Phytochemical Composition and Chronic Hypoglycemic...
Transcript of Phytochemical Composition and Chronic Hypoglycemic...

Research ArticlePhytochemical Composition and Chronic Hypoglycemic Effect ofBromelia karatas on STZ-NA-Induced Diabetic Rats
Sonia M Escandoacuten-Rivera Adolfo Andrade-Cetto and Gabriela Saacutenchez-Villasentildeor
Laboratorio de Etnofarmacologıa Facultad de Ciencias Universidad Nacional Autonoma de Mexico 4510 Ciudad de Mexico Mexico
Correspondence should be addressed to Adolfo Andrade-Cetto aaccienciasunammx
Received 25 October 2018 Revised 17 December 2018 Accepted 11 February 2019 Published 25 February 2019
Academic Editor Daniela Beghelli
Copyright copy 2019 Sonia M Escandon-Rivera et al This is an open access article distributed under the Creative CommonsAttribution License which permits unrestricted use distribution and reproduction in any medium provided the original work isproperly cited
Oral administration of an aqueous extract of the aerial parts of Bromelia karatas to STZ-NA rats showed a significant hypoglycemiceffect in a chronic trial lasting 42 days Chromatographic profiles of the active extract (WE) and an organic extract (OE) ofB karataswere obtained by high-performance liquid chromatography (HPLC) and used to identify their major components Isolation andidentification of the compounds present in the extracts were accomplished by means of various conventional chromatographic andspectroscopic techniques This process led to the identification of 120573-sitosterol-3-O-120573-D-glucopyranoside (1) and 120588-coumaric acid(3) as the major compounds present in the extracts During the isolation of 1 and 3 seven additional metabolites not previouslyreported for the plantwere obtained namely cirsiliol 41015840-O-120573-D-glucopyranoside (2) stigmasterol (4)120573-sitosterol (5) 1-O-feruloyl-3-O-120588-coumaroylglycerol (6) 120573-D-(1-O-acetyl-36-O-trans-diferuloyl) fructofuranosyl-120572-D-210158404101584061015840-O-triacetylglucopyranoside(7) 1-O-p-coumaroyl-3-O-caffeoylglycerol (8) and 2-propyl-120573-glucopyranoside (9)
1 Introduction
Diabetes mellitus is a metabolic disease that is characterizedby elevated levels of blood glucose that over time causeserious damage to the heart blood vessels eyes kidneysand nerves [1 2] Individuals with Type 2 diabetes typicallysuffer from insulin resistance and relative rather than absoluteinsulin deficiency At least initially and often throughouttheir lifetimes these individuals may not require insulintreatment to survive According to the IDF 2017 425 millionadults have diabetes worldwide andMexico ranks 6th amongthe top ten countries in the number of adults with diabetes
In Mexico the use of plants for medicinal purposes hasbeen a common practice since pre-Hispanic times Amongthe plants used in the treatment of diabetes Bromelia karatas(L) traditionally known as ldquopinuelardquo ldquochiyolrdquo ldquochicipordquoldquoaguamardquo and ldquocazuelardquo is a tropical herb that is distributedbetween 400 and 1500m elevation The leaves are elon-gated and thick and contain sharp teeth along the marginsInflorescence is sessile at the ground level Flowering ofthe plant occurs between May and October Its distributionin America ranges from Mexico to Ecuador and Brazil in
Mexico it is mainly found in the tropical zone [3] B karatasis a monocotyledon that produces a variety and diversity ofantioxidantmetabolites and glucoconjugates such as (6S9R)-vomifolyl-120573-D-glucopyranoside 345-trimethoxyphenyl-120573-D-glucopyranoside 1-O-120573-D-glucopyranosyl anthranilateand 34-dimethoxyphenyl 120573-D-glucopyranoside [4] In aprevious work we reported the traditional use of the plant inthe treatment of diabetes and its acute hypoglycemic effectwas demonstrated in STZ-NA hyperglycemic rats [5] Thatinvestigation showed that diabetic patients and healers inthe municipality of Tlanchinol Hidalgo use a decoction ofthe leaves to control blood glucose levels and confirmed thehypoglycemic effects of aqueous and hydroalcoholic extractsof the plant these extracts produced the best effects atoral doses of 350 and 300mgkg respectively The studyalso reported preliminary experiments to determine thepresence of alkaloids terpenes and phenolic compounds inthe extracts and found that phenolic compounds are themaincomponents However to date the metabolites present in theactive extract of B karatas have not been identified
The aim of the current study was to continue trials of thehypoglycemic effect of administration of a water extract of
HindawiEvidence-Based Complementary and Alternative MedicineVolume 2019 Article ID 9276953 9 pageshttpsdoiorg10115520199276953
2 Evidence-Based Complementary and Alternative Medicine
leaves of B karatas in STZ-NA rats and to evaluate the lipidprofiles and glycated hemoglobin levels of the animals afterchronic administration of the extract An additional aim ofthe study was to characterize the main components of theextract using conventional phytochemical techniques for theseparation isolation and identification of metabolites
2 Materials and Methods
