Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipidssci.utq.edu.iq/images/pdf/LIPIDS.pdf ·...
Transcript of Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipidssci.utq.edu.iq/images/pdf/LIPIDS.pdf ·...

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
1
LIPIDS
Lipids are a class of biological molecules defined by low solubility in water and high solubility in
nonpolar solvents like ether and chloroform.
FUNCTIONS OF LIPIDS
1- Storage molecules for ENERGY (fats and oils)
o Can get lots of energy from a fat
o Stored in adipose tissue
2- Structural components of cellular membranes
3- Protective molecules (waxes)
4- Hormones and vitamins
5- Intracellular messengers
6- Pigments
7- Insulation
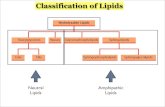
Classes of Lipids
In the year 1943 Bloor proposed the following classification of lipids based on their chemical
composition.
FATTY ACIDS (FA)
o composed of a long hydrocarbon chain (“tail”) and a terminal carboxyl group (or “head”)
o No hydrogen bonds form between the carboxylic acid functional group
Fatty Acids interact through HYDROPHOBIC INTERACTIONS
o By nature, fatty acids are AMPHIPATHIC – have both hydrophilic and hydrophobic parts
o Often have double bonds
• They are naturally occurring monocarboxylic acids that tend to have even numbers of
carbon atoms, and may be classified as:
•

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
2
– long-chain (C12 to C26)
– medium-chain (C8 and C10)
– short-chain (C4 and C6)
TYPES
Saturated fatty acids are long chain carboxylic acids and do not have double bonds.
Pack close together
Less fluid (FAs can’t move as freely)
Higher melting temperature because it takes more energy to break interactions
Likely to be solids at room temperature
Example: Arachidic acid, Palmitic acid, etc.
Unsaturated fatty acids have one or more double bonds between carbon atoms. The two
carbon atoms are bound to each other through double bonds and can occur in cis or trans
configuration. Double bonds are “cis” configuration cause a bend in the chain
Do NOT pack as closely
More fluid than saturated
Lower melting temperature than saturated
Likely to be liquid at room temperature

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
3
Saturated Unsaturated
FATTY ACIDS
o Lipids can contain many different fatty acids
o Fatty acid chain length and degree of unsaturation affect:
Melting point of lipids
Fluidity of lipids
NOMENCLATURE OF FA’s
- Referred to as a system of numbers
- # of carbons: # double bonds ∆x, y, z (position of double bonds)
- For example: oleic acid 18:1∆9
18:1Δ9
=
polar end non-polar; hydrophobic
Palmitic acid (26% of human fat)
Oleic Acid (45% of human fat)

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
4
Common Name Systematic Name Structural Formula
Butyric acid Butanoic acid CH3(CH2)2COOH
Caproic acid Hexanoic acid CH3(CH2)4COOH
Caprylic acid Octanoic acid CH3(CH2)6COOH
Capric acid Decanoic acid CH3(CH2)8COOH
Lauric acid Dodecanoic acid CH3(CH2)10COOH
Myristic acid Tetradecanoic acid CH3(CH2)12COOH
Palmitic acid Hexadecanoic acid CH3(CH2)14COOH
Stearic acid Octadecanoic acid CH3(CH2)16COOH
Arachidic acid Eicosanoic acid CH3(CH2)18COOH
Common name Chemical structure C:D =
Myristoleic acid CH3(CH2)3CH=CH(CH2)7COOH 14:1 cis-Δ9
Palmitoleic acid CH3(CH2)5CH=CH(CH2)7COOH 16:1 cis-Δ9
Sapienic acid CH3(CH2)8CH=CH(CH2)4COOH 16:1 cis-Δ6
Oleic acid CH3(CH2)7CH=CH(CH2)7COOH 18:1 cis-Δ9

