`c hryk trm dy AwXwm ie`ko ijhy hox qW smIkrn shI hY[jy kr ie`ko ijhy nhIN hn qW smIkrn shI nhIN hovygI[
-
27
-
28
Example1. Check the
correctness of the relation v
= u + a t.
Dimensions of v = [M0 L1T-1]
Dimensions of u = [M0 L1T-1]
Dimensions of a = [M0 L1T-2]
Dimensions of t = [M0 L0T1]
Dimensions of a t = [M0 L1T-2]
x [M0 L0T1] = [M0 L1T-1]
Since the dimensions of each
term in the physical relation
are the same, the equation is
correct.
Example2. Check the
correctness of the relation s
= u t + ½ a t2.
Dimensions of s = [M0 L1T0]
Dimensions of ut = [M0 L1T-1]x
[M0 L0T1]= [M0 L1T0]
Dimensions of ½ a t2 =[M0
L1T-2]x [M0 L0T1]2 = [M0 L1T0]
Since the dimensions of each
term in the physical relation
are the same, the equation is
correct.
audwhrx 1. smIkrn v = u + a t dI shIhox dI jWc kro[
v dy AwXwm = [M0 L1T-1]
u dy AwXwm = [M0 L1T-1]
a dy AwXwm = [M0 L1T-2]
t dy AwXwm = [M0 L0T1]
a t dy AwXwm = [M0 L1T-2] x [M0 L0T1] = [M0 L1T-1]
ikauN ik BOiqk smIkrn iv`c hryk trm dy AwXwm ie`ko ijhy hn ies leI smIkrn shI hY[
audwhrx 2. smIkrn s = u t + ½ a t2 dI shIhox dI jWc kro[
s dy AwXwm = [M0 L1T0]
ut dy AwXwm = [M0 L1T-1] x [M0 L0T1]= [M0 L1T0]
½ a t2 dy AwXwm = [M0 L1T-2] x [M0 L0T1]2 = [M0 L1T0]
ikauN ik BOiqk smIkrn iv`c hryk trm dy AwXwm ie`ko ijhy hn ies leI smIkrn shI hY[
-
29
2. Conversion of units from
one system to another
Dimensional equations can be
used to convert units of a
physical quantity from one
system of units to another.
We know, a physical quantity
can be expressed as
Q = n U
In system of units I,
Q = n1 U1
In system of units II
Q = n2 U2
Hence, n2 U2 = n1 U1
Or n2 = n1 U1 / U2 ....(1)
Now, U1 = [M1xL1y T1z]
U2 = [M2xL2y T2z]
From (1)
n2 = n1 [M1xL1y T1z] / [M2xL2y
T2z]
= n1[M1xL1y T1z / M2xL2y T2z]
n2=n1[M1/M2]x[L1/L2]y[T1/T]z
3. ie`k pRxwlI qoN dUjI pRxwlI iv`c iekweIAW qbdIl krnw
AwXwmI smIkrnW nUM iksy BOiqk rwSI dIAW iekweIAW nUM ie`k pRxwlI qoN dUjI pRxwlI iv`c qbdIl krn leI vriqAw jw skdw hY[
AsIN jwxdy hW ik iksy BOiqk rwSI nUM ies qrWH iliKAw jw skdw hY-
Q = n U
iekweIAW dI pRxwlI 1 iv`c,
Q = n1 U1
iekweIAW dI pRxwlI 2 iv`c
Q = n2 U2
ies leI, n2 U2 = n1 U1
Or n2 = n1 U1 / U2 ....(1)
hux, U1 = [M1xL1y T1z]
U2 = [M2xL2y T2z]
(1) qoN
n2=n1 [M1xL1y T1z] / [M2xL2y T2z]
= n1[M1xL1y T1z / M2xL2y T2z]
n2=n1[M1/M2]x[L1/L2]y[T1/T]z
-
30
Example: Convert 1 newton
into dyne using method of
dimensions.
Solution: newton is SI unit of
force. dyne is unit of force in
CGS system.
n1 = 1
n2 =?
