Provider News AK August 1, 2011 - Premera Blue Cross · Calypso Refund Requests Calypso is a...
Transcript of Provider News AK August 1, 2011 - Premera Blue Cross · Calypso Refund Requests Calypso is a...

ContentsCompany Updates page 1-3
Claims & Payment Policy Updates page 4-5
Reminders & Tips page 6-7
Administrative Resources page 8-9
Pharmacy Updates page 9
Biotech & Point of Sale Edit Expansion page 10-12
Medical Policy Updates page 13-15
August 2011
newsNETWORK
An Independent Licensee of the Blue Cross Blue Shield Association August 2011 Alaska Network News 1
News from Premera Blue Cross Blue Shield of Alaska
COMPANY Updates
Premera, Providers Team up to Streamline Refund and Corrected Billing Processes
During the week of May 9-13, healthcare providers throughout the greater Seattle area partnered with Premera
associates at Premera’s campus in Mountlake Terrace, Wash., to focus on streamlining shared processes and reducing administrative costs.
The two collaborative workshops focused on refund requests and corrected bills, as part of ongoing Lean efforts by all participants.
Calypso Refund Requests
Calypso is a Premera affi liate organization that processes refunds and overpayment requests. When Calypso identifi es an overpayment, they mail an “Overpayment Notifi cation” letter with a request for the overpaid amount. The goal of this workshop was to reduce the number of voluntary overpayment notifi cation forms that are received and to add transparency to the refund request process.
Every Lean workshop involves going out to the “gemba,” a Japanese term for observing the work area to see how the work is done. During these gemba walks, Premera and Calypso associates were able to see the process and ask questions at multiple provider sites; likewise, providers viewed the work being done and were able to ask questions at Calypso’s site.
The teams successfully implemented many of the ideas from the gemba walks. As a result, they removed waste and created a more transparent process:
◗ The overpayment notifi cation form was marked as “optional,” so providers know they have the option of using their own form or letter.
◗ A standard template was created so that providers may now combine refunds of less than $25 and submit one check on a quarterly basis.
◗ Forms and letters were modifi ed to clearly identify what information Calypso is requesting and eliminate unnecessary information.
◗ A pilot project is underway for providers to receive monthly summary reports electronically. If successful, it will help eliminate duplicate requests and rework for our providers.
Corrected Bills
Corrected bills can create signifi cant rework for both providers and Premera and may cause delays in provider payments. This team’s goal was to reduce the number of corrected bills received from the provider organizations who participated in the workshop, shorten the amount of time providers and Premera associates spend working on corrected bills, increase transparency and implement best practices.
As result of this workshop: ◗ The team collaborated with OneHealthPort (OHP) to update the Corrected Claim coversheet available on the OHP website to eliminate unnecessary information and clarify instructions.
◗ The Administrative Simplifi cation best practices for submitting corrected bills were updated and clarifi ed on the OHP and Premera websites, as well as in Premera’s internal procedures.
Continued on page 2
The two collaborative
workshops focused on
refund requests and
corrected bills, as part of
ongoing Lean efforts by
all participants.

2 August 2011 Alaska Network News
COMPANY Updates
Transition Management: Helping Members Transition from Hospital to Home
The transition from hospital to home can be a vulnerable time for members. They may
be at greater risk for potential adverse events as the following statistics show*:
◗ Most errors are the result of communication failure between the hospital team and the patient or primary care provider.
◗ Forty-nine percent of adults experience at least one medical error in medication, continuity, diagnostic work-up or test follow-up.
◗ Nineteen to 23 percent suffer an adverse event — most commonly an adverse drug event.Premera has introduced a new
process designed to support members as they transition from the hospital to the home setting. ‘Transition Management’ provides an integrated approach to
support improved care coordination and access to appropriate follow-up care and programs.
Transition Management is used by Premera’s licensed clinicians (RN and Mental Health Professionals) for inpatient management and readmission prevention. It includes:
◗ Preadmission calls to members for planned surgical procedures
◗ Outreach to facilities to assist with safe discharge plans
◗ Medical necessity reviews for admission and length of stay
◗ Post-discharge calls to members to provide education
◗ Appropriate follow-up and communication with providers
◗ Referrals to Case Management when needed.
Members have welcomed this intervention and shared how impactful the calls are in supporting their recovery. For example, one member shared that she was “impressed with the clinical knowledge of the clinician” and “grateful for the education and assistance provided to support her follow-up care.”
Premera’s integrated approach is designed to reinforce adherence to your discharge plans and medications that you prescribe and to enhance the member’s well-being. We encourage you to share information about Transition Management with your patients as appropriate.
*Source: Promoting Effective Transitions of Care at Hospital Discharge: A Review of Key Issues for Hospitalists, Journal of Hospital Medicine, Vol. 2/ No 5 / Sept/Oct 2007, Sunil Kripalani, MD, MSC, Amy Jackson, PharmD, et al.
Premera, Providers Team up to Streamline Refund and
Corrected Billing Processes Continued from page 1
◗ Premera’s procedures have been updated to no longer request medical records on all corrected bills, reducing process preparation time for providers by up to 50 percent.
◗ Premera’s procedures have been updated to ensure Customer Service accurately advises and encourages providers to submit corrected bills electronically.
◗ Participating providers are exploring ways to reduce the number of corrected bills they submit. One way they identifi ed is to hold certain claims, rather than generating late charges by submitting them separately.Through collaboration, Premera and providers are fi nding
ways to increase transparency, streamline administration, and reduce costs.
We would like to thank the following organizations for their participation in the collaborative workshops:
Evergreen HealthcareSeattle Children’s HospitalUniversity of Washington PhysiciansUniversity of Washington MedicineVirginia Mason Medical Center

