Placenta & Fetal membrane.Prof.Salah Roshdy
-
Upload
salah-roshdy-ahmed -
Category
Health & Medicine
-
view
214 -
download
5
Transcript of Placenta & Fetal membrane.Prof.Salah Roshdy
Placenta& Fetal membranes
Salah Roshdy,MDProfessor of Obstetrics & Gynecology
Sohag College of MedicineSohag University
Learning ObjectivesMention function of fetal membraneDiscuss origin of placentaDescribe normal placentaLists functions of placentaEnumerate placental hormonesDiscuss placental abnormalitiesDescribe normal & abnormal U.C
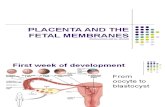
Fetal membrane • Originate from blastocyst,
don’t participate in the formation of embryo
• Including:1) Chorion2) Amnion3) Yolk sac4) Allantois5)Umbilical cord
AmnionThin but tough
Forms a fluid filled membranous amniotic sac that surrounds the embryo and fetus
Is attached to the margins of the embryonic disc
Amnion• Amniotic membrane: amniotic epi.+
extraembryonic mesoderm
•Amniotic fluid:Produce:1)amniotic cells 2) infusion of fluid from maternal blood 3) urine output from the fetus 4) pulmonary secretionsOutput: 1) absorbed by amniotic cells 2) fetus swallow
Amniotic FluidPlays a major role in fetal growth and development.Daily contribution of fluid from respiratory tract is 300-400 ml.500 ml of urine is added daily during the late pregnancy.Amniotic fluid volume is 30 ml at 10 weeks, 350 ml at 20 weeks, 700-1000 ml at 37 weeks.
Composition of Amniotic Fluid
99 % is waterDesquamated fetal epithelial cellsOrganic & inorganic saltsProtein, carbohydrates, fats, enzymes, hormonesMeconium & urine in the late stage
Significance of Amniotic FluidPermits symmetrical external growth of the embryo and fetusActs as a barrier to infection
Permits normal fetal lung development
Prevents adherence of amnion to fetus
Cushions & protects the embryo and fetus
Helps maintain the body temperature
Enables the fetus to move freely
Yolk SacIt is large at 32 daysShrinks to 5mm pear shaped remnant by 10th week & connected to the midgut by a narrow yolk stalkBecomes very small at 20 weeksUsually not visible thereafter
Significance of Yolk SacHas a role in transfer of nutrients during the 2nd and 3rd weeksBlood development first occurs hereIncorporate into the endoderm of embryo as a primordial gutPrimordial germ cells appear in the endodermal lining of the wall of the yolk sac in the 3rd week
Fate of Yolk SacAt 10 weeks lies in the chorionic cavity between chorionic and amniotic sacAtrophies as pregnancy advancesSometimes it persists throughout the pregnancy but of no significanceIn about 2% of adults the proximal intra-abdominal part of yolk stalk persists as an ileal diverticulum or Meckel diverticulum
AllantoisIn the 3rd week it appears as a sausagelike diverticulum from the caudal wall of yolk sac that extends into the connecting stalkDuring the 2nd month, the extraembryonic part of the allantois degenerates
Functions of AllantoisBlood formation occurs in the wall during the 3rd to 5th weekIts blood vessels persist as the umbilical vein and arteriesBecomes Urachus and after birth is transformed into median umbilical ligament extends from the apex of the bladder to the umbilicus
ChorionThe extraembryonic somatic mesoderm and the two layers of trophoblast form the chorionChorion forms the wall of chorionic sacEmbryo and its amniotic and yolk sacs are suspended into it by connecting stalk
ChorionGrowth of these extensions are caused by underlying extraembryonic somatic mesodermThe cellular projections form primary chorionic villi
Chorion
Chorionic villi cover the entire chorionic sac until the beginning of 8th week
As this sac grows, the villi associated with decidua capsularis are compressed, reducing the blood supply to them
These villi soon degenerates producing an avascular bare area smooth chorion (chorion laeve)
Chorion
As the villi disappear, those associated with the decidua basalis rapidly increase in numberBranch profusely and enlargeThis bushy part of the chorionic sac is villous chorion
PRIMARY CHORIONIC VILLI
At the end of 2nd week, finger-like processes formed of outer syncytiotrophoblast & inner cytotrophoblast appear
TERTIARY CHORIONIC VILLI During 3rd week, arterioles, venules & capillaries develop in the mesenchyme of villi & join umbilical vesselsBy the end of 3rd week, embryonic blood begins to flow slowly through capillaries in chorionic villi
DeciduaThe gravid endometrium is known as deciduaIt is the functional layer of endometrium in a pregnant womanThis part of the endometrium separates from the rest of the uterus after parturition
DECIDUA Decidua basalis: It lies at the site of implantation ,it forms the maternal part of the placenta
Decidua capsularis: it covers the conceptus
Decidua parietalis: the rest of the endometrium that lines the body & the fundus.