Dimensional formula of force
= [M1L1T-2]
Hence, x =1 , y= 1 , z = -2
SI CGS System
M1 = 1 kg M2 = 1 g
L1 = 1 m L2 = 1 cm
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=1[1kg/1g]1[1m/1 cm]1[1s/1s]-2
=1[1000g/1g]1[100cm/1
cm]1[1s/1s]-2
= 1 x 1000 x 100 x1
= 100000 = 105
Hence, 1 newton = 105 dyne
audwhrx :AwXwmW dy qrIky dI vrqoN nwl 1 inaUtn nUM fweIn iv`c bdlo[
h`l: inaUtn bl dI AYs. AweI. iekweI hY[fweIn sI.jI.AYs. pRxwlI iv`c bl dI iekweI hY[
n1 = 1
n2 =?
bl dw AwXwmI sUqr hY
= [M1L1T-2]
ies leI, x =1 , y= 1 , z = -2
AYs AweI sIjIAYspRxwlI
M1 = 1 kg M2 = 1 g
L1 = 1 m L2 = 1 cm
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=1[1kg/1g]1[1m/1 cm]1[1s/1s]-2
=1[1000g/1g]1[100cm/1
cm]1[1s/1s]-2
= 1 x 1000 x 100 x1
= 100000 = 105
ies leI, 1 inaUtn= 105 fweIn
-
31
Example: Convert 1 joule
into erg using method of
dimensions.
Solution: joule is SI unit of
work. erg is unit of work in
CGS system.
n1 = 1
n2 =?
Dimensional formula of work=
[M1L2T-2]
Hence, x =1 , y= 2 , z = -2
SI CGS System
M1 = 1 kg M2 = 1 g
L1 = 1 m L2 = 1 cm
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=1[1kg/1g]1[1m/1 cm]2[1s/1s]-2
=1[1000g/1g]1[100cm/1
cm]2[1s/1s]-2
= 1 x 1000 x (100)2 x1
= 10000000 = 107
Hence, 1 joule = 107 erg
audwhrx :AwXwmW dy qrIky dI vrqoN nwl 1 inaUtn nUM fweIn iv`c bdlo[
h`l: jUl kMm dI AYs. AweI. iekweI hY[Arg sI.jI.AYs. pRxwlI iv`c kMm dI iekweI hY[
n1 = 1
n2 =?
kMm dw AwXwmI sUqr hY
= [M1L2T-2]
ies leI, x =1 , y= 2 , z = -2
AYs AweI sIjIAYspRxwlI
M1 = 1 kg M2 = 1 g
L1 = 1 m L2 = 1 cm
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=1[1kg/1g]1[1m/1 cm]2[1s/1s]-2
=1[1000g/1g]1[100cm/1
cm]2[1s/1s]-2
= 1 x 1000 x (100)2 x1
= 10000000 = 107
ies leI, 1 jUl= 107 Arg
-
32
Example : Convert density
of 13.6 g/cm3 into kg/m3
using method of
dimensions.
Solution: g/cm3 is unit of
density in CGS system and
kg/m3 is SI unit of density.
n1 = 13.6
n2 =?
Dimensional formula of
density = [M1L-3T0]
Hence, x =1 , y= -3 , z = 0
CGSSystem SI
M1 = 1 g M2 = 1 kg
L1 = 1 cm L2 = 1 m
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=13.6[1g/1kg]1[1cm/1m]-
3[1s/1s]0
=13.6[1g/1000g]1[1cm/100
cm]2[1s/1s]0
= 13.6 x (1/1000)1 x (1/100)-3
x1
audwhrx: AwXwmW dy qrIky dI vrqoN nwl 13.6 g/cm3 dI Gxqw nMU kg/m3 iv`c bdlo[
h`l : g/cm3 Gxqw dw sI.jI.AYs pRxwlI iv`c and kg/m3 AYs AweI iv`c iekweI hY[
n1 = 13.6
n2 =?