August 2011 Alaska Network News 3
data indicates they are not accepting new patients.
Please verify your status online at premera.com/provider under “Find a Doctor.” To make changes, email [email protected] or call Physician and Provider Relations at 800-722-4714, option 4.
Our new mobile platform launched this summer to smart phone users, offering members another way to track health information, fi nd a doctor by specialty or location, look up driving directions, and access national networks. Features also include mobile proof of coverage that the member can email to providers. The free, secure Premera Mobile apps are available for iPhones, iPads, and Androids.
The new provider fi nder will continue to be expanded with quality information, cost estimates, and patient experience ratings coming later this year. Check the provider portal for regular updates.
COMPANY Updates
“Premera is committed to helping members take charge of their health and healthcare costs, and the new provider fi nder can play a big role. It’s comprehensive, intuitive and convenient,” said Randy Turner, Sales Director, eBusiness Strategy.
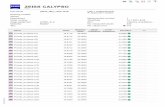
Premera launched its new online provider fi nder on June 15. In addition to listing providers, the new online directory helps our members compare provider information, and includes tools to help members better manage their healthcare. (See Figure 1)
Here are some of the new features available to members using the provider fi nder:
◗ Search for in-area doctors/professionals by condition or procedure
◗ Search for in-area hospital and clinic affi liations
◗ Maps and driving directions ◗ Links to in-area clinic and hospital websites
◗ Search in-area by extended hours and for urgent care facilities
◗ Doctor/hospital comparison on single page
◗ Seamless access to BlueCard national networks
◗ Integrated dental, vision searchesThe provider fi nder also includes a
new search function that helps members narrow their search to only those doctors who are accepting new patients. This search will not display a Premera network provider if their most current provider
Reminder: Fall Offi ce Staff Workshops: Sept. 26 – Sept. 29, 2011
Provider offi ce staff members are invited to attend the Premera Blue Cross Blue Shield of Alaska fall workshops.
The workshops are scheduled as follows:
Fairbanks: Fairbanks Princess Lodge, Sept 26 – 10:30 a.m.-1 p.m.
◗ Anchorage: Captain Cook Hotel, Sept 27 – 8:30-11 a.m. & 12-2:30 p.m.
◗ Kenai: Kenai Visitor’s & Cultural Center,Sept 28 – 12-2:30 p.m.
◗ Juneau: Juneau Arts & Culture Center,Sept 29 – 12-2:30 p.m.Workshops are for Premera contracted
providers only. Please look for your individual workshop invitation in the mail in mid-August.
We look forward to seeing you there!
Comprehensive Online ‘Provider Finder’ Now Available
Figure 1: New provider fi nder on the member/provider portal.
Company Closure Dates for 2011Premera will be closed on the following dates:
◗ Friday, Sept. 2 and Monday, Sept. 5
◗ Thursday, Nov. 24 and Friday, Nov. 25
◗ Friday, Dec. 23 and Monday, Dec.26
Premera.com/provider is the most effi cient and accurate way to access eligibility and claims status information and it is available 24/7. There you have easy access to our Prospective Review, Claims Editor, and Real-Time Estimates/Claims tools, payment policies, medical policies, forms, provider communications, and the Premera Provider Reference Manual.

4 August 2011 Alaska Network News
CLAIMS & PAYMENT POLICY Updates
Change in Calculation of Anesthesia Units
Premera is phasing in a system change that provides a more accurate calculation of anesthesia units.
For all anesthesia procedures that are based on time units, the total anesthesia minutes are calculated and any remainder is rounded to the nearest whole unit using standard rounding methodology (i.e., 0.4999 units or less rounded down to the next whole unit and 0.5 units or more rounded up to the next whole unit).
Beginning no earlier than November 2011, Premera will phase in a new calculation method which computes anesthesia units to the hundredth decimal point (example 4.33).
This modifi cation to our rounding technology will be phased in throughout 2012. You may see both rounding methods used for calculating base units during this time.
If you require additional information, please call Physician and Provider Relations at 800-722-4714, option 4.
New Maternity Services Payment PolicyA new payment policy has been
created to defi ne maternity services and its multiple components. This policy was developed utilizing the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG) coding guidelines.
Total Obstetrical Care codes should be billed at the time of delivery when antepartum, delivery and postpartum services are rendered by the same provider or the same provider group practice unless the member changes insurance or has a change in provider.
Antepartum, Delivery and Postpartum Care codes should be billed separately when only a portion of the services are provided by the same
physician or same physician group practice.
Evaluation and Management services should not be billed for routine maternity visits unless the provider is rendering three or less Antepartum visits and the patient has transferred care.
High-risk conditions or non-obstetrical related evaluations and management services should be billed consistent with AMA and ACOG coding guidelines. Unlisted procedures for maternity care and delivery must include a description of services performed to be considered for payment.
Services such as labor management, artifi cial rupture of membranes, and treatment of hemorrhage are included in Antepartum Care, Delivery and
Postpartum Care services. Services such as facility fees, supplies and cord blood collection should be billed utilizing the appropriate CPT or HCPCS code.
Services billed inconsistent with the Maternity Services policy will be subject to review and possible denial no sooner than 90 days from this notifi cation.

