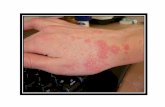
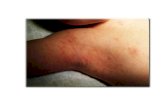
Scabies in children scabies treatment cream, how do you treat scabies
Prevalence of Scabies and Head Lice
-
Upload
dewi-masyithah-darlan -
Category
Documents
-
view
105 -
download
1
Transcript of Prevalence of Scabies and Head Lice

LETTERS TO THE EDITOR
Cytomegalovirus andTransmission via Breast
Milk
How to Support Breast Milk toPremature Infants and Prevent
Severe Infection?
To the Editors:
Recently, we published a review ontransmission of human cytomegalovi-
rus (HCMV) via breast milk to the pre-mature infant.1 Studies revealed HCMVpositivity of the infants of mothers beingHCMV-IgG positive ranging from 5.7%to 58.6%, with symptomatic HCMV dis-ease occurring in 0%–34.5% (median,3.7%) and severe sepsis-like syndrome in0%–13.8% (median, 0.7%). Evidence forlong-term sequelae was scarce, suggest-ing only mild neurologic and cognitiveimpairment without hearing impairment.Recently, Hamele et al2 reported on se-vere morbidity and mortality associatedwith breast milk-acquired HCMV infec-tion in 5 preterm infants of 24 (�5) to 27(�1) weeks gestational age. These 5 casesadd to a total of 18 infants that have beenpublished since the early 1970s when hu-man breast milk was known for the firsttime to be a potential source of HCMVinfection. In 2 cases of these 18 infants,no details were provided, another 5 casesof 29 to 33 weeks gestational age did notexperience severe disease as defined assepsis-like syndrome, and the remaining11 infants from 4 studies were reported torange from 23 to 28 weeks (23, 25, 24 –28, and 24.4 � 0.5 weeks, respectively).
Pasteurization constitutes the pre-ferred procedure to inactivate HCMV inbreast milk because freezing does notcompletely eliminate the virus.3 How-ever, heating procedures significantly de-crease protective factors contained inbreast milk, which are fundamental forthe advantages and possible long-termprotection granted by breast-feeding.4
Despite the concerns over severe HCMVdisease in a minority of very low birthweight infants, we would prefer an indi-vidual decision based on the health statusof the preterm infant instead of a generalapproach by either pasteurization or with-holding of breast milk.
We believe that parents should beinformed about the risk of HCMV acqui-sition of their preterm infant and the ad-vantages of fresh milk feeding. Providedthat informed consent is given and the
preterm infant is in stable condition, freshbreast milk might be administered (Fig.1). The suggested algorithm includes acareful risk-to-benefit evaluation of theinfant’s current health status and the ben-efits of its own mother’s breast milk thatmight, in our opinion, clearly outweighthe risk of symptomatic HCMV infection.Weekly HCMV monitoring in the infantby polymerase chain reaction from urinesamples is mandatory, and if the infantbecomes positive, feeding fresh milk mustbe stopped to reduce viral loads in case ofsymptoms and signs of HCMV infection.Otherwise, feeding of fresh breast milkmight be continued in infants of �28weeks of gestational age. At a correctedage of �32 weeks, the physician couldstop pasteurization or withholding ofbreast milk, based on the infant’s healthstatus and a more mature immune system.We still share the view of Stagno et al5
that the low risk associated with the trans-
mission of HCMV through breast milk isclearly outweighed by the well-estab-lished value of breast-feeding. Passiveimmunization with either HCMV mono-clonal antibodies or immune globulinsmight be a case of debate for high risklow birth weight infants.
Stefan Kurath, MDBernhard Resch, MD
Research Unit for Neonatal InfectiousDiseases and Epidemiology
Division of NeonatologyDepartment of Paediatrics
Medical University of GrazGraz, Austria
REFERENCES1. Kurath S, Halwachs-Baumann G, Muller W, et
al. Transmission of cytomegalovirus via breastmilk to the premature born infant: a systematicreview. Clin Microbiol Infect. 2009 �Epubahead of print�.