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
5
Elaidic acid CH3(CH2)7CH=CH(CH2)7COOH 18:1 trans-Δ9
Vaccenic acid CH3(CH2)5CH=CH(CH2)9COOH 18:1 trans-Δ11
Linoleic acid CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH 18:2 cis,cis-
Δ9,Δ
12
Linoelaidic acid CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH 18:2 trans,trans-
Δ9,Δ
12
α-Linolenic acid CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH 18:3 cis,cis,cis-
Δ9,Δ
12,Δ
15
Arachidonic acid
CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOHNIS
T
20:4
cis,cis,cis,c
is-
Δ5Δ
8,Δ
11,Δ
1
4
Essential fatty acids are those that cannot be made through any chemical pathways, known
to happen in humans. They must be obtained from the diet. Linoleic acid and linolenic acid are
the essential fatty acids.
Non-essential fatty acids are those which are not necessary to be taken through diet, they
are synthesized through chemical pathways.
Simple Lipids or Homolipids
Simple lipids are the esters of fatty acids with various alcohols.
Fats and Oils (triglycerides and triacylglycerols) - These are esters of fatty acids with a
trihydroxy alcohol, glycerol. A fat is solid at ordinary room temperature and usually from
animals and do not contain unsaturated fatty acids, an oil is liquid and usually from plants and
Contain more unsaturated fatty acids.
Simple Triglycerides - Simple triglycerides are one in which three fatty acids are similar or
are of the same type. Example: Tristearin, Triolein.
Mixed Triglycerides are one in which the three fatty acids radicles are different from each
other. Example: distearo-olein, dioleo-palmitin.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
6
Waxes
Wax is a simple lipid which is an ester of a long-chain alcohol and a fatty acid. The alcohol
may contain from 12-32 carbon atoms. Waxes are found in nature as coatings on leaves and
stems. The wax prevents the plant from losing excessive amounts of water. . Example:
Beeswax, Carnaubawax.
WAXES
Wax Alcohol Fatty Acid
Carnuba CH3(CH2)28CH2-OH CH3(CH2)24COOH
Beeswax CH3(CH2)28CH2-OH CH3(CH2)14COOH

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
7
Reactions of triglyceride
1- Hydrolysis
2- Saponification
Saponification number: is the number of milligrams of potassium hydroxide
required for the complete saponification of one gram of substance—called
also saponification value. It points to the degree of hydrocarbon chain length of lipid fatty
acids (negative relation).
3- Hydrogenation
It is the reaction by which the oil is converted to fat ( unsaturated fatty acids are converted
to saturated)
Triolein Tristearin
4- Halogenation
Unsaturated fatty acids in triglyceride accept halogen such as I2 at the double bonds.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
8
Iodine Number: is the number of grams of iodine or equivalent halogen consumed by 100
grams of the substance <the iodine numbers of linseed oil, olive oil, and coconut oil are
approximately 175–201, 77–91, and 8–9.5 respectively>
5- Formation of Acrolein (heat reaction)
6- Rancidity
rancidity, is the chemical decomposition of fats, oils and other lipids. When this process
occurs in food, undesirable odors and flavors can result. In some cases, however, the
flavors can be desirable (as in aged cheeses):
Hydrolytic rancidity
Hydrolytic rancidity occurs when water splits fatty acid chains away from the glycerol backbone
in triglycerides (fats). The chemical term is ester hydrolysis. Usually this hydrolysis process
goes unnoticed, since most fatty acids are odorless and tasteless. A particular problem arises
with butter, which contains triglycerides with a high content of butyric acid derivatives and
acetic acids(volatile short chain fatty acids).
Oxidative rancidity
Oxidative rancidity is associated with the degradation by oxygen in the air. Via a free radical
process, the double bonds of an unsaturated fatty acid can undergo cleavage, releasing
volatile aldehydes and ketones. This process can be suppressed by the exclusion of oxygen or
by the addition of antioxidants. Oxidation primarily occurs with unsaturated fats.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
9
Microbial rancidity
Microbial rancidity refers to a process in which microorganisms, such as bacteria, use their
enzymes such as lipases to break down fat. This pathway can be prevented by sterilization.
Compound Lipids or Heterolipids
Heterolipids are esters of fatty acids with alcohol and possess additional groups also. Phospholipids or Phosphatides are Very similar in structure to triacylglycerols except one of the alcohols of glycerol is esterified by phosphoric acid instead of a fatty acid = phosphatidic acid (PA). They usually possess one hydrophilic head and two non-polar tails. They are called polar lipids and are amphipathic in nature.
16-18 FAs most common Position 1 favors SATURATED FAs Position 2 favors UNSATURATED FAs