Gxqw dw AwXwm sUqr= [M1L-3T0]
ies leI, x =1 , y= -3 , z = 0
CGSSystem SI
M1 = 1 g M2 = 1 kg
L1 = 1 cm L2 = 1 m
T1 = 1 s T2 = 1 s
n2= n1[M1/M2]x[L1/L2]y[T1/T]z
=13.6[1g/1kg]1[1cm/1m]-
3[1s/1s]0
=13.6[1g/1000g]1[1cm/100
cm]2[1s/1s]0
= 13.6 x (1/1000)1 x (1/100)-3
x1
-
33
=13.6 (1/1000) x (100)3x1
= 13.6 x1/1000 x 1000000
=13.6 x1000 = 13600
Hence, 13.6 g/cm3 = 13600
kg/m3
=13.6 (1/1000) x (100)3x1
= 13.6 x1/1000 x 1000000
=13.6 x1000 = 13600
ies leI, 13.6 g/cm3 = 13600 kg/m3
-
34
III. Derivation of relations
The method of dimensions
can be used to derive the
relation for a quantity.
Example: Weight (W) of a
body depends on its mass
(m) and acceleration due to
gravity (g). Derive relation
for W.
Solution:
Suppose W α mx gy
W = k mx gy ……(1)
Dimensions of W =[M1L1T-2]
Dimensions of m = [M1L0T0]
Dimensions of g = [M0L1T-2]
Put these values in eq. (1),
[M1L1T-2] =[M1L0T0]x[M0L1T-2]y
=[MxL0T0][M0LyT-2y]
III. smIkrnW ivauNqpMn krnw
AwXwm dy qrIky nwl iksy rwSI leI smIkrn ivauNqpMn kIqI jw skdI hY[
audwhrx: iksy vsqU dw Bwr (W ) ies dy puMj (m) Aqy gurUqvI pRvyg (g) qy inrBr krdw hY[W leI sMbMD ivauNqpMn kro[
h`l :
mMn lE, W α mx gy
W = k mx gy ……(1)
W dy AwXwm=[M1L1T-2]
m dy AwXwm = [M1L0T0]
g dy AwXwm = [M0L1T-2]
ieh kImqW (1) iv`c Bro
[M1L1T-2] =[M1L0T0]x[M0L1T-2]y
=[MxL0T0][M0LyT-2y]
-
35
[M1L1T-2] =[MxLyT-2y] …(2)
Compare powers of M,L and
T on both sides of (2)
x = 1 ………(3)
y = 1 ………(4)
-2y = -2 …….(5)
Put values of x and y in eq.
(1),
W = k m1 g1
= kmg
Take k=1,
W = mg
This is the required equation.
Example2. Time period (T)
of a simple pendulum may
depend on mass (m) of its
bob, length (l) of the
pendulum and acceleration
due to gravity (g).Derive
relation for T.
Solution:
[M1L1T-2] =[MxLyT-2y] …(2)
(2) dIAW dono pwsy M,L Aqy T dIAW pwvrW dI qulnw kro
x = 1 ………(3)
y = 1 ………(4)
-2y = -2 …….(5)
(1) iv`c x Aqy y dIAW kImqW Bro
W = k m1 g1
= kmg
k=1 lE
W = mg
ieh loVINdI smIkrn hY.
