Post Thoracotomy Pain Syndrome: What Pain Management ...plan are more likely to experience acute...
Transcript of Post Thoracotomy Pain Syndrome: What Pain Management ...plan are more likely to experience acute...

CentralBringing Excellence in Open Access
Journal of Surgery & Transplantation Science
Cite this article: Hegarty D (2017) Post Thoracotomy Pain Syndrome: What Pain Management Options do we have? J Surg Transplant Sci 5(3): 1059.
*Corresponding authorHegarty D, Department Anaesthesia & Pain Medicine, Cork University Hospital, Cork, Ireland, Tel: 353-87-2886-383; Email:
Submitted: 22 August 2017
Accepted: 18 September 2017
Published: 21 September 2017
ISSN: 2379-0911
Copyright© 2017 Hegarty
OPEN ACCESS
Keywords•Post thoracotomy pain syndrome•Interventional pain management•Neuropathic pain•Intercostal nerve injection•Selective thoracic nerve root injection•Neuromodulation•Radiofrequency denervation
Review Article
Post Thoracotomy Pain Syndrome: What Pain Management Options do we have?Dominic Hegarty* Department Anaesthesia & Pain Medicine, Cork University Hospital, Ireland
Abstract
The reported incidence of post thoracotomy pain syndrome (PTPS) 3 months after surgery ranges between 22% and 67% and it has a significant impact on patients’ quality of life. The focus in the peri-operative period is to provide analgesia in order to avoid post-operative complications. Unfortunately there is a void in the literature about pain management options that may help PTPS in the weeks and months that follow surgery. All healthcare providers working in this area ought to be alert to the possibility of this syndrome. Pain physicians must be familiar with the range of conservative and interventional options from the simple intercostal blocks to neuromodulation. The objective of this article is (a) to highlight the conservative and interventional options available and (b) to underline that when treatment is utilized rationally, the possibility of controlling the pain becomes a reality.
many hospital resources including nursing, physiotherapy and radiological costs to mention but a few [7].
Perioperative anesthesia, analgesic regimes and surgical techniques can reduce the occurrence and severity of acute postoperative pain [8]. Unfortunately there is a void in the literature about the options that may help PTPS in the weeks and months that follow surgery. Mostly small case reports and anecdotal reports are the only guidance in the literature [1,2,6].
The objective of this review article is to consider the treatment options, both conservative and interventional pain procedures commonly used to diagnose and treat chronic thoracic pain syndrome.
Pathophysiology of thoracotomy pain
Pain after thoracotomy may originate from both somatic and visceral afferents that lead to a cascade of neural activity which in turn contribute to the establishment of chronic pain and ultimately PTPS [8].
Somatic afferent input: The skin incision, rib retraction, muscle splitting, injury to the parietal pleura, and chest drain insertion can, either individually or in combination, result in an escalation of nociceptive somatic afferent activity via the intercostal nerves. These in turn communicate with the ipsilateral dorsal horn of the spinal cord (T4-T10) [5]. The afferent signals are then transmitted to the limbic system and somatosensory cortices via the contralateral anterolateral system of the spinal cord. Rodent surgical models have shown that thoracotomy with
ABBREVIATIONSPPTS: Post Thoracotomy Pain Syndrome; VATS: Video-
Assisted Thoracoscopic Surgery; TENS: Transcutaneous Electrical Nerve Stimulation; ICNB: Intercostal Nerve Blocks; RF: Radiofrequency Denervation; TFJ: Thoracic Facet Joints; TSB: Thoracic Sympathetic Blocks; PVNB: Paravertebral Nerve Block; SCS: Spinal Cord Stimulation; DRG: Dorsal Root Ganglion
INTRODUCTIONThoracotomy is considered as one of the most painful surgical
procedures. The incidence of post thoracotomy pain syndrome (PTPS) 3 months after the surgery ranges between 22% and 67% [1-3]. With the longer life expectancy and improved cancer survival rates it is likely that more individuals will require thoracotomy surgery and therefore we will face the challenge of dealing with a significant chronic pain issue for many years to come [4].