FIGURE 1. A new algorithm suggested for breast-fed preterm infants �32weeks of gestational age or birth weight below 1500 g in case of HCMV-IgG–positive mother.
The Pediatric Infectious Disease Journal • Volume 29, Number 7, July 2010680 | www.pidj.com

2. Hamele M, Flanagan R, Loomins A, et al. Severemorbidity and mortality with breast milk associ-ated cytomegalovirus infection. Pediatr InfectDis J. 2010;29:84–86.
3. Hamprecht K, Maschmann J, Muller D, etal. Cytomegalovirus (CMV) inactivation inbreast milk: reassessment of pasteurization andfreeze-thawing. Pediatr Res. 2004;56:529 –535.
4. American Academy of Pediatrics, Workgroupon Breastfeeding. Breastfeeding and the use ofhuman milk (RE9729). Pediatrics. 1997;100:1035–1039.
5. Stagno S, Reynolds DW, Pass RF, et al. Breastmilk and the risk of cytomegalovirus infection.N Engl J Med. 1980;19:1073–1076.
Update onMeningococcal DiseaseMortality in the United
States Since 2002
To the Editors:
Since the publication by Sharip et al1 onnational meningococcal disease mortal-
ity (1990–2002), there has been an absenceof further analysis of mortality trends of thisdisease in the United States. The presentcommunication provides an update of USmeningococcal disease mortality trends,based on an analysis of multiple cause-of-death data2 between 2002 and 2006.
During 2002 to 2006, the age-ad-justed average annual meningococcal dis-ease mortality rate was 0.050 per 100,000person-years (95% confidence interval �CI�,0.047–0.054). Individual annual rates de-creased by 34.5% from 0.058 per 100,000person-years (95% CI: 0.049–0.067) in2002 to 0.038 per 100,000 person-years(95% CI: 0.031–0.045) in 2006. Poissonregression analysis showed that during thistime the average annual decline was 10.3%.
Four age groups experienced signif-icantly higher average annual mortalityrates than both the general population andthe non-Hispanic white racial/ethnic group(whites) between 2002 and 2006. Infants(age �1 year) had the highest mortality rate(0.33/100,000 person-years), followed bythe age groups: 85� years (0.13/100,000person-years), 1 to 4 years (0.10/100,000person-years), and 15 to 24 years (0.076/100,000 person-years). Among all racial/ethnic groups, non-Hispanic blacks (blacks)experienced the highest age-adjusted aver-age annual meningococcal disease mortalityrate of 0.069 per 100,000 person-years(95% CI: 0.057–0.081). The black racial/ethnic group had an age-adjusted rate ratioof 1.39 (95% CI: 1.24–1.55), when com-pared with whites.
In comparison with the 1990 –2002analyses by Sharip et al,1 the age-adjustedaverage annual meningococcal diseasemortality rate during 2002–2006 was sig-nificantly lower; however, group dispari-ties continued to persist. The meningo-coccal disease mortality burden remainedsubstantial for blacks, infants, childrenaged 1 to 4 years, adolescents and youngadults aged 15 to 24 years, and adultsolder than 85 years.