21 General Experimental Procedures NMR spectra includ-ing 1H 13C HSQC HMBC COSY NOESY and TOCSYwere recorded in a Varian Inova spectrometer at 400 (1H)and 95MHz (13C) or a Bruker DMX500 spectrometer oper-ated at 500MHz (1H) or 125MHz (13C) chemical shiftswere recorded as 120575 values ESI-MS were recorded on aThermo Scientific LTQ Orbitrap XL hybrid FTMS (Fouriertransform mass spectrometer) Data were collected in bothpositive and negative ionization modes via a liquid chro-matographicautosampler system that consisted of an AgilentHPLC system Analytical and preparative HPLC analyseswere performed in an Agilent 1260 Infinity system equippedwith a G1311B Quaternary Pump a G1367E Autosamplerand a G1315C DAD VL+ and controlled by Agilent Chem-Station software For analytical and semipreparative HPLCPhenomenex (Luna Omega Polar C18 50 times 21mm id16 120583m) and Macherey-Nagel (Nucleosyl C18 250 times 46mmid 5 120583m andNucleosyl C18 250 times 10mm id 5120583m) columnsrespectively were used Column chromatography (CC) wasconducted on silica gel (70-230mesh Merck) or SephadexLH-20 (Sigma-Aldrich Chemical) Thin-layer chromatogra-phy analyses were carried out on silica gel 60 F254 plates(Macherey amp Nagel) using ceric sulfate (10) solution inH2SO4 as the color reagent
22 Plant Extracts The original plant material was collectedwith the help of the healer ldquoIsabel Escalanterdquo near the townof Tamala state of Hidalgo Mexico The plant species wasverified by MC Ramiro Cruz Duran and a voucher exemplarwas deposited at the Herbarium of the ldquoInstituto Mexicanodel Seguro Socialrdquo (IMSSM 15814) Fresh plant material wascollected as needed
The water extract (WE) was prepared by boiling 20 gof the dry plant material with 500ml water followed byfiltration and lyophilization to yield 2119 g ofWEThe extractwas stored at 4∘C for further analysisTheWEand the organicextract (OE) were used for phytochemical identification ofthe main compounds of B karatas OE was prepared from365 g of plant material by consecutive extraction with amixture of dichloromethanemethanol (11) The combinedextract was concentrated in a rotary vacuum evaporator(Buchi Flawil Switzerland) at 40∘C the resulting concentratewas evaporated to dryness under reduced pressure yielding842 g of OE
23 Experimental Animals Wistar rats weighing 200-250 gwere provided by the Bioterium of the Science SchoolUNAM they were acclimated with free access to food andwater for at least one week in an air-conditioned room (25∘C
50 humidity) on a 12-h light-dark cycle prior to use in theexperiments All the experiments were conducted in accor-dance with the principles set forth in the National Institute ofHealth (NIH) Guidelines for the Care and Use of LaboratoryAnimals [6] Hyperglycemia was induced as described byMasiello [7] In brief rats that had been fasted overnightwere injected intraperitoneally with 150mgkg nicotinamide(NA) (Sigma N3376) 15min prior to intravenous injection of65mgkg streptozotocin (STZ Sigma S0130) in citrate bufferHyperglycemia was identified by polyuria and polydipsia andby measuring nonfasting plasma glucose levels 48 h afterthe injection animals that did not reach glucose levels of250mgdl were rejected
119864xperiment 1 Chronic Hypoglycemic Effect of the WaterExtract The previously described experimental design [8]was used in brief four groups of six rats were used as followsgroup I the normal control (NC) received 15ml of physio-logical NaCl solution (vehicle) group II the hyperglycemiccontrol (HC) also received 15ml of physiological NaClsolution group III was given a standard oral hypoglycemicagent glibenclamide [5mgkg body weight (bw)] in the samevehicle (CG) and group 4 received Bk-WE (218mgkg bw)dissolved in 15ml of physiological NaCl solutionThe extractor the hypoglycemic agent was orally administered twicedaily (in the morning and in the evening) over a period of 42days All groups were fed Purina Rodent Laboratory Chow5001
Blood was obtained from the tail veins of the animals theanimals were handled according to the procedures describedin [6] Glucose monitoring was performed weekly usingglucose test strips and an Accutrend Plus glucometer Gly-cated hemoglobin (HbA1c) was analyzed in a DCA VantageSiemens The lipid profile (HDL TG and cholesterol) wasmeasured using Cardio Check and Panels PTS stripsVLDL was calculated using the following equation VLDL =02 x TG HbA1c and lipid profiles were measured on days 014 28 and 42 after the initiation of treatment The data werestatistically analyzed using the unpaired t-test The plasmaglucose levels are expressed as the mean (SEM)
119864xperiment 2 Acute Hypoglycemic Effect of the IsolatedCompounds To know if the activity is based on the presenceof compounds 1-3 or on the presence of any of them inthe WE an acute experiment was performed as previouslydescribed [8]Wistar rats were divided into six groups of fourrats each Group 1 normal control orally received 15ml ofphysiologicalNaCl solution (vehicle) group 2 hyperglycemiccontrol also received 15ml of physiological NaCl solutiongroup 3 positive control received a standard oral hypo-glycemic agent [glibenclamide 5mgkg body weight (bw)] inthe same vehicle Groups 4 5 and 6 were given compounds1 (72mgkg) 2 (18mgkg) and 3 (363mgkg) all dissolvedin 15ml of physiological NaCl solution The doses of eachcompoundwere calculated according to the radio plant yieldof the compound