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
11
Phospholipids are MUCH MORE amphiphilic than triacylglycerols due to CHARGED groups at neutral pH o Has both hydrophilic and hydrophobic regions phospholipids have One POLAR HEAD and TWO NON-POLAR TAILS
Phosphoglycerides are major phospholipids, they are found in membranes. It contains fatty
acid molecules which are esterified to hydroxyl groups of glycerol. The glycerol group also
forms an ester linkage with phosphoric acid. Example: Lecithin , Cephalins.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
11
Lecithin(Phosphotidyl Cholin) Cephalins (Phosphotidyl ethanol amine)
Phosphotidyl serine
Phosphoinositides are said to occur in phospholipids of brain tissue and soybeans. They
play important role in transport processes in cells.
Phosphotidyl inositol Phosphotidyl glycerol
Cardiolipin: Cardiolipin is known as the signature phospholipid of mitochondria. It is
responsible for a wide range of mitochondrial functions, including but not limited to ATP
synthesis
Plasmalogens: are a specific glycerophospholipid class containing a vinyl ether moiety at the
sn-1-position of the glycerol backbone. It make up 18% of phospholipids mass of human.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
12
Note: Phospholipids can be degraded to their component parts by a family of enzymes called PHOSPHOLIPASES.
Some snake poisons are phospholipase such as Phospholipase A2 that catalyzes the hydrolysis of fatty acids at the C2 Position that lead to formation of lysolecithin that is “one legged” phospholipids, acts as a detergent, and dissolved membranes in red blood cells causing them to rupture
Phosphosphingosides are commonly found in nerve tissue. Example: sphingomyelins.
Sphingosine unit

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
13
Glycolipids are the compounds of fatty acids with carbohydrates and contain nitrogen but no
phosphoric acid. The glycolipids also include certain structurally related compounds
comprising the groups gangliosides, and cerebrosides.
Derived Lipids
Derived lipids are the substances derived from simple and compound lipids by hydrolysis.
These includes fatty acids, alcohols, monoglycerides and diglycerides, steroids, terpenes,
carotenoids.
The most common derived lipids are steroids, terpenes and carotenoids.
Steroids do not contain fatty acids, they are nonsaponifiable, and are not hydrolyzed on
heating. They are widely distributed in animals, where they are associated with physiological
processes. Example: Estranes, and rostranes, etc.
Function of Steroids
Glucose+ Oligosaccharides Gangliosides
β-galactocerebroside

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
14
-Source of some important biological molecules (Cholesterol, bile salts, vit. D, and sex
hormones)
- components of cell membranes.
- Energy storage
Bile acids

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
15
Terpenes in majority are found in plants. Example: Natural rubber. Geranoil, Camphor, etc.
It is built up from isoprene, a hydrocarbon(diene) consisting of five carbon atoms attached to
eight hydrogen atoms (C5H8).
The term is often extended to the terpenoids, which are oxygenated derivatives of these
hydrocarbons.
The true terpenes are usually grouped according to the number of isoprene (C5H8) units in the
molecule: monoterpenes (C10H16) contain two such units;
sesquiterpenes (C15H24);
diterpenes (C20H32),
triterpenes (C30H48),
tetraterpenes (C40H64),.
Squalene is a triterpene by which cholesterol molecule is built up in liver.

Professor Dr. Raid M. H. Al-Salih Biochemistry / Lipids
16
Carotenoids are tetraterpenes. They are widely distributed in both plants and animals. They
are exclusively of plant origin. Due to the presence of many conjugated double bonds, they are
colored red or yellow. Example: Lycopreene, carotenes, Xanthophylls.
Rubber is polyterpenes in which 1,000–5,000 isoprene units are joined in a long chain