Although these observed declines inmortality rates may in part reflect the effec-tiveness of recent public health control andprevention efforts, it is still disconcerting tosee that children aged 1 to 4 years andyoung adults aged 15 to 24 years are con-tinuing to experience higher rates of menin-gococcal death than the general population,especially given the availability of a tet-ravalent meningococcal conjugate vaccine(MCV4) since 2005. The MCV4 is pres-ently approved for use among children andadults aged 2 to 55 years.3
The present multiple cause-of-deathanalysis underscores the continual need forlongitudinal evaluations of meningococcaldisease trends and the effects of MCV4 ondecreasing disease outbreaks and mortalityand on increasing herd immunity in thefuture. More vigilant surveillance andtimely analysis of meningococcal diseasemorbidity and mortality may provide fur-ther insights into best practices for effectiveprevention and control of this potentiallyvaccine-preventable disease.4
The findings also suggest that phy-sicians should target high-risk childrenand adolescents, and educate these pa-tients and their parents about the avail-ability and potential benefits of the MCV4vaccine.4 Plausible policy strategies thatcan further reduce the burden of menin-gococcal disease in the United States in-clude publicly funded vaccination ofhigh-risk groups, mandated use of conju-gate vaccines as part of routine well childcare, and increased access to affordablevaccines in disadvantaged communities.4
Gloria Y. Kim, MPHLos Angeles County Department of Public
Health
Frank Sorvillo, PhDDepartment of Epidemiology
UCLA School of Public Health
Tony Kuo, MD, MSHSLos Angeles County Department of Public
HealthDepartment of Family Medicine
David Geffen School of Medicine at UCLALos Angeles, CA
REFERENCES1. Sharip A, Sorvillo F, Redelings MD, et al.
Population-based analysis of meningococcal dis-ease mortality in the United States. Pediatr InfectDis J. 2006;25:191–194.
2. Redelings MD, Wise M, Sorvillo F. Using mul-tiple cause-of-death data to investigate associa-tions and causality between conditions listed onthe death certificate. Am J Epidemiol. 2007;166:104–108.
3. American Academy of Pediatrics Committee onInfectious Diseases. Prevention and control ofmeningococcal disease: recommendations for useof meningococcal vaccines in pediatric patients.Pediatrics. 2005;116:496–505.
4. Moore KA, Osterholm MT. Meningococcal dis-ease and public health: a complicated road map.JAMA. 1998;279:472–473.
Chemotherapy andSurgery in Children With
Cystic Echinococcosis
To the Editors:
Cystic echinococcosis (CE), caused byEchinococcus granulosus, causes a
long-lasting infection that affects humansand a wide range of livestock species.1 Inthe human host, cysts can develop in manyanatomic sites, especially liver and lung.Because of the slow cyst growth, diagnosisof CE in children with multiorgan involve-ment is rare, even in endemic countries; thetreatment strategy in CE remains an openquestion.2
We describe a case observed in Sep-tember 2003 of a 9-year-old boy, living in afarm in the south of Italy, who was admittedto our hospital because of persistent coughand expectoration of bloody sputum. Tho-racoabdominal computed tomography scansshowed a large cyst (4 cm diameter) in themediastinum near the left ventricle, multi-ple cysts in the lung, a cyst in the liver, anda very large cyst (8 cm diameter) in thepancreas; echocardiography showed an in-trapericardial cyst (2 cm diameter). Nuclearmagnetic resonance confirmed the CE diag-nosis. Laboratory tests showed eosinophiliaof 17%, anti-Echinococcus antibody sero-positivity (hemagglutination test: antibodytiter: 1:16,384), immunoblotting: antibodiesagainst 8, 16, 20 kd (antigen B), and 55 to65 kd (antigen 5) antigenic subunits.
Treatment was started with albenda-zole (10 mg/kg/d). One month after the startof anthelminthic therapy, an echocardiogra-phy showed a volumetric increase of thepericardial cyst (3 cm diameter). After 45days of chemotherapy, complete surgicalresection of heart cyst and concomitantcleaning of liver and lung cysts becamenecessary. The patient underwent electiveoperation in 1-stage surgery, through a me-dian sternotomy incision and next xiphoum-
The Pediatric Infectious Disease Journal • Volume 29, Number 7, July 2010 Letters to the Editor
© 2010 Lippincott Williams & Wilkins www.pidj.com | 681

bilical incision. The cyst mass was seenclearly on the wall of the left atrium andcompletely dissected away. Lung cysts ofleft superior and right inferior lobes and theliver cyst of left lobe were removed. Fi-nally, the peritoneum was raised and thecyst located in the pancreas was completelyremoved. Histologic examination of thesurgical material confirmed the diagnosis ofCE. The patient was extubated on secondpostoperative day and 1 week later dis-charged home with continuous albendazoletherapy for 2 years (10 mg/kg/d). The post-operative period was uneventful.