audwhrx 2. ie`k swDwrn pYNfUlm dw foln kwl (T) ies dy bOb dy puMj (m), pYNfUlm dI lMbweI (l) Aqy gurUqvI pRvyg (g) 'qy inrBr kr skdw hY[T vwsqy sMbMD ivauNqpMn kro[
h`l :
-
36
Suppose mMn lE
T α mx ly gz T α mx ly gz
Or T = k mx ly gz ….(1) Or T = k mx ly gz ….(1)
Dimensions of T = [M0L0T1] T dy AwXwm = [M0L0T1]
Dimensions of m = [M1L0T0] m dy AwXwm = [M1L0T0]
Dimensions of l = [M0L1T0] l dy AwXwm = [M0L1T0]
Dimensions of g = [M0L1T-2] g dy AwXwm = [M0L1T-2]
Put these values in eq.(1) ieh kImqW smIkrn (1) iv`c Bro
[M0L0T1]=[M1L0T0]x[M0L1T0]y[M0L1T-2]z [M0L0T1]=[M1L0T0]x [M0L1T0]y [M0L1T-2]z
=[MxL0T0] [M0LyT0] [M0LzT-2z] =[MxL0T0] [M0LyT0] [M0LzT-2z]
[M0L0T1] =[MxLy+zT-2z] ……(2) [M0L0T1] =[MxLy+zT-2z] ……(2)
Compare powers of M, L and T on both sides M , L Aqy T dIAW pwvrW dI qulnw kro
x =0 ……..(3) x =0 ……..(3)
y + z = 0 ……(4) y + z = 0 ……(4)
-2z = 1 …….(5) -2z = 1 …….(5)
From (5), 2z =-1 or z = -1/2 (5) qoN 2z =-1 or z = -1/2
From (4) y -1/2 = 0 or y = ½ (4) qoN y -1/2 = 0 or y = ½
Put these values in eq. (1) ieh kImqW smIkrn (1) iv`c Bro
T = k m0 l1/2 g-1/2 T = k m0 l1/2 g-1/2
= k x 1 l1/2 / g1/2 = k x 1 l1/2 / g1/2
-
37
= k (l/g)1/2 = k (l/g)1/2
= k√l/g = k√l/g
Take k= 2 π Take k= 2 π
T = 2 π√l/g T = 2 π√l/g
-
38
LIMITATIONS OF
DIMENSIONAL ANALYSIS
1. The method of
dimensions does not
give the value of
dimensionless constant
(k). It has to be
calculated by some other
method.
2. The method of
dimensions cannot be
used to derive relations if
the factors on which
quantity depends are not
known.
3. The method of
dimensions cannot be
used to derive relations if
the quantity depends on
more than three factors.
4. The method of
dimensions cannot be
used to derive relations
which involve
dimensionless factors
like sin , cos, log etc.
AwXwmI ivSlySx dIAW KwmIAW
1. AwXwm dw qrIkw AwXwmhIx siQr AMk dy mùl bwry nhIN d`sdw[ies nUM iksy hor FMg nwl pqw krnw pYNdw hY[
2. AwXwm dw qrIkw PwrmUly ivauNqpMn krn leI nhIN vriqAw jw skdw jy kwrk ijnHW 'qy rwSI inrBr krdI hY, pqw nw hox[
3. AwXwm dw qrIkw PwrmUly ivauNqpMn krn leI nhIN vriqAw jw skdw jy rwSI iqMn qoN v`D kwrkW 'qy inrBr krdI hY[
4. AwXwm dw qrIkw PwrmUly ivauNqpMn krn leI nhIN vriqAw jw skdw ijnHW iv`c AwXwmhIx kwrk ijvyN sin , cos, log Awid hox[
-
39
ERRORS qrùtIAW
The difference between actual
value and measured value of
a physical quantity is called
error.
iksy BOiqk rwSI dI Asl mùl Aqy mwpy mu`l 'c Prk nUM qrùtI ikhw jWdw hY[
TYPES OF ERRORS
Errors are of three types:
1. Systematic Errors
2. Random Errors
3. Gross Errors
1. Systematic Errors:
Errors which are due to
known reasons (causes) and
which are always of same
sign or type are called
systematic errors.
(a) Instrumental Errors:
Errors which are due to some
defect in the measuring
instrument are called
instrumental errors.
(b) Environmental
Errors
qru`tIAW dIAW iksmW
qru`tIAW iqMn iksmW dIAW huMdIAW hn:
1. kRmbD qru`tIAW 2. byqrqIb qru`tIAW 3. grOs qru`tIAW
1. kRmb`D qru`tIAW
qru`tIAW ijhVIAW jwxU kwrnW kr ky Aqy hmySW ie`ko icMn /iksm dIAW huMdIAW hn, nUM kRmb`̀D qru`tIAW ikhw jWdw hY[
(a) XMqirk qru`tIAW: qru`tIAW jo mwpx vwly XMqr iv`c iksy nuks kwrn huMdIAW hn , nUM XMqirk qru`tIAW ikhw jWdw hY[
(A) vwqwvrnI qru`tIAW:
-
40
Errors which arise due to
changes in atmospheric
conditions like temperature,
pressure are called
environmental errors.
(c) Errors Due to
imperfection
Minimization of Systematic
Errors:
Because reasons of
systematic errors are known,
these errors can be minimized
by applying proper correction.