24 HPLC Analysis Elution was conducted at a flow rateof 025mLmin with water containing 01 formic acid as
Evidence-Based Complementary and Alternative Medicine 3
solvent A and acetonitrile (MeCN) as solvent B using agradient elution of 1000 (AB) for 1min 955 (AB) at 15min8515 (AB) at 15-12min 7030 (AB) at 12-20min 7030 (AB)maintained during 1min 6040 (AB) at 21-23min 2080(AB) at 23-27min 1090 (AB) at 27-28min 1090 (AB)maintained during 1min and 1000 (AB) at 29-31min Theflow rate was set to 025mLmin and the temperature of thecolumn was maintained at 35∘C System control data collec-tion and data processing were accomplished usingOpenLABLC 1260 chromatography software Working solutions ofsamples (WE OE fractions and isolated compounds) of Bkaratas were prepared by dissolving 50mg of the sample in1mL of the appropriate solvent (MeOH MeCN or H2O) andinjected (2 120583L) using an autosampler For UV detection thewavelength program was set at an acquisition of 120582 230 254280 320 and 365 nm 320 nm was selected as the optimumwavelength The equipment data acquisition processingand management of the chromatographic information werecontrolled with the OpenLab CDS ChemStatiob Edition(2001-2013) software The separation was carried out usinga Phenomenex Luna Polar C18 (50 x 21mm id 16 120583m)reverse phase column all solvents were purchased from JTBaker as HPLC grade
25 Isolation Compounds WE (1 g) was dissolved in a mix-ture ofMeOHH2O (11) from this solution 241mg of 1 spon-taneously precipitated The remainder of the WE was furtherseparated on Sephadex (500mL MeOHH2O 11) yielding 14primary fractions (WE1-WE14) WE9 (43mg) was resolvedby HPLC (Nucleosil 250 times 10mm id 5 120583m C18 Macherey-Nagel) using a 15-min gradient of MeCNMeOHH2O(60535) (23mlmin 254 and 320 nm) yielding 6 mg ofa solid yellow amorphous powder (2) with RT 953 minWE13 (465mg) was subjected to preparative TLC (EtOAcn-hexaneacetone 721) to yield compound 3 (12mg)
The OE (842 g) was partitioned by column chromatog-raphy (CC) on 336 g of silica gel (70-230mesh Merck)usingmixtures of n-hexaneCH2Cl2MeOH as eluent Elutionwas started with 100 n-hexane the polarity of the eluentwas then increased by the addition of increasing amountsof CH2Cl2 (to 100) and then MeOH This yielded 28primary fractions (OE1-OE28) Fraction OE11 (10 g) wassubjected to silica gel CC (40 g) using mixtures of n-hexaneCH2Cl2MeOH as eluent Elution was started with100 n-hexane and the polarity of the eluent was increasedby the gradual addition of CH2Cl2 (to 100) and thenMeOH This resulted in 10 subfractions (OE111-OE1110)OE1110 (40 mg) yielded a mixture of 4 and 5 OE16 (279mg)was processed with CC on silica gel (11 g) using mixturesof EtOAcMeOH as eluent starting with 100 EtOAc andincreasing the polarity with MeOH to 100 this processyielded 9 subfractions (OE161-OE169) Preparative TLC ofOE164 (32mg eluted with n-hexaneEtOAc 19) and OE17(76mg eluted with n-hexaneEtOAc 19) resulted in theisolation of 6 (10mg) and 7 (15mg) respectively FractionOE19 (347mg) was subjected to CC on silica gel (15 g)elution was started with 100 EtOAc and the polarity ofthe solvent was increased by the addition of methanol up to100 using this process it was possible to isolate compound
8 (18mg) Preparative TLC (EtOAcMeOHH2O 81505)of fraction OE22 (97mg) resulted in the isolation of 9(12mg) Finally EO23 (122mg) and EO24 (434mg) weredissolved in methanol and compounds 2 (25mg) and 1(10mg) respectively were obtained as precipitates
26 Identification of the Isolated Compounds in the Chromato-graphic Profile ofWE Isolated compounds (1-9) and extractsprepared from B karatas (WE andOE) were injected into theHPLC for identification of the compounds in the chromato-graphic profiles Peak assignments were made on the basis ofthe previously developed structure-diagnostic and supportedby examination of the UV spectrum and relative retentiontime using OpenLAB LC 1260 chromatography software
3 Results
31 Ethnobotanical Results In the field we confirmed theprevious finding that the healer ldquoIsabel Escalanterdquo and thediabetic patients of the town of Tamala use a decoction of 30 gof the dry leaves of B karatas boiled in 1 liter of water Theinfusion is cooled and drunk over the day as ldquoAgua de UsordquoThe common name of the plant in this region is ldquotimbiricherdquoAlternatively the juice of the fruit is used
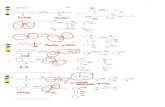
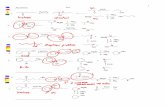
32 Phytochemical Analysis The chromatographic profileswere developed to monitor the major components of WEand OE from B karatas and to make it possible to iso-late and identify them by means of various conventionalchromatographic and spectroscopic techniques The anal-ysis led to the isolation of compounds 1-9 which wereidentified based on comparison of their 1H and 13C-NMR spectral data including data obtained in 2D experi-ments (COSY HSQC HMBC NOESY TOCSY) and theirmass spectral data with those of previously describedcompounds The major compounds were identified as asteroidal glycoside [120573-sitosterol-3-O-120573-D-glucopyranoside(1)] [9] and a phenolic acid [120588-coumaric acid (3)] [10]both compounds were isolated from the traditional extract(WE) The flavonoid [cirsiliol 41015840-O-120573-D-glucopyranoside(2)] [11] from WE along with two steroidal aglycones[stigmasterol (4) and 120573-sitosterol (5)] [12] two phenyl-propanoid glycerols [1-O-feruloyl-3-O-120588-coumaroylglycerol(6) and 1-O-120588-coumaroyl-3-O-caffeoylglycerol (8)] [13]a phenylpropanoid glycoside [120573-D-(1-O-acetyl-36-O-trans-diferuloyl)fructofuranosyl-120572-D-210158404101584061015840-O triacetylglucopy-ranoside (7)] [14] and 2-propyl-D-glucopyranoside (9) [15]from OE were some of the minor compounds Figure 1 Thecomplete spectroscopic data of the isolated compounds arepresented below