Four years’ clinical, serologic, andultrasonographic follow-up has shown norecurrence. To note, although, enzyme-linked immunosorbent assays determiningisotype antibody expression in response toE. granulosus hydatid fluid, showed no sig-nificant variations for total immunoglobulin(Ig) G and IgG1 concentrations, IgG4 and IgEconcentrations dropped at completion offollow-up (IgG4:O.D.: 1.22 vs. 0.64; IgE:O.D.:0.18 vs. 0.008).
CE is endemic in Italy, in particularin the south of the country. Because thecyst requires many years to develop, thediagnosis of pediatric echinococcosis israre, and the multiorgan localization inchild is uncommon.3,4 In particular, car-diac and pancreatic involvements, whichoccur only after the larvae pass trough thebarrier of liver and lung, are rare in chil-dren (0.2%–2%). In a Turkish study, a13-year-old girl presented hydatid cyst ofthe right atrium, cyst in the kidney, andmultiple cyst in the lungs.5 This is the firstreport of a child with hydatid with a cystin the hearth, pancreas, lung, and liver.Although operating on 4 distinct organs isa high-risk procedure, we decided for a1-stage surgery because we did not wantto risk spreading the infection and thefatal complication from the growth and/orrupture of the cardiac cyst.
This noteworthy case of a young pa-tient with multiorgan involvement showsthat combined chemotherapy and surgeryallow to successfully treating severe CE.
Maria Iannelli, MDDepartment of Pediatric Medicine (DMP)Pediatric Hospital Bambino Gesu, IRCCS
Alessandro Inserra, MDRoberto M. Di Donato, MD
Pediatric Hospital Bambino Gesu, IRCCS
Antonella Teggi, MDDepartment of Infectious Diseases
Hospital Sant’AndreaUniversity of Rome, “Sapienza”
Alessandra Siracusano, PhDDepartment of Infectious Parasitic and
Immune-mediated DiseasesIstituto Superiore di Sanita
Saverio Malena, MDPediatric Hospital Bambino Gesu, IRCCS
Renata Boldrini, MDPathology Unit-Laboratory Department (DL)
Pediatric Hospital Bambino Gesu, IRCCS
Cristina Russo, MDPediatric Hospital Bambino Gesu, IRCCS
Andrea de Zorzi, MDDepartment of Pediatric Cardiology and
Cardiac Surgery (DMCCP)Pediatric Hospital Bambino Gesu, IRCCS
Alberto Villani, MD, PhDDepartment of Pediatric Medicine (DMP)Pediatric Hospital Bambino Gesu, IRCCS
Rome, Italy
REFERENCES1. Eckert J, Deplazes P. Biological, epidemiologi-
cal, and clinical aspects of echinococcosis, azoonosis of increasing concern. Clin MicrobiolRev. 2004;17:107–135.
2. Vuitton DA, Wen H. Treatment of cystic echino-coccosis: a combination of general goals andrules, individual decisions and indications. NethJ Med. 2007;65:86–88.
3. Emilio C, Losurdo G, Mollero L, et al.Multiorgan echinococcosis in a pediatric pa-tient. Pediatr Infect Dis J. 2005;24:660 – 661.
4. Olmez D, Babayigit A, Arslan H, et al.Multiorgan involvement in a pediatric patientwith hydatid disease. J Trop Pediatr. 2008;54:417–419.
5. Aydogdu T, Sahin N, Ulusan V, et al. Right atrialhydatid cyst associated with multiple organ in-volvement: case report. J Thorac CardiovascSurg. 2001;121:1009–1011.
Prevalence of Scabies andHead Lice Among
Students of SecondaryBoarding Schools inKuching, Sarawak,
Malaysia
To the Editors:
We embarked on a study to determinethe prevalence of scabies and head
lice among secondary school students at-tending boarding schools in Kuching,Sarawak, because we had noted that mostof our clinic patients with infestationswere boarding school students. The aimwas to provide these data to the authori-ties to formulate a strategy to eliminate
these infestations from boarding schoolsin Sarawak.