Random Errors:
The errors which are due to
unknown reasons and which
are not always of the same
sign or type are called random
errors.
Minimization of Random
Errors:
These errors can be
minimized by repeating the
experiment a number of times
and then taking arithmetic
mean of all the observations.
qru`tIAW jo vwqwvrnI hwlqW ijvyN qwpmwn, dbwA Awid kwrn hoNd iv`c AwauNdIAW hn ,nUM vwqwvrnI qru`tIAW ikhw jWdw hY[
(d) gYrsMpUrnqw kwrn qru`tIAW
kRmbD qru`tIAW nUM G`t krnw:
ikauN ik kRmb`D qru`tIAW dy kwrn pqw huMdy hn, ies leI iehnW qr`utIAW nUM Xog soD lgw ky G`t kIqw jw skdw hY[
byqrqIb qru`tIAW
qru`tIAW ijhVIAW nw jwxU kwrnW kr ky Aqy hmySW ie`ko icMn /iksm dIAW nhIN huMdIAW hn, nUM byqrqIb qru`tIAW ikhw jWdw hY[
byqrqIb qru`tIAW nUM G`t krnw
iehnW qru`tIAW nUM pRXog nUM keI vwr duhrw ky Aqy swrIAW pRyKxW dw gixqk AOsq lY ky G`t kIqw jw skdw hY[
-
41
Gross Errors
The errors which arise due to
human mistakes are called
gross errors.
Minimization of Gross
Errors:
These errors can be
minimized by doing the
experiment very carefully.
ABSOLUTE ERROR
The magnitude of the
difference between the true
value and measured value of
a physical quantity is called
absolute error.
This is denoted by | Δa |.
RELATIVE ERROR
The error obtained by dividing
absolute error by actual value
is called relative error.
Relative error = Δamean/ amean
PERCENTAGE ERROR
The error obtained by taking
percentage of relative error is
called percentage error.
grOs qru`tIAW
ieh qru`tIAW mnu`KI glqIAW kwrn hoNd iv`c AwauNdIAW hn[
grOs qru`tIAW nUM G`t krnw
ieh qru`tIAW pRXog nUM iDAwn nwl krn nwl G`t kIqIAW jw skdw hY[
inrpyK qru`tI
iksy BOiqk rwSI dI Asl mùl Aqy mwpy gey mu`l iv`c Prk dI mwqrw nUM inrpyK qru`tI ikhw jWdw hY[
ies nUM | Δa | nwl drswieAw jWdw hY[
swpyKI qru`tI
qru`tI jo inrpyK qru`tI nUM Asl mùl nwl Bwg krn 'qy pRwpq huMdI hY nUM swpyKI qru`tI ikhw jWdw hY[
swpyKI qru`tI = Δamean/ amean
pRqISq qru`tI
qru`tI jo swpyKI qru`tI dI pRqISqqw lYx nwl pRwpq huMdI hY nUM pRqISq qru`tI ikhw jWdw hY[
pRqISq qru`tI δa = (Δamean/amean) × 100%
-
42
Percentage error, δa = (Δamean/amean)×100%
SIGNIFICANT FIGURES
The digits which are used to express the result with proper
accuracy are called significant figures.
Rules for Significant Figures:
1. All non-zero digits are significant.
Example: The number of significant figures in 463298 is 6.
2. All zeros between nonzero digits are also significant.
Example: The number of significant figures in 6032 is 4.
3. The zeros after the decimal point and before non-zero digit
are not significant.
Example: The number of significant figures in 0.007 is 1.
4. The zeros to the right of decimal point and also right of
nonzero digits are significant.
Example: The number of significant figures in 0.2370 is 4.
5. Zeros after non zero digit are not significant.
The number of significant figures in 100000000 is 1.