120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Colorless powderFAB-MSmz 577 [M+H]+ for C35H60O6 EI-MSmz 414 [M-C6H10O5]
+ 1H NMR (400MHz Pyridine-d5) 120575 536 (1H tJ = 26Hz H-6) 507 (1H d J = 78Hz H-11015840) 458 (1H dd J= 118 25Hz H-61015840) 443 (1H dd J = 118 51 Hz H-61015840) 430(2H m H-31015840 and H-41015840) 408 (1H t J = 82Hz H-21015840) 399(2H m H-3 and H-51015840) 275 (1H ddd J = 135 48 22HzH-12) 250 (1H m H-12) 215 (2H m H-2) 199 (1H dd J =126 35Hz H-4) 186 (1Hm H-4) 172 (1H dtd J = 180 79
4 Evidence-Based Complementary and Alternative Medicine
HO
OH
O
O
OHR
OOHO
OHOH
HO
OOH
O
OOH
OOHO
O
O
OHOHHO
OH
HO HO
HO
O
O
1 23
54
6 R = OH8 R = OMe
OO
OH
OH
OHHO
9
OOO
OOH
O
O
O
OH
OCH3O
HOOCH3
O
O
O
HO
O
O
O
O
7
Figure 1 Isolated compounds from Bromelia karatas 120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Cirsiliol 41015840-O-glucoside (2) 120588-Coumaric acid (3) Stigmasterol (4) 120573-Sitosterol (5) 1-O-Feruloyl-3-O-p-coumaroylglycerol (6) 120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) 1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) 2-Propyl-120573-glucopyranoside (9)
69 38Hz H-1) 168 (1Hm H-25) 141 (1H ddt J = 158 9843Hz H-20) 136 (1H m H-8) 110 (1H m H-17) 102 (1Hm H-24) 100 (3H d J = 65Hz H-21) 095 (3H s H-19)095 (1H m H-14) 092(3H d J = 73Hz H-26) 089 (3H t J= 74Hz H-29) 088 (3H d J = 69Hz H-27) 067 (3H s H-18) 13C NMR (100MHz Pyridine-d5) 120575 14091 (C-5) 12192(C-6) 10257 (C-11015840) 7859 (C-3) 7847 (C-31015840) 7812 (C-51015840)7530 (C-21015840) 7168 (C-41015840) 6283 (C-61015840) 5684 (C-14) 5625(C-17) 5035 (C-9) 4605 (C-24) 4249 (C-13) 3996 (C-4)3934 (C-12) 3749 (C-1) 3693 (C-10) 3640 (C-20) 3421 (C-22) 3219 (C-7) 3206 (C-8) 3026 (C-2) 2947 (C-25) 2856(C-16) 2639 (C-23) 2452 (C-15) 2340 (C-28) 2130 (C-11)2000 (C-27) 1943 (C-19) 1922 (C-26) 1903 (C-21) 1217 (C-29) 1199 (C-18)
Cirsiliol 41015840-O-glucoside (2) Yellow prisms HR-ESIMS ion atmz 5151204 [M+Na]+ for C23H24O12
1H NMR (400MHzPyridine-d5) 120575 796 (1H d J = 22Hz H-21015840) 772 (1H d J =86Hz H-51015840) 752 (1H dd J = 86 23Hz H-61015840) 703 (1H sH-3) 673 (1H s H-8) 580 (1H d J = 77Hz H-110158401015840) 461 (1Hdd J = 120 21 Hz H-6a
10158401015840) 444 (1H dd J = 121 53Hz H-6b10158401015840) 438 (1H t J = 89Hz H-310158401015840) 434 (1H d J = 89Hz H-
410158401015840) 426 (1H t J = 89Hz H-210158401015840) 418 (1H ddd J = 90 52
21 Hz H-510158401015840) 402 (3H s OCH3-6) 391 (s OCH3-7)13C
NMR (100MHz Pyridine-d5) 120575 18355 (C-4) 16476 (C-2)15983 (C-7) 15402 (C-9) 15391 (C-5) 15054 (C-41015840) 14900(C-31015840) 13357 (C-6) 12689 (C-11015840) 1197 (C-61015840) 11825 (C-51015840)11583 (C-21015840) 10687 (C-10) 10537 (C-3) 10354 (C-110158401015840) 9197(C-8) 7968 (C-510158401015840) 7891 (C-310158401015840) 7518 (C-210158401015840) 7157 (C-410158401015840)6269 (C-610158401015840) 6096 (6-OCH3) 5684 (7-OCH3)
120588-Coumaric Acid (3) Colorless amorphous powder ESI-MSion at mz 163 [M-H]minus for C9H8O3
1H NMR (400MHzCD3OD) 120575 750 (1H d J = 157Hz H-7) 741 (2H d J =86Hz H-2 H-6) 678 (2H d J= 86Hz H-3 H-5) 633 (1Hd J = 158Hz H-8) 13C NMR (100MHz CD3OD) 120575 17520(C-9) 15842 (C-4) 14418 (C-7) 13054 (C-2 C-6) 12801 (C-1) 11666 (C-3 C-5) 11576 (C-7)
Stigmasterol (4) White powder ESI-MS ion at mz 413[M+H]+ for C29H48O
1H NMR (500MHz CDCl3) 120575 535(1HmH-6) 515 (1H dd J = 152 80Hz H-21) 502 (1H ddJ = 152 85Hz H-20) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 084 (3Ht J = 76Hz H-24) 081 (3H d J = 68Hz H-26) 080 (3Hd J = 68Hz H-27) 068 (3H s H-28) 13C NMR (125 MHz
Evidence-Based Complementary and Alternative Medicine 5
CDCl3) 120575 14092 (C-5) 1385 (C-22) 1294 (C-23) 12186 (C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5139 (C-24) 5031(C-9) 4248 (C-4) 4238 (C-13) 4064 (C-20) 3985 (C-12)3742 (C-1) 3667 (C-10) 3208 (C-7 C-8 C-25) 3184 (C-2)2901 (C-16) 2446 (C-15) 2556 (C-28) 2137 (C-11) 2036 (C-26) 1956 (C-27) 1894 (C-21) 1894 (C-19) 1210 (C-29) 1202(C-18)
120573-Sitosterol (5) White powder ESI-MS ion at mz 415[M+H]+ for C29H50O
1H NMR (500 MHz CDCl3) 120575 535(1H d J = 53Hz H-5) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 083 (3Hd J = 68Hz H-24) 081 (3H d J = 68Hz H-26) 069 (3Hs H-28) 13C NMR (125 MHz CDCl3) 120575 14092 (C-5) 12186(C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5031 (C-9) 4601(C-24) 4248 (C-13) 4238 (C-4) 3985 (C-12) 3742 (C-1)3667 (C-10) 3631 (C-20) 3412 (C-22) 3208 (C-2) 3184 (C-7 C-8) 2933 (C-25) 2841 (C-16) 2626 (C-23) 2446 (C-15)2324 (C-28) 2137 (C-11) 1998 (C-26) 1914 (C-27) 1913 (C-19) 1894 (C-21) 1221 (C-29) 1202 (C-18)