We randomly selected 2 of 9 board-ing schools in Kuching, Sarawak, an Is-lamic school and a technical school. Theprotocol for this cross-sectional study wasapproved by the school authorities and theState Health and Education Department.The study was conducted between Marchand May 2009. All students who voluntarilyconsented were surveyed. The diagnoseswere made clinically. Mass treatment of allthe students was conducted after the survey.Data were subjected to descriptive analysis.
A total of 944 of 950 students con-sented to the survey. Of these, 488 (51.7%)were males and 456 (48.3%) were females.The median age was 16 years, ranging from13 to 17. Malays constituted the majoritywith 708 (75.0%), followed by Bidayuhs121 (12.8%) and Ibans 65 (6.9%).
We found that 233 (24.7%) studentshad head lice, and 76 (8.1%) students hadscabies. All the students with head lice werefemales. This constituted 48.9% of the totalfemale students surveyed. Scabies wereseen in 61 males and 15 females. Theseconstituted 12.5% of the male students and3.3% of the female students studied. Thepoint prevalences for head lice and scabieswere 40% and 10.4% in the Islamic schooland 7.1% and 5.7% in the technical school,respectively.
Our point prevalence for head lice of24.7% was twice that for the prevalence of12.9% among primary school children inKuala Lumpur, Malaysia.1 The higher ratemight have resulted from the fact that thesurvey was done in boarding schools wherestudents were in close contacts with eachothers in the classes and in the dormitories.
We found that only female studentswere infested with head lice. Interestingly,we also found that the infestation by headlice was more common in the Islamicschool than in the technical school. Wesuspect that the prevalence of head lice washigher in Muslim females because of thepractice of wearing a head scarf, keepinglong hair, infrequent hair washing, and theproximity among these students duringmass prayers. Moreover, the dormitories inthe Islamic schools were more over-crowded, housing 12 students per roomcompared with 8 in the technical school. InNigeria, it was also found that the infesta-tion rate was higher in the Islamic commu-nity with 4.1% compared with the Christiancommunity rate of 3%.2 Females, longerhair, less frequent hair washing, sharingcleaning implements, and overcrowdingwere also identified as risk factors for headlice infestation.3,4
Letters to the Editors The Pediatric Infectious Disease Journal • Volume 29, Number 7, July 2010
© 2010 Lippincott Williams & Wilkins682 | www.pidj.com

Scabies was found in 8.1% of thestudents in our study. In a survey amongprimary school children, the prevalence was4% in urban Mali, 0.7% in Malawi, and4.3% in rural Cambodia.5 The higher prev-alence seen here might be due to the prox-imity and frequent handshaking amongMuslim students in the boarding schools.
In conclusion, the prevalence ofthese infestations is high, warranting masstreatment by the education and healthauthorities to contain the transmission in allthe boarding schools in Sarawak. Control ofthese infestations is important to allow abetter quality of life and improve the edu-cational and cocurricular performance ofthe boarding school students. We recom-mend that school authorities screen all newstudents for these infestations and referthem for appropriate treatment before start-ing school to prevent transmission of thesediseases.
Felix Boon-Bin Yap, MD, MRCPEstrellita M. T. Elena, MB BS
Muniandy Pubalan, MB BS, MRCPDepartment of Dermatology
Sarawak General HospitalSarawak, Malaysia
REFERENCES1. Sinniah B, Sinniah D, Rajeswari B. Epidemiology and
control of human head louse in Malaysia. Trop GeogrMed. 1983;35:337–342.
2. Ebomoyi EW. Pediculosis capitis among urbanschool children in Ilorin, Nigeria. J Natl MedAssoc. 1994;86:861–864.
3. Counahan M, Andrews R, Buttner P, et al.Head lice prevalence in primary schools inVictoria, Australia. J Paediatr Child Health.2004;40:616 – 619.