25
DeciduaThe full significance of decidual cells is not understoodThey may protect the maternal tissue against uncontrolled invasion by the syncytiotrophoblastThey may be involved in hormonal production
27
PLACENTAThis is a fetomaternal organ.It has two components:
Fetal part – develops from the chorionic sac ( chorion frondosum )
Maternal part – derived from the endometrium ( functional layer – decidua basalis )
During the 4th and 5th month, the decidua forms a number of decidual septa, which project into the intervillous space. As a result of this septum formation, the placenta is divided into a number of compartments (cotyledons).
PLACENTAL MEMBRANE This is a composite structure that separating the fetal blood from the maternal blood.It has four layers:SyncytiotrophoblastCytotrophoblastConnective tissue of
villusEndothelium of fetal
capillariesAfter the 20th week, the cytotrophoblastic cells disappear and the placental membrane consists only of three layers.
30
31
It separates fetal from maternal blood.It prevents mixing of them.
It is an incomplete barrier as it only prevents large molecules to pass ( heparin & bacteria)
But cannot prevents passage of viruses(e.g. rubella), micro-organisms(toxoplama, treponema pallidum) drugs and hormones.
Functions of placental barrier:
1. It prevents most organisms from passing to the fetus, so it acts as a protective mechanism against damaging factors, many viruses such as Rubella, Coxackie virus, German measles and poliomylitis virus traverse the placenta. These viruses may result in congenital malformations.
2. Most of the drugs cross the placenta and cause serious damage.
The full term placenta is discoid in shape. Diameter = 15-25 cm, 2-3 cm thick, Weight = 0.5 kg. Umbilical cord is attached to its center. Position : in the upper uterine segment (99.5%), either in the posterior surface (2/3) or the anterior surface (1/3).
Surfaces: 1- Fetal surface: which is
smooth and shinny because it is covered by an amniotic membrane. The umbilical cord is attached centrally to this surface.
2- Maternal surface: which is rough, reddish, and has 15 – 20 elevated areas called cotyledons with deep grooves in between made by the decidual septa.
Function of placenta:-1. Respiratory function2. Excretory function3. Nutritional function4. Endocrine function:- placenta acts as
endocrine gland 5. Barrier function:- prevents transfer of
maternal infection. 6. Enzymatic action- 7. Immunological function:- ig G.