Compounding the high incidence of PTPS and its refractoriness to treatment is the dearth of clinical studies to guide treatment. The literature highlights the importance of providing effective analgesia in the peri-operative period in order to avoid post-operative complications and rightly so [5,6]. Complications following thoracotomy surgery, such as impaired deep breathing, coughing, and remobilization culminating in atelectasis and pneumonia are issues that negatively impact on duration of hospital stay, very often causing a delay in discharge from the intensive care setting and results in the absorption of

CentralBringing Excellence in Open Access
Hegarty (2017)Email:
J Surg Transplant Sci 5(3): 1059 (2017) 2/3
rib retraction results in cutaneous mechanical hypersensitivity lasting for several months [9,10]. The bilateral hypersensitivity associated with this injury is characterized by changes in behavioral responses to mechanical stimulation [10].
Visceral afferents input: Visceral afferent arises from the phrenic and vagus nerves after injury to the bronchi, visceral pleura, and pericardium. The injured tissue releases inflammatory mediators, such as prostaglandins, histamine, bradykinin, and potassium. These mediators directly activate nociceptors, enhance their activity, and reduce the pain threshold. This amplified response to pain is called primary sensitization and leads to intensified pain on breathing or coughing after the operation [5].
Unfortunately, in response to the continued nociceptive input during the perioperative period the dorsal horn neurons and higher pain centers, through activation of NMDA receptors in response to substance P, calcitonin gene-related peptide, and glutamate become hypersensitive. This leads to another well-recognized issue namely central sensitization [5].
The combination of the somatic and visceral input results in an amplification of the pain cascade and may result in the development of neuropathic and chronic pain. The difficulty is that there are no predictive markers to help identify individuals who might be at a greater risk than others.
Factors associated with increased risk of PPTP
Over the years the surgical technique and inherent patient variability have being blamed for the high incidence of PTPS. Table 1 provides an overview of the possible causes of chronic thoracic pain.
Possible surgical factors: While the posterolateral approach to thoracotomy may provide the best surgical access this can result in one of the most painful surgical incisions when the latissimusdorsi, and at times the serratus anterior and trapezius muscles are divided. In general muscle-sparing approaches are used where the incision of the muscles is replaced with dissection and reflection onto the ribs. This may result in two issues; firstly the reduction in the field of view may lead to excessive rib retraction, fracture, dislocation, costovertebral disruption, and damage to the intercostal nerves; secondly, these incisions may span multiple dermatomes as opposed to the single dermatome of the posterolateral approach; for example, the axillary incision extends vertically downwards. This exposes the patients to multi sites of nociceptive input bombarding the spinothalamic pain ways [1,12,13].
There are an increasing number of video-assisted thoracoscopic surgery (VATS) being undertaken. It would be reasonable to expect a reduction in acute pain if intercostal nerve damage is avoided by limiting the number and size of intercostal ports used. Unfortunately, despite the best efforts to limit injury the incidence of chronic pain appears to be similar to open thoracotomy [13,14,15].
Patient factors: Although the literature suggests that patients who are young, of female gender, with a history of depression and anxiety and who are poorly informed about their management plan are more likely to experience acute post-surgical pain, these risks have not been demonstrated in thoracotomy patients [14].
Based on logistic regression analysis, risk factors for developing chronic pain after thoracic surgery include age below 60 years old (OR: 1.51, 95% CI: 1.13–2.02), female gender (OR: 1.77, 95% CI: 1.36–2.31), hypertension (OR: 1.86, 95% CI: 1.35–2.57), lack of PCA for post-operation analgesia (OR: 1.31, 95% CI: 1.00–1.71), and prolonged duration of chest tube drainage (>4 days) (OR: 1.55, 95% CI: 1.14–2.10) [1].
Diagnosis of PTPS
PTPS is not always unambiguously derived from the clinical history or physical examination, therefore additional examination or investigation is often indicated. As with any clinical situation a complete history and examination is important to understand the nature of the symptoms and to identify any possible physical signs. Exclusion of red flags is a very important element of the diagnosis and investigations should also be focused to out rule all underlying issues [1,2,3]. Issues include:
a) In the event of a collapsed vertebra, an X-ray of the spinal column may sufficient. Along with a clinical history of a trauma, with or without a history of osteoporosis, the diagnostics can be completed.
b) Magnetic resonance imaging (MRI) could be necessary to rule out malignant causes of the pain or metastases. This is particularly important if there is a history of malignancy, or in cases of acute development of severe pain or progressive pain symptoms including the development of symptoms suggestive of neurological impairment.
c) A thoracic X-ray can be useful in the event of thoracic wall pathology. If there are abnormalities, the patient should be referred to a pulmonary physician for further evaluation.