4. Ríos SM, Fernandez JA, Rivas F, et al.�Pediculosis prevalence and associated risk fac-tors in a nursery school, Bogota, Colombia� �Ar-ticle in Spanish�. Biomedica. 2008;28:245–251.
5. Landwehr D, Keita SM, Ponnighaus JM, et al.Epidemiologic aspects of scabies in Mali,Malawi, and Cambodia. Int J Dermatol. 1998;37:588–590.
Lack of Sensitivity ofQuantiFERON-TB GoldTest in Tube in a Child
With TuberculousMeningitis
To the Editors:
Diagnosis of tuberculous meningitis re-mains an important clinical challenge.
About half of patients will present withnormal chest radiography or a negativetuberculin skin test (TST). Negative smearsfor acid-fast bacilli and lack of isolation of
Mycobacterium tuberculosis in cerebral spi-nal fluid (CSF) culture are observed in morethan 50% of cases. The CSF polymerasechain reaction (PCR) assay represents adiagnostic advance, but is insensitive toconfidently exclude the diagnosis. Recently,interferon-gamma release assays (IGRAS)have been reported to improve diagnosticsensitivity of tuberculosis in adults and chil-dren, but studies in tuberculous meningitisare lacking.
We have recently diagnosed a tuber-culous meningitis in a 2-year-old Rumaniangirl who presented with a negative TST (0mm), normal chest radiograph, negativeCSF smears for acid-fast bacilli, negativeCSF-PCR assay for M. tuberculosis andnegative QuantiFERON-TB Gold Test InTube (QTF) (0.27 UI/mL; positive value�0.35 UI/mL) with proper interferon pro-duction in mitogen control (6.21 UI/mL).At time of admission, CSF had a mildlyelevated pleocytosis (60 leukocytes/mm3;85% neutrophils), a slight raised protein(90.7 mg/dL) and a low glucose value (14mg/dL) and computed tomography scan re-vealed mild basilar meningeal enhance-ment. There was no evidence of tuberculo-sis exposure. One month after admission,M. tuberculosis was isolated in CSF andgastric aspirate contents. A new QTF andTST were again performed and were posi-tive (0.72 UI/mL and 13 mm, respectively).
The few available data of the use ofIGRAS for diagnosing extrapulmonary tu-berculosis suggest that these assays havethe same sensitivity as in pulmonarydisease.1Regarding the use of QFT for tu-berculous meningitis diagnosis, few articleshave been published.2,3 All of them esti-mate that QTF sensitivity is higher thanacid-fast-bacilli-CSF smears, CSF cultureor CSF-PCR assay for M. tuberculosis. TheT-SPOT. TB test (Oxford Immunotec) fortuberculous meningitis diagnosis has ahigher sensitivity (62%–100%) than con-ventional diagnosis techniques and betterthan that described for TST.1,4–7 Some au-thors suggest that IGRAS could be per-formed in CSF instead of in plasma withbetter sensitivity.2,5,6
In young children, deficiencies in den-dritic cell and TH-1-type T-cells function,which play a central role in avoiding lympho-hematogenous spread of M. tuberculosis afterthe infection, contribute to the susceptibilityto develop disseminated disease.8 Up to50% of children with TB meningitis havenegative TST results9 and this is believed tobe consequence of diminished functioningof T lymphocytes with less cytokine pro-duction. For this reason, in young childrenwith TB meningitis and negative TST wewould expect to find a high percentage of
indeterminate IGRA results due to absenceof response in mitogen control. Neverthe-less, our patient presented an initial nega-tive result with a good IFN-� production inthe mitogen control, and a positive resultafter 6 weeks of evolution. Considering thattuberculous meningitis are early developedafter the infection,9 some negative TST andIGRAS results observed will be probablydue to prompt spread of the mycobacteria innew acquired infections without an effec-tive T lymphocytes response development.In a child with suspected tuberculousmeningitis and a negative IGRA, we be-lieve it is prudent to initiate antitubercu-lous therapy.