1-O-Feruloyl-3-O-p-coumaroylglycerol (6) Colorless oil ESI-MS ion at mz 4374 [M+Na]+ for C22H22O8
1H NMR(400MHz CD3OD) 120575 766 (2H d J = 160Hz H-71015840 H-710158401015840)744 (2H d J = 87Hz H-21015840 H-61015840) 718 (1H d J = 20Hz H-210158401015840) 707 (1H dd J = 83 21 Hz H-610158401015840) 680 (2H d J = 82HzH-31015840 H-51015840) 679 (1H d J = 86Hz H-510158401015840) 639 (1H d J =142Hz H-810158401015840) 635 (1H d J = 143Hz H-81015840) 428 (4H dd J= 53 19Hz H-1 H-3) 416 (1H m H-2) 387 (3H s OCH3-310158401015840) 13CNMR (100MHz CD3OD) 120575 16901 (C-9
10158401015840) 16899 (C-91015840) 16134 (C-41015840) 15071 (C-310158401015840) 14938 (C-410158401015840) 14725 (C-710158401015840)14697 (C-71015840) 13121 (C-21015840 C-61015840) 12766 (C-110158401015840) 12711 (C-11015840)12419 (C-610158401015840) 11684 (C-31015840 C-51015840) 11648 (C-510158401015840) 11510 (C-810158401015840)11480 (C-81015840) 11175 (C-210158401015840) 6861 (C-2) 6639 (C-3) 6637 (C-1) 5644 (OCH3-3
10158401015840)
120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) Colorlessamorphous solid ESI-MS ion at mz 8852 [M+Na]+ forC40H46O21
1H NMR (500MHz CD3OD) 120575 772 (1H d J =159Hz H-710158401015840) 768 (1H d J = 159Hz H-7101584010158401015840) 728 (2H d J= 20Hz H-210158401015840) 722 (2H d J = 20Hz H-2101584010158401015840) 713 (1H dd J= 85 201Hz H-610158401015840) 711 (1H dd J = 85 19Hz H-6101584010158401015840) 683(2H d J = 82Hz H-510158401015840 H-5101584010158401015840) 646 (1H d J = 1442HzH-810158401015840) 643 (1H d J = 144Hz H-8101584010158401015840) 570 (1H d J = 38HzH-11015840) 539 (1H d J = 77Hz H-3) 480 (1H d J = 102HzH-41015840) 468 (1H dd J = 102 38Hz H-21015840) 450 (1H dd J =121 327Hz H-6a) 445 (1H m H-6b) 446 (1H t J = 79HzH-4) 426 (d J = 115Hz H-1a) 424 (1H m H-51015840) 421(d br J = 114Hz H-61015840a) 410 (1H dd J = 116 35Hz H-61015840b)409 (1H d J = 115Hz H-1b) 391 (3H s OCH3-3
101584010158401015840) 391(3H s OCH3-3
10158401015840) 388 (1H d J = 76Hz H-31015840) 213 (3H sOAc-1) 210 (3H s OAc-21015840) 203 (3H s OAc-61015840) 187 (3Hs OAc-41015840) 13C NMR (125 MHz CD3OD) 120575 17260 (OAc-6
1015840)17214 (OAc-21015840) 17202 (OAc-1) 17172 (OAc-41015840) 16877(C-9101584010158401015840) 16798 (C-910158401015840) 15112 (C-410158401015840) 15076 (C-4101584010158401015840) 14951(C-310158401015840) 14943 (C-3101584010158401015840) 14823 (C-710158401015840) 14725 (C-7101584010158401015840) 12774(C-110158401015840) 12750 (C-1101584010158401015840) 12462 (C-610158401015840) 12431 (C-6101584010158401015840) 11657(C-510158401015840) 11652 (C-5101584010158401015840) 11515 (C-8101584010158401015840) 11430 (C-810158401015840) 11183
(C-210158401015840) 11168 (C-2101584010158401015840) 10391 (C-2) 9046 (C-11015840) 8152 (C-5)7955 (C-3) 7395 (C-21015840) 7379 (C-4) 7225 (C-41015840) 7006(C-5) 6982 (C-31015840) 6661 (C-1) 6453 (C-6) 6409 (C-61015840)5656 (OCH3-3
10158401015840 OCH3-3101584010158401015840) 2092 (OAc-CH3-2
1015840) 2079(OAc-CH3-1) 2068 (OAc-CH3-6
1015840) 2062 (OAc-CH3-41015840)
Assignments of the acetate and feruloyl units at positions1 21015840 41015840 61015840 (for acetyl) and 3 6 (for feruloyl) respectivelyin the sucrose molecule were corroborated by the HMBCtwo-dimensional spectrum this showed a correlationbetween methine and methylene protons at 1 21015840 41015840 and 61015840of the sucrose with methyl protons and the carbonyl carbonof the acetyl groups (1H13C1H 426 40917202213for 1 46817214210 for 21015840 48017172187 for 41015840 42141017260203 for 61015840) and between methine and methyleneprotons at 3 and 6 of the fructose with the protons at 810158401015840and 8101584010158401015840 of the feruloyl moiety and the carbonyl carbons at910158401015840 and 9101584010158401015840 (1H13C1H 64616798539 for C-3 feruloyl64316877450 445 for C-3 feruloyl)
1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) Colorless oil ESI-MS ion at mz 3998 [M-H]minus for C21H20O8
1H NMR(400MHz CD3OD) 120575 766 (1H d J = 160Hz H-71015840) 760 (1Hd J = 159Hz H-710158401015840) 744 (2H dd J = 89 23Hz H-21015840 H-61015840)705 (1H d J = 21 Hz H-210158401015840) 694 (1H dd J = 83 20Hz H-610158401015840) 679 (2H dd J = 91 25Hz H31015840 H-51015840) 677 (1H d J =84Hz H-510158401015840) 635 (1H d J = 159Hz H-81015840) 630 (1H d J =159Hz H-810158401015840) 427 (4H d J = 54Hz H-1 H-3) 416 (1H qJ = 52Hz H-2) 13C NMR (100MHz CD3OD) d 16901 (C-91015840 C-910158401015840) 16131 (C-41015840) 14965 (C-310158401015840) 14736 (C-710158401015840) 14697(C-71015840) 14681 (C-410158401015840) 13122 (C-21015840 C-61015840) 12770 (C-110158401015840) 12713(C-11015840) 12302 (C-610158401015840) 11683 (C-31015840 C-51015840) 11651 (C-510158401015840) 11520(C-210158401015840) 11482 (C-81015840) 11476 (C-810158401015840) 6865 (C-2) 6635 (C-1C-3)
2-Propyl-120573-glucopyranoside (9) Colorless amorphous solidESI-MS ion at mz 2452 [M+Na]+ for C9H18O6
1H NMR(400 MHz CD3OD) 120575 434 (1H d J = 78Hz H-1) 404 (1Hhept J = 61 Hz H-21015840) 385 (1H dd J = 119 20Hz H-6b)366 (1H dd J = 119 54Hz H-6a) 335 (1H m H-3) 327(1H m H-4) 325 (1H m H-5) 314 (1H dd J = 91 78HzH-2) 123 (3H d J = 62Hz CH3-1
1015840) 119 (3H d J = 61HzCH3-3
1015840) 13C NMR (100MHz CD3OD) 120575 10255 (C-1) 7811(C-3) 7786 (C-5) 7514 (C-2) 7258 (C-21015840) 7170 (C-4) 6279(C-6) 2381 (C-11015840) 2204 (C-31015840)