Table 1: Causes of PTPS / chronic thoracic pain Neuralgia
• Intercostal neuralgia• Neuralgia of the abdominal wall• Pain radiating from the spinal cord• Osteoporosis• Vertebral collapse• Vertebral metastases
Scar pain• Post-thoracotomy• Post-mastectomy• Post-thoracoscopy• Intercostobrachial neuralgia• Post-lobectomy• Pfannenstiel incision
Rib pathology• Fracture/pseudarthrosis• Rib resection
Table 2: Examples of Interventional Pain Procedures for PTPS• Intercostal Nerve Injection / Denervation• Selective thoracic nerve root injection • Thoracic medial branch block (facet joints)• Thoracic sympathetic blocks • Paravertebral Block• Other targeted injections (Table 3)
• Neuromodulation

CentralBringing Excellence in Open Access
Hegarty (2017)Email:
J Surg Transplant Sci 5(3): 1059 (2017) 3/3
d) In cases of doubt or when intra-abdominal pathology is suspected, then an ultrasound or a computed tomography (CT) scan may be indicated.
e) Blood tests for biomarkers, liver function and full blood screen including coagulation and infective screening should always be considered
This is not an exhaustive list of investigations and each case should be considered on clinical merit.
Conservative treatment options
Medical treatment with analgesics can be applied according to the World Health Organization pain ladder. The description of the pain can be very useful in guiding the choice of agents [16]. In cases of neuropathic pain (where terms such as “burning”, pins & needles or hypersensitivity are reported), consideration of analgesics such as anti-epileptics and antidepressants can be administered. Pregabalin, gabapentin or amitriptylines are examples of first line choices recommended dealing with neuropathic pain. [17]. Questionnaires can be used as tools to help identify possible neuropathic cases [12].
Pain aggravated by movement usually indicates a role for agents such as regular paracetemol, tramadol, codeine or non-steroidal inflammatory agents once these are not contraindicated. The dual action of tapentadol (an opioid and noradrenaline reuptake inhibitor) has shown to be very useful in providing analgesia while offering steady analgesic in PPTS [17]. In general the choice of the agents and the dosage required needs to be established in most cases.
Hypersensitivity over healed scar tissue would suggest a role for lignocaine patches. Transcutaneous electrical nerve stimulation (TENS) is an option for the treatment of thoracic radicular pain. Hydrotherapy and acupuncture can be considered depending on the degree of pain intensity. Physical therapy is usually applied in the form of manual therapy and focuses on the musculoskeletal tissue
Unfortunately evidence based conservative treatment are very limited.
Interventional options for PPTP
If conservative treatment fails to control or resolve matters then there are a number of procedures that may lead to a significant improvement. The success of any pain procedure requires careful patient selection in order to optimize the clinical outcome and reduce the risk to the patient [18]. Table 2 highlights some interventional options that exist.
Intercostal Nerve Injection / Denervation
Intercostal blocks (ICNB) can be carried out above the Th10 level to localize the level of the pain with a target of > 50% reduction of pain during the local anesthetic blockade. Determining which nerve is causing the pain based on clinical examination is sometimes difficult because the innervations of intercostal nerve overlap [19] (Figure 1).
Therefore, diagnostic blocks of intercostal nerves are usually performed at 3 levels. The injection can be done under
fluoroscopy or with ultrasound guidance [20]. A study done in cadavers [21] confirmed that US confers higher accuracy and allows use of lower volumes of injectate compared with anatomic landmarks as a guide method for ICNBs. The intercostal neurovascular bundle runs in the lower part of the inner side of the rib and cannot be visualized with US, the use of US guidance is recommended since imaging of the needle tip and the pleura in real-time could potentially reduce pneumothorax formation during block.