Nutritive functionFetus obtains its nutrients from the maternal blood
Glucose- transferred to the fetus by facilitated diffusion
Lipids for fetal growth and development has dual origin. They are transferred across the fetal membrane or synthesised in the fetus
Amino acids are transferred by active transport
Water and electrolytes- Na, K ,Cl cross by simple diffusion, Ca , P, and Fe cross by active transport
Water soluble vitamins are transferred by active transport but the fat soluble vitamins are transferred slowly
Respiratory functionAlthough fetal respiratory movement occurs, no active exchange of gases takes place
Intake of oxygen and output of carbon dioxide take place by simple diffusion across the fetal membrane
O2 delivery to the fetus is at the rate of 8 ml/kg which is achieved by cord blood flow of 160-320ml/min
Excretory functionWaste products from the fetus such as urea, uric acid, cretinine are excreted to the maternal blood by simple diffusion
Barrier FunctionSubstances with large molecular weight or size like insulin or heparin are transferred minimally
Only IgG ( not IgA or Ig M )antibodies and antigens can cross the placental barrier
Most drugs can cross the placental barrier and some can be teratogenic
Various viruses, bacteria, protozoa can cross the placenta and affect the fetus in utero
Immunological functionInspite of foreign paternally inherited antigens in the fetus and placenta, there is no graft rejection due to immunological protection provided by the placenta
Endocrine and Enzymatic functionPlacenta secretes various hormones – Protein hormones like HCG, human placental lactogen,pregnancy specific beta 1 glycoprotein,,pregnancy associated plasma protein, steroidal hormones like estrogen and progestroneEnzymes secreted are diamine oxidase-which activates the circulatory pressor amines,oxytocinase which neutralizes oxytocin, phospholipase A2 which synthesizes arachidonic acid
PLACENTAL PROTEIN HORMONES
1. placental lactogen (hPL) 2. chorionic gonadotropin
(hCG)3. Adenocorticotropin (ACTH)4. Growth hormone variant
(hGH-V)5. Parathyroid hormone-related
protein (PTH-rP)6. Calcitonin7. Relaxin8. Inhibins9. Activins10. Atrial natriuretic peptide
PLACENTAL PROTEIN HORMONES
11. Hypothalamic-like releasing and inhibiting hormones Thyrotropin releasing hormone
(TRH) Gonadotropin releasing hormone
(GnRH) Corticotropin-releasing hormone
(CRH) Growth hormone-releasing
hormone (GHRH)12. fetal compartment – alpha feto-
protein13. Maternal compartment – prolactin,
relaxin and other decidual proteins
• Glycoprotein with biological activity similar to luteinizing hormone• Both act via the plasma membrane LH-hCG receptor• Produced in the placenta, but also synthesized in fetal
kidney and a number of fetal tissues may produce the β-subunit or intact hCG molecule .
• Also produced by malignant tumors• Presence of hCG in blood and urine of reproductive age women is almost indicative of the presence of fetal trophoblasts either in pregnancy or in neoplastic disease
Human chorionic gonadotropin (hCG)
Chemical Characteristics of hCGCarbohydrate component protects the molecule from catabolismPlasma half life of the intact molecule: 36-hour Composed of 2 dissimilar subunits (α and β)No biological activity of either separated subunitBioactivity which is binding to the LH receptor is only present if the two units are combinedStructurally identical to 3 other glycoprotein hormones: LH, FSH and TSHAmino acid sequences of the beta subunit of hCG is distinctively dissimilar from those of LH, FSH, and TSH
Biosynthesis of hCGPlasma levels of free β-subunits increase steadily until the 36th week of pregnancy and then plateaus till the end of pregnancySecretion of β-hCG corresponds roughly to the placental massRate of secretionof the complete hCG molecule is maximal at 8 to 10 weeks of gestationPlacental GnRH, produced in cytotrophoblast, acts in paracrine manner on syncitiotrophoblast to stimulate hCG production
Biosynthesis of hCGOther agents that believed to influence hCG secretion in trophoblast:Interleukin-6Epidermal growth factorCyclic AMP
Activin stimulates and inhibin inhibits production of GnRH and hCG
Cellular Origin of hCG< 5 weeks hCG is expressed in both syncytiotrophoblasts and cytotrophoblast cellsAt the peak of maternal levels later in gestationhCG is produced almost exclusively in the syncitiotrophoblast