August 2011 Alaska Network News 5
CLAIMS & PAYMENT POLICY Updates
Benefi t Changes: Frequency Limits for Preventive Services
In the coming months, as groups renew, Premera is updating frequency limits for preventive services based
upon clinical practice guidelines set forth by the federal government. In the best interest of our members and providers, Premera will apply frequency limits more conservatively than requirements allow for the majority of services.
The schedule and list of select preventive services that the new frequency limits will apply to are listed below. Once the frequency limitation(s) are exceeded, Premera will deny any further claims for those procedures if they are coded as preventive.
Selected Preventive Services and
Limits
◗ Preventive exams for those age 4 and above are limited to one per year (calendar/or plan)
◗ Men’s health exams are limited to one per year (calendar/or plan)
◗ Colonoscopy (one every 5 years) ◗ Sigmoidoscopy (one every 5 years) ◗ Fecal Occult Blood Home Test (one per year)
◗ Bone Density Study (one every 2 years; women only)
◗ Aortic Ultrasound (one per lifetime; men age 65-75)
◗ Women’s Annual GYN/OB Exam (one per year)
Other Important Notes
◗ Age and gender limitations are only applicable to Bone Density Study and Aortic Ultrasound.
◗ Colonoscopy and Sigmoidoscopy services that exceed frequency limits will be pended for medical review.
◗ No retroactive billing will be allowed for services already provided prior to frequency limit implementation date.
◗ All preventive service frequency limits are subject to change and are based on calendar/plan year measurement.
◗ All other select preventive services that exceed frequency limits will be denied.
Verifying Member Benefi ts
Providers should verify member benefi ts online at premera.com/provider, using the Real-Time Claims/Estimates tool on the secure portal. The tool will accurately refl ect the member’s preventive services benefi t, their associated copays for preventive services, and any age and/or frequency limits that apply. Providers can also call the Customer Service number on the back of the member’s ID card to verify frequency limits for preventive benefi ts.
Reminder: Frequency Limits
Recently, we identifi ed situations in which our stated policy limitations were being exceeded. We have taken the necessary steps to correct this issue.
Please note that the following policies have stated daily frequency limitations:
◗ Acupuncture Services ◗ After Hours Service (99050) ◗ Blood Draw (36415) ◗ Chiropractic ◗ Group Psychotherapy ◗ Multiple Births ◗ Osteopathic Services ◗ Physical Therapy Services

6 August 2011 Alaska Network News
REMINDERS & Tips
ICD-10 Preparedness: Premera and Providers
The migration from ICD-9 to ICD-10 will require a great deal of hard work. It’s an effort that’s been compared to the work required to implement system
changes for Y2K and HIPAA. Premera’s implementation planning is well underway, and we will meet the federal compliance date of Oct. 1, 2013.
What You Should Know:
• Your awareness and preparedness are crucial in making a smooth transition to ICD-10.
• Premera encourages you to develop an implementation strategy that includes an assessment of the impact on your organization, a detailed timeline and budget.
• If your offi ce is not prepared by Oct. 1, 2013, you may experience delays in claims processing.
Steps You Can Take to Prepare:
• Assess staff training needs.• Budget for time and costs related to ICD-10
implementation, including expenses for system changes, resource materials, and training.
• Conduct test transactions using version 5010/ICD-10 codes with payers and clearinghouses.
• Stay up-to-date on ICD-10 resources and information from CMS.
• Identify your current systems and work processes that use ICD-9 codes.
• Talk with your practice management system vendor about accommodations for both Version 5010 and ICD-10 codes.
• Discuss implementation plans with your clearinghouses, billing services, and payers to ensure a smooth transition.
Centers for Medicare &Medicaid Services (CMS) Resources:
• The CMS ICD-10 website: cms.gov/icd10 provides the latest ICD-10 information and links to resources for providers to prepare for ICD-10 implementation in a 5010 environment.
• The CMS Sponsored ICD-10 Teleconferences web page:cms.gov/ICD10/Tel10/list provides information on upcoming and previous CMS national provider ICD-10 teleconferences, including registration, presentation materials, written transcripts and audio recordings.
• Medicare Fee-for-Service Provider Resources: http://www.cms.gov/ICD10/06_MedicareFeeforServiceProviderResources.aspand
• Provider Resources (for all providers): cms.gov/ICD10/05a_ProviderResources web pages provide links to a variety of related educational resources and information.
Additional Resources:
• WEDI (Workgroup for Electronic Data Interchange): wedi.org
• HIMSS (Health Information and Management Systems Society): himss.org/icd10
Do You Submit Paper Claim Forms For Professional Services?
Try using our Real-Time Estimates/Claims tool for faster submission and processing. To enable real-time claim functionality, send us a completed ‘Request to Enable Real-Time Claims’ form located under Claims and Billing forms on the left navigation of the provider portal.
To launch the tool, select ‘Submit Estimate/Claim’ on the left navigation menu of the provider portal at premera.com/provider.