Once ICNBs are completed, the patient needs to be observed at least 30 minutes for symptoms or signs of local anesthetics toxicity; development of hematoma, cough, and shortness of breath, low oxygen saturation, or wheezing that may indicate pneumothorax. Complications of the ICNBs are quite rare: local anaesthetics toxicity, pneumothorax, bleeding, infection, neuropathic pain, paravertebral spread, hypotension, spinal anesthesia [22].
The option of using advanced radiofrequency denervation (RF) can be considered depending on the outcome following the injections. Good results are reported following RF treatment in thoracic radicular pain management. A significant and prolonged (>12 months) reduction in pain can be expected in 52-70% cases [23]. The more segments involved the effectiveness of the treatment was smaller [23].
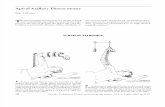
Figure 1 Left sided Intercostal nerve injection under fluoroscopy with contrast.
Figure 2 Shows a single level thoracic nerve root injection (also known as a transforminal epidural injection).

CentralBringing Excellence in Open Access
Hegarty (2017)Email:
J Surg Transplant Sci 5(3): 1059 (2017) 4/3
Selective thoracic nerve root treatment
Selective thoracic nerve root blocks (STNRBs) are helpful procedures [24] (Figure 2). The most common indication is where there is radiculopathy and ICNBs have not relieved the symptoms. If an MRI scan has not being completed then it is useful to eliminate underlying red flags such as a vertebral facture / collapse, disc herniation, entrapment of the nerve root, metastatic disease or infection in advance of the procedure.
When steroids are added to the local anaesthetic, the goal is to generate a long-term pain relief, mostly in patients with radiculopathy symptoms. After a successful STNRB (i.e. 50% reduction in pain), it is recommended to go on with pulsed radiofrequency of DRG for a definite pain relief. Thoracic DRG radiofrequency is difficult because of being located anteriority in the intervertebral foramen [23].
The anatomical location of the foraminal arteries is variable; the study by Kroszczynski et al. [26], found that at thoracolumbar levels, the artery is almost always (92% ± 5%) located anterosuperior to the nerve. At typical thoracic levels is less often anterosuperior (38% ± 19%), but more often anterior to the nerve. It is known that the location of the Adamkiewicz artery is very irregular, which makes even more dangerous the possibility of secondary spinal cord injury and paraplegia as this is the main blood supply of the thoracolumbar spinal cord. It is noteworthy to mention that the foraminal arteries supply the spinal cord, so the intra-arterial injection is very dangerous [27,28]
Thoracic facet & medical branch block
The thoracic facet joints (TFJ) are an important source of mid back and upper back pain. In a population with localized chest pain, the prevalence of TFJ pain amounts up to 42% [28]. The incidence in the subgroup of post-thoracotomy pain patients is unknown. In the context of the PPTS one has to assume that the mechanical strain on the facet joint results in an inflammatory response that fails to resolve. Fortuitously, TFJ’s are innervated by the medial branch nerve of the dorsal rami, making them suitable targets to help manage pain [29].
An individual with thoracic facet pain will complain of paravertebral pain that worsens with prolonged standing, extension, or rotation of the thoracic spinal column. The pain is often bilateral and affects several segments [30]. Sometimes, the pain is felt more centrally and hyperesthesia occasionally occurs in the adjacent dermatomes. Location of marked tenderness in the region with paravertebral pressure is usually the only sign [30].
The close proximity of the costovertebral junction needs to be considered and one may mimic the other clinical. Investigation using MRI imagery may be helpful but often a diagnostic block is the best way of identifying the source of pain.
In general facet blocks/medial branch blocks are helpful. A study by Manchikanti et al. [31] included 48 patients with thoracic facet join pain; treatment with bupivacaine or bupivacaine-betamethasone resulted in experienced significant pain relief (79% - 83%) lasting 46 to 50 weeks, requiring 3 to 4 treatments.
Thoracic sympathetic blocks
Thoracic sympathetic blocks (TSBs) (Figure 3) are usually
helpful to diagnose and treat chronic benign and malignant thoracic and mediastinal pain syndromes, including neuropathic pain, chest wall pain, thoracic visceral pain, herpes zoster, post-herpetic pain, phantom breast pain. Diagnostic and therapeutic blocks of the sympathetic chain from T2-8 can be used in patients with severe intractable pain caused by cancer of the esophagus heart, bronchi, trachea, lung, pleura or other chronic esophageal pain [32,33]
Anatomically the sympathetic trunks are two ganglionated nerve structures that extend the entire length of the vertebral column. The thoracic part of the sympathetic chain runs downward and leaves the thorax behind the medial arcuate ligament [34].