Concentration of hCG in Serum and Urine
• Intact hCG molecule is detectable in plasma of pregnant women about 7 to 9 days after ovulation
• hCG enters maternal blood at time of blastocyst implantation
• Blood levels increase rapidly, doubling every 2 days• Maximal levels attained at about 8 to 10 weeks’
gestation• Between the 60th and 80th days after the last menses
- peak levels reach about 100,000 mIU/mL
Concentration of hCG in Serum and Urine
• When the hCG titers exceeds 1,000-1,500 IU/L, vaginal ultrasonography should identify an intrauterine gestation
• 10-12 weeks gestation – maternal plasma levels begin to decline
• Nadir - about 20 weeks• Plasma levels are maintained at this lower level for
the rest of the pregnancy• Urine concentration of hCG follows the pattern of
maternal plasma
Metabolic Clearance of hCG
30 percent through the kidneys the rest cleared by the liver and other pathways
Biological Functions of hCG
1. Rescue and maintenance of function of the corpus luteum (continued progesterone production) • progesterone producing life span of the corpus
luteum of menstruation could be prolonged for 2 weeks by hCG administration
• about the 8th day after ovulation or 1 day after implantation- hCG takes over for the corpus luteum
• Continued survival of the corpus luteum is totally dependent on hCG
Biological Functions of hCG
• Survival of the pregnancy is dependent on corpus luteum progesterone until the 7th week of pregnancy
• Progesterone luteal synthesis begins to decline at about 6 weeks despite continued and increasing hCG production
• Down regulation of hCG-LH receptors in the corpus luteum when trophoblasts produce sufficient progesterone for pregnancy maintenance
2. Stimulation of fetal testicular testosterone secretion
• Before 110 days – no fetal anterior pituitary LH• At a critical time in sexual differentiation of the
male fetus, hCG enters fetal plasma from the syncitiotrophoblast, acts as an LH surrogate and stimulates replication of testicular Leydig cells and testosterone synthesis to promote male sexual differentiation
Biological Functions of hCG
3. Stimulation of maternal thyroid activityhCG binds to the TSH receptors of thyroid cellsLH-hCG receptor is expressed in the thyroidPossibly, hCG stimulates thyroid activity via the LH-hCG receptor and by the TSH receptorhCG has intrinsic thyroid activity and maybe the 2nd placental thyrotropic substance
Biological Functions of hCG
4. Promotion of relaxin secretion by the corpus luteum
5. Promote uterine vascular vasodilatation and myometrial smooth muscle relaxation via LH-hCG receptors.
6.Suppresses maternal immune function& reduces possibility of fetus immunorejection.
Biological Functions of hCG
Human Chorionic Somammotropin (hCS)or Placental Lactogen
Structure similar to growth hormone
Produced by the placenta
Levels throughout pregnancy
Large amounts in maternal blood but DO NOT reach the fetus
- single non-glycosylated polypeptide chain
- similar to hPRL (prolactin)
1. Chemical Characteristics
potent lactogenic and GH-like bioactivity
HUMAN PLACENTAL LACTOGEN
- hPL – on chromosome 17
2. Gene Structure
3. Serum Concentration• demonstrable in placenta within 5 to 10 days after conception• detected as early as 3 weeks after fertilization• rises until about 34 to 36 weeks
HUMAN PLACENTAL LACTOGEN
- stimulated : insulin, cAMP- inhibited : PGE2, PGF2α
4. Regulation of hPL Biosynthesis
5. Metabolic Actions① lipolysis and increase FFA② anti-insulin action
HUMAN PLACENTAL LACTOGEN
Human Chorionic Somammotropin (hCS)
or Placental LactogenBiological effects are reverse of those of insulin: utilization of lipids; make glucose more readily available to fetus, and for milk production.
hCS levels proportionate to placental size
hCS levels placental insuffiency
Progesterone • After 6 to 7 weeks of gestation ovarian
progesterone production is minimal • After about 8 weeks – placenta replaces the
ovary as the source of progesterone & continues to increase production throughout pregnancy
• daily production rate is 250 mg• In pregnancies with multiple fetuses, daily
production rate may be >6000 mg/day
Source of Cholesterol for Placental Progesterone Biosynthesis
• Progesterone - synthesized from cholesterol in a two-step enzymatic reaction
• 1st cholesterol is converted to pregnenolone within the mitochondria.