33 Chromatographic Profiles In the HPLC profiles of theWE and the OE of B karatas (Figure 1) the majority ofcomponents showed maximum absorption between 280 and320 nmThe two chromatograms were qualitatively differenthowever the main components of WE [TR 133 (3) 142 and219 (2)min] were also observed in the OE [TR 133 (3) 142and 219 (2)min] but in different proportion Compounds 45 and 9 were not observed in any of the chromatographicprofiles Figure 2
Compound 1 was not observed as a major compound inthe profile of the WE (Figure 2(a)) because it precipitatedduring the process of sample preparation before the samplewas injected into the HPLC equipment its remnant was
6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

2 Evidence-Based Complementary and Alternative Medicine
leaves of B karatas in STZ-NA rats and to evaluate the lipidprofiles and glycated hemoglobin levels of the animals afterchronic administration of the extract An additional aim ofthe study was to characterize the main components of theextract using conventional phytochemical techniques for theseparation isolation and identification of metabolites
2 Materials and Methods
21 General Experimental Procedures NMR spectra includ-ing 1H 13C HSQC HMBC COSY NOESY and TOCSYwere recorded in a Varian Inova spectrometer at 400 (1H)and 95MHz (13C) or a Bruker DMX500 spectrometer oper-ated at 500MHz (1H) or 125MHz (13C) chemical shiftswere recorded as 120575 values ESI-MS were recorded on aThermo Scientific LTQ Orbitrap XL hybrid FTMS (Fouriertransform mass spectrometer) Data were collected in bothpositive and negative ionization modes via a liquid chro-matographicautosampler system that consisted of an AgilentHPLC system Analytical and preparative HPLC analyseswere performed in an Agilent 1260 Infinity system equippedwith a G1311B Quaternary Pump a G1367E Autosamplerand a G1315C DAD VL+ and controlled by Agilent Chem-Station software For analytical and semipreparative HPLCPhenomenex (Luna Omega Polar C18 50 times 21mm id16 120583m) and Macherey-Nagel (Nucleosyl C18 250 times 46mmid 5 120583m andNucleosyl C18 250 times 10mm id 5120583m) columnsrespectively were used Column chromatography (CC) wasconducted on silica gel (70-230mesh Merck) or SephadexLH-20 (Sigma-Aldrich Chemical) Thin-layer chromatogra-phy analyses were carried out on silica gel 60 F254 plates(Macherey amp Nagel) using ceric sulfate (10) solution inH2SO4 as the color reagent
22 Plant Extracts The original plant material was collectedwith the help of the healer ldquoIsabel Escalanterdquo near the townof Tamala state of Hidalgo Mexico The plant species wasverified by MC Ramiro Cruz Duran and a voucher exemplarwas deposited at the Herbarium of the ldquoInstituto Mexicanodel Seguro Socialrdquo (IMSSM 15814) Fresh plant material wascollected as needed
The water extract (WE) was prepared by boiling 20 gof the dry plant material with 500ml water followed byfiltration and lyophilization to yield 2119 g ofWEThe extractwas stored at 4∘C for further analysisTheWEand the organicextract (OE) were used for phytochemical identification ofthe main compounds of B karatas OE was prepared from365 g of plant material by consecutive extraction with amixture of dichloromethanemethanol (11) The combinedextract was concentrated in a rotary vacuum evaporator(Buchi Flawil Switzerland) at 40∘C the resulting concentratewas evaporated to dryness under reduced pressure yielding842 g of OE
23 Experimental Animals Wistar rats weighing 200-250 gwere provided by the Bioterium of the Science SchoolUNAM they were acclimated with free access to food andwater for at least one week in an air-conditioned room (25∘C
50 humidity) on a 12-h light-dark cycle prior to use in theexperiments All the experiments were conducted in accor-dance with the principles set forth in the National Institute ofHealth (NIH) Guidelines for the Care and Use of LaboratoryAnimals [6] Hyperglycemia was induced as described byMasiello [7] In brief rats that had been fasted overnightwere injected intraperitoneally with 150mgkg nicotinamide(NA) (Sigma N3376) 15min prior to intravenous injection of65mgkg streptozotocin (STZ Sigma S0130) in citrate bufferHyperglycemia was identified by polyuria and polydipsia andby measuring nonfasting plasma glucose levels 48 h afterthe injection animals that did not reach glucose levels of250mgdl were rejected
119864xperiment 1 Chronic Hypoglycemic Effect of the WaterExtract The previously described experimental design [8]was used in brief four groups of six rats were used as followsgroup I the normal control (NC) received 15ml of physio-logical NaCl solution (vehicle) group II the hyperglycemiccontrol (HC) also received 15ml of physiological NaClsolution group III was given a standard oral hypoglycemicagent glibenclamide [5mgkg body weight (bw)] in the samevehicle (CG) and group 4 received Bk-WE (218mgkg bw)dissolved in 15ml of physiological NaCl solutionThe extractor the hypoglycemic agent was orally administered twicedaily (in the morning and in the evening) over a period of 42days All groups were fed Purina Rodent Laboratory Chow5001