Paravertebral nerve blocks
PVNBs can provide excellent intraoperative anaesthetic, postoperative analgesic conditions, and may offer good chronic pain control in some situations. It is a technique used to block spinal nerves, including the dorsal and ventral rami, and the sympathetic chain ganglion. Compared to neuraxial nerve blocks PVNBs have much less unfavorable effects and fewer contraindications [35]. The thoracic paravertebral space is a triangular area running the length of the thoracic vertebral
Figure 3 Left side thoracic sympathetic injection under fluoroscopy with contrast.
Figure 4 Positioning of 2 spinal cord stimulation lead over the dorsal horn column (2 x 8 electrode)) in the AP and lateral view.

CentralBringing Excellence in Open Access
Hegarty (2017)Email:
J Surg Transplant Sci 5(3): 1059 (2017) 5/3
Table 3: Shows the differential diagnosis and treatment options [39].
Syndrome Leading Features Action required
Costosternal SyndromeInjury to the thin articular cartilage where the rib articulates with the sternum. Can involve any level. Older patients (>40years)
Injection with X-ray or ultrasound
Tietzes Syndrome Acute painful swelling of the 2nd& 3rd costal cartilages. Typically in the younger patients (<40years) Ultrasound Injection
Manubriosternal Joint Pain Tenderness at the Angle of LouisThis is a fibrocartilaginous joint or synchondrosis. Infiltration of the region may be more useful
Sternalis Syndrome The muscle lies parallel to the sternum and innervated by the anterior thoracic nerves. It is not present in all individuals
Ultrasound guided injection along the lateral to the sternal boarder
Xiphodynia Tenderness at the Xiphisternaljoint. Innervation by the T4-T& intercostal nerves and the phrenic nerve
Ultrasound guided injection over the painful joint
Slipping Rib Syndrome“Clicking” sensation with movement of the effected ribs using the hooking maneuver. Associated with trauma to the lower costal cartilages
X-ray guidance can help identify the level but ultrasound is useful
LatissimusDorsi Syndrome Trigger points in the muscle belly Myofascial Trigger pint injection with ultrasound
Costovertebral Joint Syndrome
Synovial plane joint with articulation between the ribs and the vertebrae. Tenderness to touch with associated “splinting” of the backSymptoms may mimic thoracic facet joint because of the close proximity
It is important to rule out additional issues including:OsteoarthritisRheumatoid ArthritisPsoriatic AthritisReiters SyndromeAnkylosing Arthritis
column bilaterally, from T1 to T12. This anatomical space is in continuity with adjacent vertebral levels, allowing for spread of the injected solutions. The injection or injections can be done blindly using the anatomical landmarks, using fluoroscopy, or with ultrasound guidance.
Neuromodulation
The recent advancement in neuromodulation equipment and programming platforms opens the opportunity to use this technology to deal with the chronic pain condition. At present we have to rely on case studies and reported clinical evidence to understand the potential that exists. The majority of the use has being off label use. At present options include the using traditional spinal cord stimulation (SCS) with electrode placement over the dorsal column [36], dorsal root ganglion (DRG) stimulation [37] or peripheral field / plexus stimulation [38] (Figure 4). Each case should be evaluated on an individual basis and all the options considered by a pain physician and multidisciplinary team experienced in this area
Differential diagnosis
There are several possible causes of thoracic pain that need to be included in the differential diagnosis as the treatment is specific and the prognosis good. Table 3 outlines the key clinical elements with proposed treatment options.
CONCLUSIONPTPS is a very challenging chronic pain condition that affects
many patients on a daily basis. All healthcare providers working in this area ought to be alert to the possibility of this syndrome and must be prepared to recognize the treatment options. Pain physicians must be familiar with the range of conservative and interventional options from the simple intercostal blocks to
neuromodulation. When treatment is utilized rationally, they have the most probability to alleviate pain, and finally to improve the quality of life for patients.