August 2011 Alaska Network News 7
REMINDERS & Tips
HIPAA 5010: Complete Address Ensures Provider Payment
When submitting HIPAA 5010-compliant transactions, the billing provider address must be a complete, physical street address and can no longer
be a P.O. Box or lock-box.
Billing Provider Address (Loop 2010AA)• Must be a street address.• P.O. Box or lock-box addresses is to be sent in the Pay-To
Address Loop(Loop ID-2010AB), if needed.
If you need payments directed to a different address, use the pay-to- address fi elds.
Pay-To Address (Loop ID-2010AB) ◗ Required when the address for payment is different than that of the Billing Provider.Premera encourages providers to check with their
clearinghouse or system vendor to request assistance with these changes and avoid warning messages or rejection errors for incorrect street address fi elds.
If you have questions, contact EDI at [email protected] or call 800-435-2715.
Use of Modifi ers 25 and 59 Must Be Clinically Justifi ed
Under certain circumstances it may be necessary to report that a procedure or service was distinct or independent from other E/M or non E/M services performed on the same day. Modifi ers 25 or 59 should be added only when appropriate, to indicate a separate and distinct service. However, the use of these modifi ers must be supported and justifi ed in the clinical notes.
See payment policy: Modifi er 59 – Distinct Procedural Service (Non E/M)
• Use this only if your documentation supports using a modifi er
• Examples: o Separate encounters or operative sessions on the
same date o Separate lesion o Separate incision or excision o Separate site or organ system o Separate injury
See payment policy: Modifi er 25 – Separately identifi able E/M service by the same physician on the same day of the procedure or other service
• Modifi er 25 can only be appended to E/M Services codes• Use this modifi er only when the E/M service is separate
and distinct from any procedure or other service provided on the same day.
o Above and beyond the other service provided or beyond the usual preoperative and postoperative care associated with the other procedure performed
o Documentation must meet the criteria for separate reporting of the E/M service
Please do not send medical records when billing for these services. Random audits may be performed to ensure services are billed appropriately. As part of the audit process, we may request medical records supporting the use of this modifi er.
FEP Secondary to Medicare ClaimsFEP (Federal Employee Program) secondary to Medicare
claims are automatically sent to Premera by Medicare after Medicare has processed the claim. To prevent duplicate claim submission, please do not bill these claims to Premera FEP if the Medicare Explanation of Benefi ts indicates the claims were forwarded (or crossed over) to the secondary payer.

8 August 2011 Alaska Network News
ADMINISTRATIVE Resources
Premera Begins Downloading ProviderSource™ Credentialing DataProviderSource is now Premera’s
preferred method for receiving provider credentialing and recredentialing information. Effective May 1, 2011, Premera began downloading attested* provider credentialing data from ProviderSource.
ProviderSource is an easy-to-use online portal to a statewide system for centralized collection, verifi cation and distribution of all provider credentialing data. This service is hosted by OneHealthPort and paid for by health plans and hospitals that use the data.
There is no fee for providers to input their credentialing data. With ProviderSource, you simply enter provider data, review it, and then attest to its accuracy. Throughout the year, the service will prompt you to keep your information current and complete the attestation process. You can streamline your practice by avoiding the hassle of completing multiple hard-copy applications each year. You may use the data to complete other standard forms and to print or download records at no cost.
We thank the providers who have completed loading their credentialing
data and the attestation process. Please note: OHP has informed us that many providers have loaded their data, but have not yet completed the attestation process. If you have begun the process, please confi rm that you have completed the attestation so that Premera, other payers, and hospitals can access your records electronically.
You will fi nd information, tools and tips on how to use ProviderSource using this link: www.onehealthport.com/services/providersource_live.
*Premera cannot access provider credentialing data until the provider has attested the information in the application.
Credentialing Notifi cationFollowing the initial credentialing process,
providers who are in the recredentialing cycle are considered approved unless the provider is otherwise notifi ed.
If you have specifi c questions regarding credentialing, please call Physician and Provider Relations at 800-722-4714, option 4.

August 2011 Alaska Network News 9
ADMINISTRATIVE Resources
Refer to Contracted Air Ambulance Providers When Possible
We would like to remind you of the importance of referring members to providers who are in-network with Premera Blue Cross Blue Shield of Alaska. When members receive air transport services by an out-of-network provider, their coinsurance amounts are higher and they can incur a signifi cant balance bill.
Our contracted air ambulance providers include: ◗ Airlift Northwest: 800-426-2430 ◗ American Care: 800-941-2582 ◗ LifeMed Alaska: 800-478-5433
By using these contracted providers, our members are guaranteed the lowest out-of-pocket costs possible and protected against balance billing.
Sign-up for OneHealthPort’s Electronic Newsletter
OneHealthPort emails periodic announcements and newsletters with news and information about healthcare technology, security developments, and community events. Providers and offi ce staff can subscribe at: onehealthport.com/news/newsletter_sign_up.php
PHARMACY Updates
Average Wholesale Price Sourcing
We have been informed that First DataBank (FDB), a national drug pricing publisher, is planning to stop publishing the drug pricing benchmark Average Wholesale Price (AWP) sometime around September 2011. This is an industry-wide change and is occurring as a result of the
FDB AWP Settlement.Our vendor that supplies drug pricing benchmark information for offi ce administered drugs currently
uses multiple publishing sources of AWP including FDB, MediSpan, and Redbook. Therefore we are anticipating no change to provider reimbursement when one AWP source leaves the market.
Premera is working to assess the impacts of this change, if any.
About the Settlement
A lawsuit, brought by New England Carpenters Health Benefi ts Fund, et al., alleged that McKesson and FDB unlawfully conspired to infl ate the mark-up factor, thereby wrongfully increasing the published AWP for many drugs. The lawsuit alleges this in turn increased the prices paid by third-party payers — like Premera — and consumers for certain drugs. McKesson and FDB have denied any wrongdoing, but have elected to enter into a settlement rather than pursue their defense.

