Production of x ray
-
Upload
daisyfaithy-clare -
Category
Education
-
view
1.634 -
download
1
description
Transcript of Production of x ray

Production of X-raysWhere do x-rays come from?
An x-ray machine, like that used in a doctor's or a dentist's office, is really
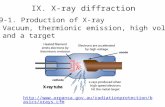
very simple. Inside the machine is an x-ray tube. An electron gun inside the tube shoots high energy electrons at a target made of heavy atoms, such as tungsten. X-rays come out because of atomic processes induced by the energetic electrons shot at the target.
X-rays are just like any other kind of electromagnetic radiation. They can be produced in parcels of energy called photons, just like light. There are two different atomic processes that can
produce x-ray photons. One is called Bremsstrahlung, which is a fancy German name meaning "braking radiation." The other is called K-shell emission. They can both occur in heavy atoms like tungsten.

So do both ways of making x-rays involve a change in the state of electrons?
That's right. But Bremsstrahlung is easier to understand using the classical idea that radiation is emitted when the velocity of the electron shot at the tungsten changes. This electron slows down after
swinging around the nucleus of a tungsten atom and loses energy by radiating x-rays. In the quantum picture, a lot of photons of different wavelengths are produced, but none of the photons has more energy than the electron had to begin with. After emitting the spectrum of x-ray radiation the original electron is slowed down or stopped.
What is the "K-shell" in the other way of making x-rays?
Do you remember that atoms have their electrons arranged in closed "shells" of different energies? Well, the K-shell is the lowest energy state of an
atom.
What can the incoming electron from the electron gun do to a K-shell electron in a tungsten target atom?

It can give it enough energy to knock it out of its energy state. Then, a tungsten electron of higher energy (from an outer shell) can fall into the K-shell.
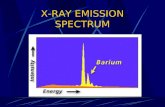
The energy lost by the falling electron shows up in an emitted x-ray photon. Meanwhile, higher energy electrons fall into the vacated energy state in the outer shell, and so on. K-shell emission produces higher-intensity x-rays than Bremsstrahlung, and the x-ray photon comes out at a single wavelength. Have a look at both mechanisms in the experiment below.
Bremsstrahlung
Why is tungsten used in x-ray tubes? Can't other elements produce x-rays?
Most elements emit x-rays when properly bombarded with electrons. Heavier elements (like tungsten) are best because they emit a higher intensity through bremsstrahlung, but there are
plenty of heavy elements to choose from. The real issue is engineering: Most electrons that hit the tungsten don't do anything special at all -- no bremsstrahlung, no K-shell emission. All of the energy from the electrons' impact then goes into heating the tungsten. Tungsten is used because it can withstand this bombardment, as it has a high melting point and can conduct heat away very well.

What would happen if you replaced the tungsten with something else?
The bremsstrahlung pattern looks very similar no matter what element you use. The K-shell emission spectrum is unique and different for each element.
In bremsstrahlung, why is a range of photons emitted instead of just one wavelength?
The incoming electron is accelerated and strikes the tungsten at a high speed and has a lot of energy. Recall that we called it "braking radiation." The electron might be slowed a little or a lot.
So the amount of "braking" determines which wavelength of photons are emitted.
Yes, but there is more. If we represent all of the energy of the electron as a pie, there are bazillions of different ways of cutting up this pie.

But one thing's for sure, you can never end up with more pie than you started with. If all of the energy goes into producing only
one photon, there is no way you could have a photon with more energy than that!
So there should be a sharp cut-off in the spectrum. It looked like there was a cut-off on each end.

No, there is only one cut-off that corresponds to a minimum wavelength. There is no limit to a maximum wavelength emitted. Go back to the bremsstrahlung spectrum and see how it fades
gradually to zero for long wavelengths.
K-Shell Emission
In the K-shell process, what's with that electron that drifts in from nowhere after the electron gets knocked-out?
A heavy atom has lots of electrons surrounding the nucleus in various shells. To keep it simple I didn't show them all. Really that electron that drifts in comes from one of the other shells of the atom. The
K-shell knock-out affects the innermost electron, so its like having a hole in the bottom. This "hole" causes a domino effect where the electrons above it cascade down to fill the hole.
But why the innermost electron? I would have guessed the outermost electron would be the easiest to knock out.
An x-ray photon has a lot of energy in it, and only transitions of the inner electrons release that much energy. Transitions of the outer electrons, which can happen, might be in the infrared or visible part of the
spectrum. For the electron energies used in x-ray tubes, it

turns out the inner electrons are the most likely to be knocked out.
You said earlier that the K-shell spectrum depends on the target element. Why?
Remember that we said each elements has its team colors? We're looking at the transition between two states, so we are looking at the team
colors in the x-ray part of the spectrum. These fingerprints were used by Moseley to help understand atoms with lots of electrons.
What did Moseley actually do?
Back in 1913 when he was a graduate student he looked at the K-shell radiation coming out from various elements from aluminum to gold. He found a connection between the wavelength of the emitted
K-shell x-ray and the element (atomic number) that helped us understand atoms better.

How can you find the wavelength of an x-ray? That seems like a hard thing to measure.
That's another story in itself. He used the (then) recently developed technique called Bragg scattering, where you scatter x-rays off a crystal. He was able to predict the existence of elements not yet observed,
such as technetium, promethium and rhenium. And even today, because of the uniqueness of the fingerprints of each element, x-rays are used for chemical analysis as it is very sensitive to impurities.