-
43
mh`qvpUrx AMk
AMk, jo iksy irjlt nUM Xog Su`Dqw nwl pRgt krn leI vrqy jWdy hn, nUM mh`qvpUrx AMk ikhw jWdw hY[
mh`qvpUrx AMkW leI inXm
1. swry gYr-isPr AMk mh`qvpUrx huMdy hn[ audwhrx: 463298 iv`c 6 mh`qvpUrx AMk hn[
2. gYr-isPr AMkW ivckwr AwauNdIAW swrIAW isPrW vI mh`qvpUrx huMdIAW hn[ audwhrx: 6032 iv`c 4 mh`qvpUrx AMk hn[
3. dSmlv ibMdU dy s`jy pwsy pr gYr-isPr AMk dy K`by pwsy isPrW mh`qvpUrx nhIN huMdIAW hn[ audwhrx: 0.007 iv`c 1 mh`qvpUrx AMk hY[
4. dSmlv ibMdU dy s`jy pwsy Aqy gYr-isPr AMk dy s`jy pwsy isPrW mh`qvpUrx huMdIAW hn[ audwhrx: 0.2370 iv`c 4 mh`qvpUrx AMk hn[
5. gYr-isPr AMkW dy s`jy pwsy isPrW mh`qvpUrx nhIN huMdIAW[ audwhrx: 100000 iv`c 1 mh`qvpUrx AMk hY[
-
44
QUESTIONS FROM PREVIOUS
EXAMINATIONS
SECTION A
Fill in the blanks:
1. Dimensional formula of potential energy is......
[May'19]
2. S.I. unit of Planck's constant is .............
[May'19]
3. Dimensional formula of pressure is........... [Nov'18]
4. Dimensional formula of force is ............
[Nov'18]
5. The dimensional formula of energy is ....... [May'18]
6. Number of fundamental and base units in S.I. system is ....
[May'18]
7. Dimensional formula of Gravitation constant is ........
[May'17]
8. Energy and power have ......................dimensions.
[Nov'16]
9. SI unit of temperature is..............
[May'16]
10. 1 nanometer is equal to ...................
[May'15]
11. The dimensional formula of compressibility is .........
[Nov'14]
12. Number of significant figures in 0.345200 are ..........
[Nov'14]
13. A physical quantity can be completely measured if we know the
numerical value and its ...............
[Dec'14]
14. The dimensional formula of torque is ...........
[May'13]
15. The dimensional formula of kinetic energy is ..........
[Dec'12]
-
45
16. The dimensional formula of pressure is ...............
[May'11]
17. Plane angle and solid angle are the two ........... units in SI
system. [Dec'10]
18. If zero of vernier scale and main scale do not coincide the type
of error is called..........
[Dec'10]
State True or Force:
1. One Fermi is equal to 10-15 m. [May'18][May '19][May'13]
2. Arithmetic mean of all the absolute errors is called mean absolute
error. [Nov'18]
3. Dimensional formula of work and energy is not same. [Nov'18]
4. The equation v2 + u2 = 2as is dimensionally correct.
[Nov'16]
5. SI unit of pressure is dyne/cm2. [Nov'14]
6. One nanometre is equal to 10-9 m.
[Dec'14]
7. Young's modulus has the same dimension as pressure.
[May'12]
8. SI unit of compressibility is Nm-1.
[Dec'12]
9. SI is coherent systems of units. [May,11]
10. Dimensions of work and torque are same.
[Dec'10]
Multiple Choice questions:
1. The significant figures in 0.09 are
a) 1 b) 2 c) 3 d) 4 [May'19]
2. Dimensional formula of angular velocity is same as that of
a) Linear velocity b) acceleration c) frequency d) speed
[Nov '18]
3. The significant figures in 96.000 are
a) 3 b)4 c) 5 d) 6 [May'18]
-
46
4. How many significant figures are there in 40.00?
a) 1 b) 2 c) 3 d) 4 [Nov'16]
5. The significant figures in 0.07805 is
a) 3 b) 4 c) 5 d) 6 [May'16]
6. Which of the following is dimensionless constant/quantity?
a) Young's modulus b) Poisson ratio c) Bulk modulus d) Modulus
of rigidity [Dec'14]
7. The significant figures in 0.09806 is
a) 3 b) 4 c) 5 d)6 [May'13]
8. The formula P= (x2-b)/at relates power (P) distance (d) and time
(t) . The dimensional formula of 'a' is
a) [ML1T2] b) [M1L1T-2] c) [M1L0T2] d) [M1L2T-2] [May '12]