10 August 2011 Alaska Network News
BIOTECH & POINT OF SALE (POS) Edit Expansion
Premera has added new review criteria based on clinical best practice and approval by an independent Pharmacy and Therapeutics Committee. The program is designed to promote appropriate drug selection, length of therapy and utilization of specifi c drugs while improving the overall quality of care.
Newly added Biotech and POS Edit and POS Program drugs may be added or deleted from this list without prior notifi cation. If you have questions, please call the Pharmacy Services Center at 888-261-1756 or fax us at 888-260-9836, Monday through Friday, 8 a.m. – 5 p.m. Pacifi c Time.
New Edits in the Biotech Edit ProgramEffective July 1, 2011
Afi nitor® (everolimus)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_125858.pdf
Coverage Criteria:Afi nitor® (everolimus) may be approved for use as a single agent for advanced renal cell carcinoma when both the following conditions are met:
• Tumor is unresectable, and• Patient has progressed on prior tyrosine kinase inhibitor
therapy, i.e., sunitinib (Sutent®) or sorafenib (Nexavar®).
Afi nitor® (everolimus) may be approved for use as a single agent for subependymal giant cell astrocytoma (SEGA) in patients with tuberous sclerosis when the tumor is unresectable.
Afi nitor® (everolimus) may be considered medically necessary for off-label use in treating Waldenström’s macroglobulinemia/lymphoplasmocytic lymphoma (WM) (NCCN Category 2A), or advanced neuroendocrine tumors (NCCN Category 2A).
The initial approval period will be three months. Continued approval beyond the fi rst three months of therapy will require documentation that there has been no disease progression on current therapy.
Afi nitor® is a specialty pharmacy drug covered under the pharmacy benefi t.
Targretin® (bexarotene)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_125859.pdf
Coverage Criteria:Targretin® (bexarotene) may be approved for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma when one or more systemic therapies have failed (including but not limited to the following list of agents recommended by NCCN guidelines):
For simple skin involvement:• Systemic retinoids (isotretinoin, acitretin, etc.)• Interferon (alpha, gamma)• Methotrexate• Alemtuzumab (Campath)
For late stage disease usually with solid organ involvement, more aggressive therapy is usually required, such as:
• Gemcitabine,• Liposomal doxorubicin
The initial approval period will be three months. Continued approval beyond the fi rst three months of therapy will require documentation that there has been no disease progression while on the current therapy.
Votrient® (Pazopanib)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_125861.pdf
Coverage Criteria:Votrient® (pazopanib) may be considered medically necessary for:
• Treatment of patients with advanced renal cell carcinoma (RCC); and
• Treatment of patients with thyroid carcinoma.
The initial approval period will be three months. Continued approval beyond the fi rst three months of therapy will require documentation that there has been no disease progression while on the current therapy.
Votrient® is a specialty pharmacy drug covered under the pharmacy benefi t.

August 2011 Alaska Network News 11
BIOTECH & POINT OF SALE (POS) Edit Expansion
Zolinza® (vorinostat)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_125859.pdf
Coverage Criteria:Zolinza® (vorinostat) may be approved for treatment of cutaneous manifestations of cutaneous T-cell lymphoma when two or more systemic therapies have failed (including but not limited to the following list of agents recommended by NCCN guidelines):
For simple skin involvement:• Systemic retinoids (isotretinoin, acitretin, etc.)• Interferon (alpha, gamma)• Methotrexate• Alemtuzumab (Campath)
For late stage disease usually with solid organ involvement, more aggressive therapy is usually required, e.g.:
• Gemcitabine,• Liposomal doxorubicin• Bexarotene (Targretin)
The initial approval period will be three months. Continued approval beyond the fi rst three months of therapy will require documentation that there has been no disease progression while on the current therapy.
Zolinza® is a specialty pharmacy drug covered under the pharmacy benefi t.
New Edits in the POS Edit Program:Effective Aug. 1, 2011
Branded Antidepressants (excluding Lexapro and Pristiq)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_060793.pdf
Branded SSRI (selective serotonin reuptake inhibitor), SNRI (serotonin/norepinephrine reuptake inhibitor), and any other second generation antidepressants (i.e., antidepressants other than tricyclic and MAOI agents) may be considered medically necessary when the following criteria have been met:
Major Depressive Disorder/Generalized Anxiety Disorder• May be considered medically necessary when there has
been a trial and failure of two generically available second generation antidepressants.
Lexapro and Pristiq may be considered medically necessary when the following criteria have been met:
• Lexapro® (Escitalopram) may be considered medically necessary when there has been a trial and failure of citalopram in addition to one other generically available second generation antidepressant.
• Pristiq® (Desvenlafaxine) may be considered medically necessary when there has been a trial and failure of venlafaxine in addition to one other generically available second generation antidepressant.
Cymbalta® (Duloxetine)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_060796.pdf
Cymbalta® (Duloxetine) may be considered medical necessary for the indication of major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathy, chronic musculoskeletal pain or fi bromyalgia when the following criteria have been met:
Major Depressive Disorder/Generalized Anxiety Disorder• May be considered medically necessary when there has
been a trial and failure of two generically available second generation antidepressants.
Diabetic Peripheral Neuropathy• May be considered medically necessary when there has
been a trial and failure of gabapentin AND a tricyclic antidepressant (e.g, amitriptyline) unless contraindicated.
Chronic Musculoskeletal Pain• May be considered medically necessary when there has
been a trial and failure of at least THREE nonsteroidal anti-infl ammatory drugs (NSAIDs).
Fibromyalgia• May be considered medically necessary when there has
been a trial and failure of gabapentin and at least two ofthe following:
o A tricyclic antidepressant (e.g amitriptyline) o Cyclobenzaprine o Tramadol