• Pregnenolone leaves the mitochondria and converted to progesterone in the endoplasmic reticulum by 3β-hydroxysteroid dehydrogenase
• Progesterone is released immediately through a process of diffusion
Source of Cholesterol for Placental Progesterone Biosynthesis
• limited capacity for the biosynthesis of cholesterol in trophoblast
• maternal plasma cholesterol was the principal precursor (90 %) of progesterone biosynthesis in the placenta
• trophoblast preferentially uses LDL cholesterol for progesterone biosynthesis
Source of Cholesterol for Placental Progesterone Biosynthesis
• Hydrolysis of LDL releases essential amino acids and cholesterol esters, which in turn yield fatty acids and cholesterol
• Essential amino acids and fatty acids are transported to the fetus and cholesterol is used for placental progesterone biosynthesis
• Fetus contributes essentially no precursor• Pregnenolone sulfate may be the most important
precursor for synthesis and metabolism of progesterone in human decidua and fetal membranes
Progesterone and Fetal Well-Being
No relationship between placental progesterone synthesis and fetal well beingProgesterone biosynthesis may persist several weeks after fetal death
Progesterone Metabolism During Pregnancy
• Same as in men and nonpregnant women• During pregnancy - a disproportionate increase in the
plasma concentration of 5α-dihydroprogesterone as a result of synthesis in syncytiotrophoblast from both placenta-produced progesterone and fetal-derived precursor
• 5α-reduced metabolite contributes to the resistance in pregnancy against the vasopressor action of angiotensis II
• Progesterone is also converted to the potent mineralocorticoid deoxycorticosterone in pregnant women and in the fetus, thus an increase in deoxycorticosterone in the maternal and fetal compartments
Role of Progesterone • Prepares and maintains the endometrium to
allow implantation• Has a role in suppressing the maternal
immunologic response to fetal antigens thereby preventing maternal rejection of the trophoblast and has a role in parturition
• serves as a substrate for fetal adrenal gland production of glucocorticoids and mineralocorticoids
Support of the endometrium to provide an environment conducive to fetal survival. If the endometrium is deprived of progestins, the pregnancy will inevitably be terminated.Suppression of contractility in uterine smooth muscle, which, if unchecked, would clearly be a disaster. This is often called the "progesterone block" on the myometrium. Toward the end of gestation, this myometrial-quieting effect is antagonized by rising levels of estrogens, thereby facilitating parturition.
Progesterone potently inhibit secretion of the pituitary gonadotropins LH and FSH. This effect almost always prevents ovulation from occuring during pregnancy.
Placental Estrogen Production• produces huge amounts of estrogens
using blood-borne steroidal precursors from the maternal and fetal adrenal glands
• Normal human pregnancy is hyperestrogenic state, continually increasing as pregnancy progresses terminating abruptly after birth
Placental Estrogen Production
• first 2 to 4 weeks of pregnancy - rising levels of hCG maintain production of estradiol in the maternal corpus luteum
• seventh week of pregnancy – maternal corpus luteum production of both progesterone and estrogen decreases significantly
• there is a luteal–placental transition by the seventh week, more than 50 percent of estrogen entering the maternal circulation is produced in the placenta
Placental Estrogen Biosynthesis
• pathways for estrogen synthesis in the human placenta differ from those in the ovary of non pregnant women
• production occurs in the follicular and luteal • Ovarian theca cells synthesize androstenedione
granulosa cells estradiol • Androstenedione is produced de novo from
acetate and cholesterol, catalyzed by aromatase 450 estrone, acted upon by estradiol dehydrogenase estradiol
Schematic presentation of the
biosynthesis of estrogens in the human placenta
• DHEA-S secreted in large amounts by the fetal adrenal glands is converted to 16 α hydroxydehydroepiandrosterone sulfate (16 α OHDHEA-S) in the fetal liver
• DHEAS and 16 α OHDHEA-S are converted in the placenta to estrogens viz., 17 β estradiol (E2) and estriol (E3)
• Near term, half of E2 is derived from fetal adrenal DHEA-S and half from maternal DHEA-S
• 90 % of E3 in the placenta arises from fetal 16 α OHDHEA-S and only 10 % from other sources
Two of the principle effects of placental estrogens are: Stimulate growth of the myometrium and antagonize the myometrial-suppressing activity of progesterone. In late gestation induces myometrial oxytocin receptors, thereby preparing the uterus for parturition.Stimulate mammary gland development.