Blood was obtained from the tail veins of the animals theanimals were handled according to the procedures describedin [6] Glucose monitoring was performed weekly usingglucose test strips and an Accutrend Plus glucometer Gly-cated hemoglobin (HbA1c) was analyzed in a DCA VantageSiemens The lipid profile (HDL TG and cholesterol) wasmeasured using Cardio Check and Panels PTS stripsVLDL was calculated using the following equation VLDL =02 x TG HbA1c and lipid profiles were measured on days 014 28 and 42 after the initiation of treatment The data werestatistically analyzed using the unpaired t-test The plasmaglucose levels are expressed as the mean (SEM)
119864xperiment 2 Acute Hypoglycemic Effect of the IsolatedCompounds To know if the activity is based on the presenceof compounds 1-3 or on the presence of any of them inthe WE an acute experiment was performed as previouslydescribed [8]Wistar rats were divided into six groups of fourrats each Group 1 normal control orally received 15ml ofphysiologicalNaCl solution (vehicle) group 2 hyperglycemiccontrol also received 15ml of physiological NaCl solutiongroup 3 positive control received a standard oral hypo-glycemic agent [glibenclamide 5mgkg body weight (bw)] inthe same vehicle Groups 4 5 and 6 were given compounds1 (72mgkg) 2 (18mgkg) and 3 (363mgkg) all dissolvedin 15ml of physiological NaCl solution The doses of eachcompoundwere calculated according to the radio plant yieldof the compound
24 HPLC Analysis Elution was conducted at a flow rateof 025mLmin with water containing 01 formic acid as
Evidence-Based Complementary and Alternative Medicine 3
solvent A and acetonitrile (MeCN) as solvent B using agradient elution of 1000 (AB) for 1min 955 (AB) at 15min8515 (AB) at 15-12min 7030 (AB) at 12-20min 7030 (AB)maintained during 1min 6040 (AB) at 21-23min 2080(AB) at 23-27min 1090 (AB) at 27-28min 1090 (AB)maintained during 1min and 1000 (AB) at 29-31min Theflow rate was set to 025mLmin and the temperature of thecolumn was maintained at 35∘C System control data collec-tion and data processing were accomplished usingOpenLABLC 1260 chromatography software Working solutions ofsamples (WE OE fractions and isolated compounds) of Bkaratas were prepared by dissolving 50mg of the sample in1mL of the appropriate solvent (MeOH MeCN or H2O) andinjected (2 120583L) using an autosampler For UV detection thewavelength program was set at an acquisition of 120582 230 254280 320 and 365 nm 320 nm was selected as the optimumwavelength The equipment data acquisition processingand management of the chromatographic information werecontrolled with the OpenLab CDS ChemStatiob Edition(2001-2013) software The separation was carried out usinga Phenomenex Luna Polar C18 (50 x 21mm id 16 120583m)reverse phase column all solvents were purchased from JTBaker as HPLC grade
25 Isolation Compounds WE (1 g) was dissolved in a mix-ture ofMeOHH2O (11) from this solution 241mg of 1 spon-taneously precipitated The remainder of the WE was furtherseparated on Sephadex (500mL MeOHH2O 11) yielding 14primary fractions (WE1-WE14) WE9 (43mg) was resolvedby HPLC (Nucleosil 250 times 10mm id 5 120583m C18 Macherey-Nagel) using a 15-min gradient of MeCNMeOHH2O(60535) (23mlmin 254 and 320 nm) yielding 6 mg ofa solid yellow amorphous powder (2) with RT 953 minWE13 (465mg) was subjected to preparative TLC (EtOAcn-hexaneacetone 721) to yield compound 3 (12mg)
The OE (842 g) was partitioned by column chromatog-raphy (CC) on 336 g of silica gel (70-230mesh Merck)usingmixtures of n-hexaneCH2Cl2MeOH as eluent Elutionwas started with 100 n-hexane the polarity of the eluentwas then increased by the addition of increasing amountsof CH2Cl2 (to 100) and then MeOH This yielded 28primary fractions (OE1-OE28) Fraction OE11 (10 g) wassubjected to silica gel CC (40 g) using mixtures of n-hexaneCH2Cl2MeOH as eluent Elution was started with100 n-hexane and the polarity of the eluent was increasedby the gradual addition of CH2Cl2 (to 100) and thenMeOH This resulted in 10 subfractions (OE111-OE1110)OE1110 (40 mg) yielded a mixture of 4 and 5 OE16 (279mg)was processed with CC on silica gel (11 g) using mixturesof EtOAcMeOH as eluent starting with 100 EtOAc andincreasing the polarity with MeOH to 100 this processyielded 9 subfractions (OE161-OE169) Preparative TLC ofOE164 (32mg eluted with n-hexaneEtOAc 19) and OE17(76mg eluted with n-hexaneEtOAc 19) resulted in theisolation of 6 (10mg) and 7 (15mg) respectively FractionOE19 (347mg) was subjected to CC on silica gel (15 g)elution was started with 100 EtOAc and the polarity ofthe solvent was increased by the addition of methanol up to100 using this process it was possible to isolate compound
8 (18mg) Preparative TLC (EtOAcMeOHH2O 81505)of fraction OE22 (97mg) resulted in the isolation of 9(12mg) Finally EO23 (122mg) and EO24 (434mg) weredissolved in methanol and compounds 2 (25mg) and 1(10mg) respectively were obtained as precipitates
26 Identification of the Isolated Compounds in the Chromato-graphic Profile ofWE Isolated compounds (1-9) and extractsprepared from B karatas (WE andOE) were injected into theHPLC for identification of the compounds in the chromato-graphic profiles Peak assignments were made on the basis ofthe previously developed structure-diagnostic and supportedby examination of the UV spectrum and relative retentiontime using OpenLAB LC 1260 chromatography software