12 August 2011 Alaska Network News
BIOTECH AND POINT OF SALE (POS) Edit Expansion
Lyrica® (Pregabalin)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_060796.pdf
Lyrica® (pregabalin) may be considered medically necessary for the indication of diabetic peripheral neuropathy, post herpetic neuropathy, seizure disorder and fi bromyalgia when the following criteria have been met:
Diabetic Peripheral Neuropathy/Post Herpetic Neuropathy• May be considered medically necessary when there has
been a trial and failure of gabapentin AND a tricyclic antidepressant (e.g, amitriptyline) unless contraindicated.
Seizure Disorder• Will be considered on an individual case basis.• Fibromyalgia• May be considered medically necessary when there has
been a trial and failure of gabapentin and at least two of the following:
o A tricyclic antidepressant (e.g amitriptyline) o Cyclobenzaprine o Tramadol
Solodyn® (minocycline extended release)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_037976.pdf
Coverage Criteria:Coverage of Solodyn may be considered medically necessary when there has been a trial and failure of a generic tetracycline product such as doxycycline or minocycline
Please note:• There will be no grandfathering of current members on
doses that exceed the doses listed above• Medco sent a targeted mailing on July 1, 2011 to members
who may be affected by the change in the amounts allowed. The letter included a fax back form with instructions to follow up with their provider for prior authorization.
Effective Sept. 1, 2011
Atypical Antipsychotics
Fanapt® (Iloperidone)Invega® (Paliperidone)Latuda™ (Lurasidone)Saphris® (Asenapine)
https://www.premera.com/stellent/groups/public/documents/medicalpolicy/cmi_037976.pdf
Coverage Criteria:Fanapt®, Invega®, Latuda™ or Saphris®, may be considered medically necessary when there has been a trial and failure of either Risperidone, Seroquel, Zyprexa, Geodon or Abilify.
Please note: This criteria will apply to new therapy requests only. Current members who are on the medication prior to the Sept. 1, 2011 effective date will be automatically grandfathered.

August 2011 Alaska Network News 13
MEDICAL POLICY Updates
Physicians, Providers and Offi ce Staff
Premera medical policies are guides used to evaluate the medical necessity of a particular service or treatment. We adopt policies after careful review of published, peer-reviewed scientifi c literature, national guidelines and local standards of practice. Since medical technology is constantly changing, we reserve the right to review and update our policies as appropriate. When there
are differences between the member’s contract and medical policy, the member’s contract prevails. The existence of a medical policy regarding a specifi c service or treatment does not guarantee that the member’s contract covers that service.
Medical policies are available on premera.com/provider; click on Medical Policies under Reference Info. If you would like a copy of a particular medical policy and are unable to obtain it online, email your request to [email protected].
Note: All policy numbers begin with CP.MP and are listed here in numeric order.The following policy changes are effective for dates of service of May 10, 2011 and later:
BC.1.01.19 Threshold Electrical Stimulation as a Treatment of Motor Disorders. This treatment, previously considered investigational, is now considered not medically necessary.
BC.2.01.54 Percutaneous Transluminal Angioplasty of Intracranial Atherosclerotic Stenoses With or Without Stenting. Title has been changed to Endovascular Procedures for Intracranial Arterial Disease (Atherosclerosis and Aneurysms). Intracranial stent placement as treatment for intracranial aneurysms, previously not addressed, may now be considered medically necessary when criteria are met.
BC.2.01.82 Bioimpedance Devices for Detection of Lymphedema. New policy. Devices using Bioimpedance are considered investigational for use in the diagnosis, surveillance, or treatment of patients with lymphedema, including use in subclinical secondary lymphedema.
PR.2. 01.514 Actinic Keratoses Treatments. Title has been changed to Dermatologic Applications of Photodynamic Therapy. Superfi cial basal cell and Bowen’s disease, previously considered investigational, may now be considered medically necessary when criteria are met.
BC.2.04.67 KIF6 Genotyping for Predicting Cardiovascular Risk and/or Effectiveness of Statin Therapy. New policy. KIF6 Genotyping is considered investigational for predicting cardiovascular risk and/or the effectiveness of statin therapy.
PR.5.01.514 HER2 Inhibitors. Title changed to Trastuzumab and Other HER-2 Inhibitors. Neoadjuvant therapy added as medically necessary for the treatment of patients with breast cancer whose tumors overexpress the HER2 protein.
PR.5.01.520 Pharmacy Medically Necessary Criteria for Branded Antidepressants. Policy statement on branded SSRI, SNRI and any second generation antidepressants, previously considered medically necessary after a trial and failure of at least one generic second generation antidepressant, has now been updated to require trial and failure of at least two generic second generation antidepressants.
PR.5.01.521 Pharmacologic Treatment of Neuropathy, Fibromyalgia and Seizure Disorders. Duloxetine to treat chronic musculoskeletal pain, may now be considered medically necessary when criteria are met. Use of branded agents (duloxetine, pregabalin) for neuropathic pain now requires trial and failure of two generic alternatives.
Referring Patients for Sleep TestingWe are revising our medical policy for Diagnosis and
Management of Sleep Apnea (CP.MP.PR.2.01.503) as of Sept. 1, 2011. The policy change will impact how you refer our members for diagnosis of sleep apnea. We now consider home sleep testing medically necessary and the preferred initial evaluation for all members likely to have Obstructive Sleep Apnea.
Referrals for in-facility tests are considered medically necessary for diagnosing sleep conditions other than Obstructive Sleep Apnea, and when initial home testing was not diagnostic.
Initial in-facility testing referral is appropriate for specifi c conditions including:
• Children (younger than 18 years old)• Individuals with risk factors that preclude a home study
o Congestive heart failure o Advanced COPD o Morbid obesity o Neuromuscular disordersIf you refer the member for an in-facility test, we
recommend that the facility request a Benefi t Advisory to ensure that the services are reviewed for medical necessity prior to the service being performed.
Find a contracted Home Sleep Testing provider using our provider fi nder at premera.com/provider.