Relaxin• Expressed in: human corpus luteum, decidua, and
placenta • structurally similar to insulin and insulin-like growth factor • relaxin along with rising progesterone levels acts on
myometrial smooth muscle to promote uterine relaxation and the quiescence observed in early pregnancy
• relaxin and relaxin-like factors in the placenta and fetal membranes may play an autocrine–paracrine role in regulation of extracellular matrix degradation in the puerperium
Leptin• normally secreted by adipocytes • initially believed to be an anti-obesity hormone • now regulates bone growth and immune function • secreted by both cytotrophoblast cells and
syncytiotrophoblast and maternal levels are significantly higher than in non pregnant women and that in the fetal circulation
• Fetal leptin levels – correlated positively with fetal birthweight
• play an important role in fetal development and growth
Inhibin • glycoprotein hormone, inhibit pituitary FSH release • produced by the testis, ovarian granulosa cells and the
corpus luteum • placenta produces inhibin alpha-, and beta A and beta B-
subunits• Inhibin A – principal bioactive inhibin secreted during
pregnancy• Highest level is at term• Placental inhibin production together with large amounts of
placental sex steroids inhibit FSH secretion and preclude ovulation during pregnancy
Inhibin Trophoblastic inhibin synthesis inhibited by activin A stimulated by hCg, GnRH, epidermal
growth factor, transforming growth factor-alpha and PGF 2 β and PGE 2
may act via GnRH to regulate hCG synthesis and secretion in the placenta
Activin • closely related to inhibin • enhances FSH synthesis and secretion and
participates in the regulation of the menstrual cycle • roles in cell proliferation, embryogenesis,
osteogenesis, differentiation, apoptosis, metabolism, homeostasis, immune response, wound repair and endocrine function
• nerve cell survival factors • has 3 forms: A, B and AB
Activin • Chorionic activin and inhibin - regulators within the
placenta for the production of GnRH, hCG and steroids
• Inhibin – inhibitory, Activin - stimulatory• may serve functions in placental metabolic processes
other than GnRH synthesis, but are still under study• Placental and decidual inhibin and activin early in
pregnancy – indicate their possible roles in embryogenesis and local immune responses
• Activin levels actively decline after delivery
Fetomaternal circulation
2 umbilical arteries deoxygenated, or "venous-like" blood flows to the
placenta 1 umbilical vein with a significantly higher oxygen content(80%
saturation)
As fetal lungs are not functioning, the fetus obtains oxygen and nutrients from the mother through the placenta and the umbilical cord
The core concept behind fetal circulation is that fetal hemoglobin has a higher affinity for oxygen than does adult hemoglobin, which allows a diffusion of oxygen from the mother's circulatory system to the fetus.
Circulatory system of the mother is not directly connected to that of the fetus . Water, glucose, amino acids, vitamins, and inorganic salts freely diffuse across the placenta along with oxygen
CirculationBlood from the placenta is carried to the fetus by the umbilical vein. About half of this enters the fetal ductus venosus and is carried to the inferior vena cava, while the other half enters the liver proper from the inferior border of the liver. The branch of the umbilical vein that supplies the right lobe of the liver first joins with the portal vein
The blood then moves to the right atrium of the heart. Between the right and left atrium, the foramen ovale allow most of the blood flows through it directly into the left atrium from the right atrium, thus bypassing pulmonary circulation.
The continuation of this blood flow is into the left ventricle, and from there it is pumped through the aorta into the body.
Some of the blood entering the right atrium does not pass directly to the left atrium through the foramen ovale, but enters the right ventricle and is pumped into the pulmonary artery. In the fetus, connection between the pulmonary artery and the aorta, called the ductus arteriosus, which directs most of this blood away from the fluid filled, non functioning lungs
Changes of fetal circulation at birth
1. Closure of umbilical arteries: Functionally, soon after birth, but actual obliteration takes by 2-3 months
2. Closure of umbilical vein: little later than arteries and allows few extra volume of blood to go to fetus from placenta
3. Closure of ductus arteriosus: within few hrs of respiration
4. Closure of foramen ovale: functionally , soon after birth but anatomically, closes in about 1 year
Changes of fetal circulation at birth
Fetal Structure
Foramen ovaleDuctus arteriosusLeft umbilical vein Extra-hepatic portion Intra-hepatic portion (ductus
venosus)
Left and right umbilical arteries Proximal portions Distal portions
Adult Remnant
Fossa ovalis of the heartLigamentum arteriosum
Ligamentum teres hepatis Ligamentum venosum
Superior vesical arteriesMedial umbilical ligaments
Abnormalities Of The Placenta(A) Abnormal Shape
(B) Abnormal Diameter
(C) Abnormal Weight
(D) Abnormal Position
(E) Abnormal Adhesion
Abnormalities of placenta1- Abnormal position: Placenta Praeviathe placenta is attached to the lower uterine segment (due to low level of implantation of the blastocyst). It causes severe antepartum haemorrhage. There are three types:
1- Placenta accreta: due to abnormal adhesion between the chorionic villi and the uterine wall.2- Placenta percreta: The chorionic villi penetrate the myometrium all the way to the perimetrium.- the placenta fails to separate from the uterus after birth and may cause severe postpartum hemorrhage.