REFERENCES1. Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S, et al. A Retrospective Study
of Chronic Post-Surgical Pain following Thoracic Surgery: Prevalence, Risk Factors, Incidence of Neuropathic Component, and Impact on Qualify of Life. PLoS One. 2014; 9: e90014.
2. Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014; 15: 887-897.
3. Mongardon N, Pinton-Gonnet C, Szekely B, Michel-Cherqui M, Dreyfus JF, Fischler M. Assessment of chronic pain after thoracotomy: a 1-year prevalence study. Clin J Pain. 2011; 27: 677-681.
4. Kinney MA, Hooten WM, Cassivi SD, Allen MS, Passe MA, Hanson AC, et al. Chronic postthoracotomy pain and health-related quality of life. Ann Thorac Surg. 2012; 93: 1242-1247.
5. Mesbah A, Yeung J, Goa F. Pain after Thoracotomy. BJA Education. 2016; 16: 1-7.
6. Bottiger BA, Esper SA, Stafford-Smith M. Pain management strategies for thoracotomy and thoracic pain syndromes. Semin Cardiothorac Vasc Anesth. 2014; 18: 45-56.
7. Shelley B, Macfie A, Kinsella J. Anesthesia for thoracic surgery: a survey of UK practice. J Cardiothorac Vasc Anesth. 2011; 25: 1014-1017.
8. Searle RD, Simpson MP, Simpson KH, Milton R, Bennett MI. Can chronic neuropathic pain following thoracic surgery be predicted during the postoperative period? Interact Cardiovasc Thorac Surg. 2009; 9: 999-1002.
9. Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006; 444: 894-898.

CentralBringing Excellence in Open Access
Hegarty (2017)Email:
J Surg Transplant Sci 5(3): 1059 (2017) 6/3
Hegarty D (2017) Post Thoracotomy Pain Syndrome: What Pain Management Options do we have? J Surg Transplant Sci 5(3): 1059.
Cite this article
10. Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009; 36: 170-180.
11. Wang HT, Liu W, Luo AL, Ma C, Huang YG. Prevalence and risk factors of chronic post-thoracotomy pain in Chinese patients from Peking Union Medical College Hospital. Chin Med J (Engl). 2012; 125: 3033-3038.
12. Brennan TJ, Kehlet H. Preventive analgesia to reduce wound hyperalgesia and persistent postsurgical pain: not an easy path. Anesthesiology. 2005; 103: 681-683.
13. Guastella V, Mick G, Soriano C, Vallet L, Escande G, Dubray C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011; 152: 74-81.
14. Enck RE. Postsurgical chronic pain. Am J Hosp Palliat Care. 2010; 27: 301-2.
15. Buchheit T, Pyati S. Prevention of chronic pain after surgical nerve injury: amputation and thoracotomy. Surg Clin North Am. 2012; 92: 393-407.
16. Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011; 152: 14-27.
17. Fenton R, Hegarty D, O’Leary M. Tapentadol consumption in post operative cardiothoracic surgery: an observational retrospective study to establish initial dose. Abstract Presented at Irish Pain Society ASM 2015.
18. Rathmell JP. Atlas of image guided intervention in regional anesthesia and pain medicine. 1st edn. Lippincott Williams & Wilkins, Philadelphia. 2006.
19. Abrahams MS, Horn JL, Noles LM, Aziz MF. Evidence-based medicine: ultrasound guidance for truncal blocks. Reg Anesth Pain Med. 2010; 35: 36-42.
20. Shankar H, Eastwood D. Retrospective comparison of ultrasound and fluoroscopic image guidance for intercostal steroid injections. Pain Pract. 2010; 10: 312-317.
21. Bhatia A, Gofeld M, Ganapathy S, Hanlon J, Johnson M. Comparison of anatomic landmarks and ultrasound guidance for intercostal nerve injections in cadavers. Reg Anesth Pain Med. 2013; 38: 503-507.
22. Ho AM, Karmakar MK, Critchley LA. Acute pain management of patients with multiple fractured ribs: a focus on regional techniques. Curr Opin Crit Care. 2011; 17: 323-327.