ACKNOWLEDGMENTSThe authors thank Spanish Pediatric
Association (AEP) for the 19th Ausonia-Arbora Grant that has contributed toperform all the studies of the SpanishCollaborative Group for the study ofQuantiFERON-TB Gold Test In Tube inchildren.
Ana Mendez Echevarría, MD, PhDFernando Baquero-Artigao, MD
Paediatric Infectious Disease UnitLa Paz Hospital
Miguel Gonzalez-Munoz, MD, PhDImmunology Department
Carlos III Hospital
Fernando De Castillo, MD, PhDPaediatric Infectious Disease Unit
La Paz Hospital
Maria Jose Mellado Pena, MD, PhDPaediatric Department
Carlos III Hospital
Ramon Velazquez-Fragua, MDPaediatric Neurology Department
La Paz HospitalMadrid, Spain
REFERENCES1. Liao CH, Chou CH, Lai CC, et al. Diagnostic
performance of an enzyme-linked immunospotassay for interferon-gamma in extrapulmonarytuberculosis varies between different sites of dis-ease. J Infect. 2009;59:402–408.
2. Luca MC, Petrovici CM, Vata A, et al. Gammainterferon testing in blood and cerebrospinal flu-id–rapid method for the diagnosis of tuberculousmeningitis �in Romanian�. Rev Med Chir SocMed Nat Iasi. 2008;112:108–110.
3. Iguchi M, Maruyama K, Tsutsumi Y, et al.QuantiFERON: an early diagnostic tool for cerebraltuberculosis �in Japanese�. Rinsho Shinkeigaku.2008;48:259–262.
4. Quan C, Lu CZ, Qiao J, et al. Comparativeevaluation of early diagnosis of tuberculous men-ingitis by different assays. J Clin Microbiol.2006;44:3160–3166.
The Pediatric Infectious Disease Journal • Volume 29, Number 7, July 2010 Letters to the Editor
© 2010 Lippincott Williams & Wilkins www.pidj.com | 683

5. Kosters K, Nau R, Bossink A, et al. Rapid diag-nosis of CNS tuberculosis by a T-cell interferon-gamma release assay on cerebrospinal fluidmononuclear cells. Infection. 2008;36:597–600.
6. Thomas MM, Hinks TS, Raghuraman S, et al.Rapid diagnosis of Mycobacterium tuberculosismeningitis by enumeration of cerebrospinal fluid
antigen-specific T-cells. Int J Tuberc Lung Dis.2008;12:651–657.
7. Kim SH, Chu K, Choi SJ, et al. Diagnosis ofcentral nervous system tuberculosis by T-cell-based assays on peripheral blood and cerebrospi-nal fluid mononuclear cells. Clin Vaccine Immu-nol. 2008;15:1356–1362.
8. Lewinsohn DA, Gennaro ML, Scholvinck L, etal. Tuberculosis immunology in children: diag-nostic and therapeutic challenges and opportuni-ties. Int J Tuberc Lung Dis. 2004;8:658–674.
9. Carrol ED, Clark JE, Cant AJ. Non-pulmonarytuberculosis. Paediatr Respir Rev. 2001;2:113–119.
ERRATUM
Multiple Brain Abscesses Caused by Pseudomonas luteola: ERRATUM
In the Letter to the Editor that appeared on page 1144 of volume 27, number 12, the authors were listed in theincorrect order. The author list should have appeared as Anne Gaschet, PD, Charlotte Engrand, MD, Caroline Piau,PS, Jeremie Violette, PS, Pierre Betremieux, PhD, Pierre Tattevin, PhD, Patrick Pladys, PhD, Pierre-Yves Donnio,PhD, and Anne Jolivet-Gougeon, PhD.
Reference:Gaschet A, Piau C, Violette J, et al. Multiple Brain Abscesses Caused by Pseudomonas luteola. Pediatr Infect Dis J.2008;27:1144–1146.
Letters to the Editor The Pediatric Infectious Disease Journal • Volume 29, Number 7, July 2010
© 2010 Lippincott Williams & Wilkins684 | www.pidj.com