14 August 2011 Alaska Network News
MEDICAL POLICY Updates
PR.5.01.531 Pharmacotherapy of Lupus. New policy. Belimumab (Benlysta®) may be considered medically necessary for the treatment of adult patients with active, autoantibody-positive, systemic lupus erythematosus who are receiving standard therapy. All other uses of belimumab (Benlysta®) are considered investigational.
PR.5.01.532 Cutaneous T-Cell Lymphomas (CTCL): Systemic Therapies. New policy. Bexarotene (Targretin®) may be considered medically necessary for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy. All other uses of bexarotene are considered investigational. Vorinostat (Zolinza®) may be considered medically necessary for treatment of cutaneous manifestations in patients who are refractory to at least two prior systemic therapies. All other uses of vorinostat are considered investigational.
PR.5.01.533 mTOR Kinase Inhibitors. New policy. Use in Cancer Patients: Everolimus (Afi nitor®) may be considered medically necessary for the labeled indications, treatment of patients with advanced renal cell carcinoma (RCC) after treatment failure with sunitinib (Sutent®) or sorafenib (Nexavar®), and subependymal giant cell astrocytoma (SEGA) in patients with tuberous sclerosis. Everolimus (Afi nitor®) may be considered medically necessary for off-label use in treating Waldenström’s macroglobulinemia/lymphoplasmocytic lymphoma, or advanced neuroendocrine tumors. All other oncology uses of everolimus (Afi nitor®) are considered investigational. Temsirolimus (Torisel®) may be considered medically necessary for the labeled indication, treatment of patients with advanced renal cell carcinoma (RCC). All other uses of temsirolimus (Torisel®) are considered investigational.
Use in Organ Transplant Patients: Everolimus (Zortress®)* is considered medically necessary for
prophylaxis of organ rejection in allogeneic transplant patients. *Note: This form of everolimus does NOT require a benefi t advisory. Prograf (tacrolimus®)* and sirolimus (Rapamune®)* are considered medically necessary for the prophylaxis of organ rejection in patients receiving allogeneic transplants. *Note: These drugs do not require a benefi t advisory.
PR.5.01.534 Multiple Receptor Tyrosine Kinase Inhibitors. New policy. Indications for sorafenib and sunitinib imported unchanged . New statement added: Pazopanib (Votrient®) may be considered medically necessary for treatment of patients with advanced renal cell carcinoma (RCC); and treatment of patients with thyroid carcinoma.
BC.7.01.86 Endovascular Stent Grafts for Thoracic Aortic Aneurysms of Dissections. Policy statement edited to clarify that medically necessary use is for specifi c types of aneurysms without dissection, using devices approved by the U.S. Food and Drug Administration for their approved specifi cations.
BC.7.01.126 Image-Guided Minimally Invasive Lumbar Decompression (IG-MLD) for Spinal Stenosis. New policy. Image-guided minimally invasive lumbar decompression is considered investigational.
PR.7.01.516 Morbid Obesity Surgery. Sleeve gastrectomy, previously considered investigational, may now be considered medically necessary when criteria are met.
PR.8.01.503 Intravenous Immune Globulin Therapy. Use of IVIg for Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococci (PANDAS), previously not addressed, is now considered a specifi c investigational indication.
PR.8.03.503 Occupational Therapy. Home-based occupational therapy, previously not addressed, may now be considered medically necessary when the member is homebound. See Policy Guidelines for the defi nition of homebound.
The following policy changes are effective for dates of service of June 13, 2011 and later:
PR.1.01.522 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid. Intermittent monitoring (72 hour) previously considered medically necessary only when criteria were met, is now considered medically necessary without qualifying criteria.
BC.2.02.10 Biventricular Pacemakers for the Treatment of Heart Failure. Cardiac resynchronization therapy in patients with NYHA class II, previously considered investigational, may now be considered medically necessary when criteria are met.
BC.2.02.26 Left-Atrial Appendage Closure Devices for Stroke Prevention in Atrial Fibrillation. New policy. The use of left-atrial appendage closure devices for the prevention of stroke in atrial fi brillation is considered investigational.
BC.2.04.62 Proteomics-based Testing for the Evaluation of Ovarian (Adnexal) Masses. New policy. The proteomics-based OVA1™ test may be considered medically necessary as an aid to further assess
the likelihood that malignancy is present when the physician’s (other than gynecologic oncologist) independent clinical and radiological preoperative evaluations do not indicate malignancy in a patient with an ovarian (adnexal) mass. The use of the OVA1™ test is considered investigational for other indications due to lack of clinical utility.
PR.2.04.506 Genetic Testing for Inherited Susceptibility to Colon Cancer, Including Microsatellite Instability Testing. Policy statement clarifi ed to indicate that APC testing of the index patient is considered medically necessary. In addition, MMR mutation testing for Lynch syndrome no longer requires a positive family history.
BC.4.01.16 Progesterone Therapy as a Technique to Reduce Preterm Birth in High-Risk Pregnancies. For women with a singleton pregnancy and a short cervix, a condition previously considered investigational, daily vaginal progesterone between 20 and 23 6/7 weeks may now be considered medically necessary.