2- Abnormal adhesion:
3- Abnormal attachment of umbilical cord:a- Velamentous attachment:The cord does not reach the placenta itself but is attached to amniotic membrane over the fetal surface of placenta. The umbilical vessels pass in the membrane to reach the placenta. It is easly torn.
(4) Abnormal Shape:
1. Placenta Bilobate2. Placenta Bipartite 3. Placenta Succenturiate4. Placenta Circumvallate5. Placenta Fenestrate
2. Placenta Bipartite:
The placenta consists of two equal parts connected by membranes.The umbilical cord is inserted in one lobe and branches from its vessels cross the membranes to the other lobe. Rarely, the umbilical cord divides into two branches, each supplies a lobe.
The placenta consists of a large lobe and a smaller one connecting together by membranes. The umbilical cord is inserted into the large lobe and branches of its vessels cross the membranes to the small succenturiate (accessory) lobe.
3. Placenta Succenturiate:
3. Placenta Succenturiata:
The accessory lobe may be retained in the uterus after delivery leading to postpartum haemorrhage. This is suspected if a circular gap is detected in the membranes from which blood vessels pass towards the edge of the main placenta.
A whitish ring composed of decidua, is seen around the placenta from its foetal surface.
This may result when the chorion frondosum is two small for the nutrition of the foetus, so the peripheral villi grow in such a way splitting the decidua basalis into a superficial layer ( the whitish ring) and a deep layer.
4. Placenta Circumvallate:
It can be a cause of :1. Abortion,2. Ante partum haemorrhage,3. Preterm labour and 4. Intrauterine foetal death.
4. Placenta Circumvallate:
5. Placenta Fenestrata:
A gap is seen in the placenta covered by membranes giving the
appearance of a window.
Placenta membranacea: A great part of the chorion develops into placental tissue. The placenta is large, thin and may measure 30-40 cm in diameter. It may encroach on the lower uterine segment i.e. placenta praevia.
(4) Abnormal Diameter:
The Umbilical CordAnatomy
•Origin : It develops from the connecting stalk.•Length: At term, it measures about 50 cm.•Diameter: 2 cm.
Structure: It consists of mesodermal connective tissue called Wharton's jelly, covered by amnion.
It contains:1. One umbilical vein carries oxygenated blood
from the placenta to the foetus2. Two umbilical arteries carry deoxygenated
blood from the foetus to the placenta,3. Remnants of the yolk sac and allantois.
The Umbilical Cord
Insertion: The cord is inserted in the foetal surface of the placenta near the center "eccentric insertion" (70%) Or at the center "central insertion" (30%).
The Umbilical Cord
1. Marginal insertion : in the placenta ( battledore insertion).
2. Velamentous insertion: in the membranes and vessels connect the cord to the edge of the placenta. If these vessels pass at the region of the internal os , the condition is called " Vasa praevia".
(A) Abnormal cord insertion:
Vasa praeviaVasa praevia can occur also when the vessels connecting a succenturiate lobe with the
main placenta pass at the region of the internal os
1. Short cord which may lead to :i-Intrapartum haemorrhage due to
premature separation of the placenta,ii-Delayed descent of the foetus druing
labour,iii-Inversion of the uterus.
(B) Abnormal cord length:
2. Long cord which may lead to:i-Cord presentation and cord
prolapse,ii-Coiling of the cord around the neck,iii-True knots of the cord.
(B) Abnormal cord length:
(C) Knots of the cord:1. True knot:
when the foetus passes through a loop of the cord. If pulled tight, foetal asphyxia may result.
2. False knot: localized collection of Wharton’s jelly
containing a loop of umbilical vessels.