23. van Kleef M, Stolker RJ, Lataster A, Geurts J, Benzon HT, Mekhail N. 10. Thoracic pain. Pain Pract. 2010; 10: 327-338.
24. Rosas HG, Gilula LA. Performing thoracic transforaminal injections: a new technique. Radiology. 2010; 254: 595-600.
25. Wang A, Pilgram TK, Gilula LA. Immediate complications and pain relief associated with 296 fluoroscopically guided thoracic foraminal
nerve blocks. AJR Am J Roentgenol. 2011; 197: 1410-1416.
26. Kroszczynski AC, Kohan K, Kurowski M, Olson TR, Downie SA. Intraforaminal location of thoracolumbar anterior medullary arteries. Pain Med. 2013; 14: 808-812.
27. Yin W, Bogduk N. Retrograde filling of a thoracic spinal artery during transforaminal injection. Pain Med 2009; 10: 689-692.
28. van Kleef M, Barendse GA, Dingemans WA, Wingen C, Lousberg R, de Lange S, et al. The effects of producing a radiofrequency lesion adjacent to the dorsal root ganglion in patients with thoracic segmental pain by radiofrequency percutanious partial rhizotomy. Clin J Pain. 1995; 11: 325-332.
29. Nelson JW. Thoracic facet joint blocks and neurotmy. In: Prithvi PR (edn) Interventional Pain Management: Image-Guided Procedures. (2nd edn), Saunders, Philadelphia, USA, 2008; 279-286.
30. Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. 1997; 22: 332-336.
31. Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Effectiveness of thoracic medial branch blocks in managing chronic pain: a preliminary report of a randomized, double-blind controlled trial. Pain Physician 2008; 11: 491-504.
32. Krumova EK, Gussone C, Regeniter S, Westermann A, Zenz M, Maier C. Are sympathetic blocks useful for diagnostic purposes? Reg Anesth Pain Med. 2011; 36: 560-567.
33. Ohseto K. Efficacy of thoracic sympathetic ganglion block and prediction of complications: clinical evaluation of the anterior paratracheal and posterior paravertebral approaches in 234 patients. J Anesth. 1992; 6: 316-331.
34. Richardson J, Lönnqvist PA, Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth. 2011; 106: 164-171.
35. Karmakar MK, Samy W, Li JW, Lee A, Chan WC, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med 2014; 39: 289-298.
36. Graybill J, Conermann T, Kabazie AJ, Chandy S. Spinal cord stimulation for treatment of pain in a patient with post thoracotomy pain syndrome. Pain Physician. 2011; 14: 441-445.
37. Ali R, Epstin L, Khelemsky Y. Successful treatment of chronic post thoracotomy pain syndrome with dorsal root ganglion stimulation North American Neuromodulation Society Abstract 2017 (online nans2017 0.0070006).
38. Hegarty D, Goroszeniuk T. Peripheral nerve stimulation of the thoracic paravertebral plexus for chronic neuropathic pain. Pain Physician. 2011; 14: 295-300.
39. Waldman S. Atlas of Pain Management Injection Techniques. 2nd edn. Phikadelphia: Saunder Elsevier. 2007.
![BMJ Open...severe post-operative pain insult and persistent chronic pain many months after surgery is common.[1-5] Chronic post-thoracotomy pain (CPTP) is defined by the International](https://static.fdocuments.us/doc/165x107/6014ecdc3e1bfd5bdd1baba4/bmj-open-severe-post-operative-pain-insult-and-persistent-chronic-pain-many.jpg)







![Stephen H. Pennefather and James McKevith · vagal nerve stimulation can suppress pain [20]. Blocking the vagus nerve might actually increase post-thoracotomy pain by reducing vagally](https://static.fdocuments.us/doc/165x107/5d5b9bd488c993bd048bb481/stephen-h-pennefather-and-james-mckevith-vagal-nerve-stimulation-can-suppress.jpg)









![Factors Associated with Chronic Post-Thoracotomy Pain · The surgical incision, ... acute and chronic pain related to ... It is a risk factor for chronic post-surgical pain [28].](https://static.fdocuments.us/doc/165x107/5b4d2b2c7f8b9ac6118bbe3f/factors-associated-with-chronic-post-thoracotomy-the-surgical-incision-.jpg)