August 2011 Alaska Network News 15
MEDICAL POLICY Updates
BC.5.01.17 Repository Corticotrophin Injection. New policy. Repository corticotropin injection may be considered medically necessary for treatment of infantile spasms (West’s syndrome). Repository corticotropin injection is considered not medically necessary for use in diagnostic testing of adrenocortical function. Use of repository corticotropin injection is considered not medically necessary as treatment of corticosteroid-response conditions, unless there are medical contraindications or intolerance to corticosteroids that are not also expected to occur with use of repository corticotropin injection. Except as noted here, use of repository corticotropin injection is considered investigational for conditions that are not responsive to corticosteroid therapy including, but not limited to, use in tobacco cessation, acute gout and childhood epilepsy.
PR.5.01.605 Medical Necessity Criteria for Pharmacy Edits. List of point-of-sale program drugs updated;
antiemetics removed from the list. Non-benzodiazepine hypnotic agents (branded single source), require prior trial of zolpidem or zaleplon unless these would be inappropriate.
Phased-in additional changes are: August: Solodyn® (extended-release minocycline) for
acne requires a failed trial of any generic tetracycline product (e.g., doxycycline or minocycline).
September: (1) Initiating therapy with a nonpreferred atypical antipsychotic (Fanapt, Invega, Latuda or Saphris) requires failed trial of a preferred atypical antipsychotic agent (risperidone, Abilify, Geodon,
Seroquel or Zyprexa). This does not apply to patients already stabilized on a nonpreferred agent.
(2) Orally-administered brand Bisphosphonate products require a failed trial of generic alendronate.
October: Nonpreferred ARBs (Atacand, Avapro, Cozaar, Benicar, Edarbi or Teveten, as well as combinations of these agents) require failed trial of a preferred ARB (generic losartan, Diovan or Micardis, or a combination of one of these).
For more information, see our formulary search tool at https://www.premera.com/stellent/groups/public/documents/xcpproject/pvd_drugsearch.asp.
BC.7.01.84 Semi-Implantable and Fully Implantable Middle Ear Hearing Aid for Moderate to Severe Sensorineural Hearing Loss. Fully implantable middle ear hearing aids, previously not addressed, are now considered investigational.
BC.8.01.516 Breast Brachytherapy. Ductal carcinoma in situ (DCIS), previously considered investigational, may now be considered medically necessary when criteria are met. Criteria for stages 0, I and II revised to refl ect the American Society of Breast Surgeons recommendations.
BC.9.03.23 Intravitreal Corticosteroid Implants. Ozurdex, a dexamethasone intravitreal implant not previously addressed, may now be considered medically necessary for the treatment of non-infectious ocular infl ammation, or uveitis, affecting the posterior segment of the eye or for macular edema following branch or central retinal vein occlusion.
The following policy changes are effective for dates of service of November 4, 2011 and later:
BC.2.04.51 Genetic Testing for Tamoxifen Treatment. New policy. Genotyping to determine cytochrome p450 (CYP2D6) genetic polymorphisms is considered investigational for the purpose of managing treatment with tamoxifen for women at high risk for, or with, breast cancer.
BC.2.04.68 Laboratory Testing to Allow Area Under the Curve (AUC) - Targeted 5-Fluorouracil (5-FU) Dosing for Patients Administered 5-FU for Cancer. New policy. OnDose testing or other types of assays for determining 5-fl uorouracil area under the curve in order to adjust 5-FU dose for colorectal cancer patients or other cancer patients is considered investigational.
BC.2.04.69 Fecal Calprotectin Testing. New policy. Fecal Calprotectin testing is considered investigational in the diagnosis and management of intestinal conditions, including the diagnosis and management of infl ammatory bowel disease.
BC.6.01.53 Digital Breast Tomosynthesis. New policy. Digital breast tomosynthesis is considered investigational in the screening or diagnosis of breast cancer.
PR.8.03.502 Physical Medicine and Rehabilitation – Physical Therapy and Massage Therapy.
Title revised to include massage therapy, which must meet the same coverage standards as other physical medicine and rehabilitation services. Massage therapy requires a prescription that must state the diagnosed condition to be treated, the number of visits, and the time period in which the massage will be provided. There must be a treatment plan, and treatment notes must meet the “Plan of Care and Documentation” as outlined in the policy. All therapies must achieve a specifi c diagnosis-related goal for a patient who has a reasonable expectation of achieving measurable improvement in a reasonable and predictable period of time. In addition, massage therapy, when provided as a stand-alone procedure rather than as part of a comprehensive therapeutic treatment plan, is considered not medically necessary.
Home-based physical therapy, previously not addressed, may now be considered medically necessary when the member is homebound. See Policy Guidelines for the defi nition of homebound.

16 August 2011 Alaska Network News
Please post or circulate this newsletter in your offi ce
Network NewsBack issues of Network News are on our web site at premera.com/provider in the Library under Communications.
PRESORTED STANDARD
U.S. POSTAGE PAIDSEATTLE, WA
PERMIT NO. 2944
Premera Blue Cross Blue Shield of Alaska
P.O. Box 327
Seattle, WA 98111
012335 (08-2011)DR 2482