9. How many significant figures are there in 30.00?
a) 1 b) 2 c) 3 d) 4 [May'11]
10. The wavelength associated with a particle of mass 'm' and
moving with velocity v is given by λ = h/mv where h is Planck's
constant. The dimensional formula of 'h' is
a) ML2T b) M1L-1T-1 c) ML1T-1 d) ML2T-1
[Dec 11]
11. Dimensional formula for density is
a) [ML3T] b) [ML-3T0] C) [M3LT3] d) [MLT]
[Dec'10]
SECTION B
1. Convert 1 newton of force into dyne using dimensional
analysis. [May'19][May'18][Dec'10]
2. What is meant by error? Define systematic and random
error.
[May'19]
3. The Vander Waal gas equation is (P+ a/V2)(V-b) = RT. Find
the dimensions of 'a' and 'b' by using dimensional analysis.
[May'19]
4. Check the accuracy of the relation v2-u2=2as
[Nov'18]
5. Check the accuracy of the relation s= ut +1/2 at2.
[Nov'18]
-
47
6. What are significant figures? Give rules for finding significant
figures. [May'17]
7. Check the correctness of relation t = 2 π √l/g where l is
length and g is acceleration due to gravity.
[Nov'16]
8. State and prove principle of homogeneity of Dimensions.
[May'16]
9. Derive the expression for centripetal force (F) acting on a
body of mass (m) moving with velocity (v) in a circle of radius
(r) using dimensional analysis.
[Nov'14]
10. What is meant by error? Define systematic and random
errors. [Nov'14]
11. Write six pairs of physical quantities which have same
dimensions. [Dec'14]
12. Using method of dimensions, convert 1 joule into ergs.
[May'12]
13. What is a unit? Give different systems of units.
[Dec'12]
14. Check the correctness of the relation, v = u + at by the
method of dimensions.
[May'11]
15. What are limitations of dimensional analysis.
[Dec'10]
SECTION C
1. Check the correctness of relation t = 2 π√l/g where l is length
and g is acceleration due to gravity.
[May'19]
2. A body of mass m is moving with uniform speed v in a circle of
radius r. Find an expression for centripetal force F by method of
dimensions. [Nov'18]
3. a) Check the correctness of the relation t = 2 π √l/g where t is
time period of pendulum, l is length and g is acceleration due to
gravity.
b) Explain different systems of units. [May'18]
-
48
4. The density of mercury is 13.6 gm/cm3. Convert its value into
kg/m3. [Nov'14] [Dec '12]
5. a) The wavelength λ associated with a moving particles
depends upon its mass m, velocity v and Planck's constant h.
Show dimensionally that λ α h/mv .
c) The maximum error in the measurement of mass and length
are 3% and 2% respectively. Find the maximum error in the
measurement of density.
[Nov'16]
6. The wavelength of a moving electron depends upon its mass
m, its velocity v and Planck's constant h. Prove that λ α h/mv.
[May'16][Dec'10]
7. Define fundamental and derived units. [Dec'14]
8. What are significant figures? Give rules for finding significant
figures with examples.
[May'13]
9. a) Write five pairs of physical quantities which have same
dimensions.
b) Deduce the dimensions of gravitational constant. [Dec'13]
10. Define
(a) Instrumental error
(b) Error due to imperfect theory or formulae
(c) Random error. Give example of each type. How can the
above error be removed or minimised.
[May'12]
11. Prove that 1 joule = 107 ergs using dimensional equation.
[May'11]
12. The frequency of vibration (n) of a stretched string depends
upon the load applied T, length l, and its mass per unit length u.
Find dimensionally the formula of frequency. [Dec '11]
13. The Pressure P exerted by a liquid column depends on its
height h, density d and acceleration due to gravity g. Derive an
expression for P with the help of dimensions.
[Dec'10]
-
49
-
50




![Dimensional Analysis hdt [????] · 2 Dimensional Analysis: Definition Dimensional Analysis—a tool to find relationships among physical quantities by using their dimensions. The](https://static.fdocuments.us/doc/165x107/5ad85c687f8b9af9068d69b7/dimensional-analysis-hdt-dimensional-analysis-definition-dimensional-analysisa.jpg)