3 Results
31 Ethnobotanical Results In the field we confirmed theprevious finding that the healer ldquoIsabel Escalanterdquo and thediabetic patients of the town of Tamala use a decoction of 30 gof the dry leaves of B karatas boiled in 1 liter of water Theinfusion is cooled and drunk over the day as ldquoAgua de UsordquoThe common name of the plant in this region is ldquotimbiricherdquoAlternatively the juice of the fruit is used
32 Phytochemical Analysis The chromatographic profileswere developed to monitor the major components of WEand OE from B karatas and to make it possible to iso-late and identify them by means of various conventionalchromatographic and spectroscopic techniques The anal-ysis led to the isolation of compounds 1-9 which wereidentified based on comparison of their 1H and 13C-NMR spectral data including data obtained in 2D experi-ments (COSY HSQC HMBC NOESY TOCSY) and theirmass spectral data with those of previously describedcompounds The major compounds were identified as asteroidal glycoside [120573-sitosterol-3-O-120573-D-glucopyranoside(1)] [9] and a phenolic acid [120588-coumaric acid (3)] [10]both compounds were isolated from the traditional extract(WE) The flavonoid [cirsiliol 41015840-O-120573-D-glucopyranoside(2)] [11] from WE along with two steroidal aglycones[stigmasterol (4) and 120573-sitosterol (5)] [12] two phenyl-propanoid glycerols [1-O-feruloyl-3-O-120588-coumaroylglycerol(6) and 1-O-120588-coumaroyl-3-O-caffeoylglycerol (8)] [13]a phenylpropanoid glycoside [120573-D-(1-O-acetyl-36-O-trans-diferuloyl)fructofuranosyl-120572-D-210158404101584061015840-O triacetylglucopy-ranoside (7)] [14] and 2-propyl-D-glucopyranoside (9) [15]from OE were some of the minor compounds Figure 1 Thecomplete spectroscopic data of the isolated compounds arepresented below
120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Colorless powderFAB-MSmz 577 [M+H]+ for C35H60O6 EI-MSmz 414 [M-C6H10O5]
+ 1H NMR (400MHz Pyridine-d5) 120575 536 (1H tJ = 26Hz H-6) 507 (1H d J = 78Hz H-11015840) 458 (1H dd J= 118 25Hz H-61015840) 443 (1H dd J = 118 51 Hz H-61015840) 430(2H m H-31015840 and H-41015840) 408 (1H t J = 82Hz H-21015840) 399(2H m H-3 and H-51015840) 275 (1H ddd J = 135 48 22HzH-12) 250 (1H m H-12) 215 (2H m H-2) 199 (1H dd J =126 35Hz H-4) 186 (1Hm H-4) 172 (1H dtd J = 180 79
4 Evidence-Based Complementary and Alternative Medicine
HO
OH
O
O
OHR
OOHO
OHOH
HO
OOH
O
OOH
OOHO
O
O
OHOHHO
OH
HO HO
HO
O
O
1 23
54
6 R = OH8 R = OMe
OO
OH
OH
OHHO
9
OOO
OOH
O
O
O
OH
OCH3O
HOOCH3
O
O
O
HO
O
O
O
O
7
Figure 1 Isolated compounds from Bromelia karatas 120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Cirsiliol 41015840-O-glucoside (2) 120588-Coumaric acid (3) Stigmasterol (4) 120573-Sitosterol (5) 1-O-Feruloyl-3-O-p-coumaroylglycerol (6) 120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) 1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) 2-Propyl-120573-glucopyranoside (9)
69 38Hz H-1) 168 (1Hm H-25) 141 (1H ddt J = 158 9843Hz H-20) 136 (1H m H-8) 110 (1H m H-17) 102 (1Hm H-24) 100 (3H d J = 65Hz H-21) 095 (3H s H-19)095 (1H m H-14) 092(3H d J = 73Hz H-26) 089 (3H t J= 74Hz H-29) 088 (3H d J = 69Hz H-27) 067 (3H s H-18) 13C NMR (100MHz Pyridine-d5) 120575 14091 (C-5) 12192(C-6) 10257 (C-11015840) 7859 (C-3) 7847 (C-31015840) 7812 (C-51015840)7530 (C-21015840) 7168 (C-41015840) 6283 (C-61015840) 5684 (C-14) 5625(C-17) 5035 (C-9) 4605 (C-24) 4249 (C-13) 3996 (C-4)3934 (C-12) 3749 (C-1) 3693 (C-10) 3640 (C-20) 3421 (C-22) 3219 (C-7) 3206 (C-8) 3026 (C-2) 2947 (C-25) 2856(C-16) 2639 (C-23) 2452 (C-15) 2340 (C-28) 2130 (C-11)2000 (C-27) 1943 (C-19) 1922 (C-26) 1903 (C-21) 1217 (C-29) 1199 (C-18)
Cirsiliol 41015840-O-glucoside (2) Yellow prisms HR-ESIMS ion atmz 5151204 [M+Na]+ for C23H24O12
1H NMR (400MHzPyridine-d5) 120575 796 (1H d J = 22Hz H-21015840) 772 (1H d J =86Hz H-51015840) 752 (1H dd J = 86 23Hz H-61015840) 703 (1H sH-3) 673 (1H s H-8) 580 (1H d J = 77Hz H-110158401015840) 461 (1Hdd J = 120 21 Hz H-6a
10158401015840) 444 (1H dd J = 121 53Hz H-6b10158401015840) 438 (1H t J = 89Hz H-310158401015840) 434 (1H d J = 89Hz H-
410158401015840) 426 (1H t J = 89Hz H-210158401015840) 418 (1H ddd J = 90 52
21 Hz H-510158401015840) 402 (3H s OCH3-6) 391 (s OCH3-7)13C
NMR (100MHz Pyridine-d5) 120575 18355 (C-4) 16476 (C-2)15983 (C-7) 15402 (C-9) 15391 (C-5) 15054 (C-41015840) 14900(C-31015840) 13357 (C-6) 12689 (C-11015840) 1197 (C-61015840) 11825 (C-51015840)11583 (C-21015840) 10687 (C-10) 10537 (C-3) 10354 (C-110158401015840) 9197(C-8) 7968 (C-510158401015840) 7891 (C-310158401015840) 7518 (C-210158401015840) 7157 (C-410158401015840)6269 (C-610158401015840) 6096 (6-OCH3) 5684 (7-OCH3)
120588-Coumaric Acid (3) Colorless amorphous powder ESI-MSion at mz 163 [M-H]minus for C9H8O3
1H NMR (400MHzCD3OD) 120575 750 (1H d J = 157Hz H-7) 741 (2H d J =86Hz H-2 H-6) 678 (2H d J= 86Hz H-3 H-5) 633 (1Hd J = 158Hz H-8) 13C NMR (100MHz CD3OD) 120575 17520(C-9) 15842 (C-4) 14418 (C-7) 13054 (C-2 C-6) 12801 (C-1) 11666 (C-3 C-5) 11576 (C-7)
Stigmasterol (4) White powder ESI-MS ion at mz 413[M+H]+ for C29H48O
1H NMR (500MHz CDCl3) 120575 535(1HmH-6) 515 (1H dd J = 152 80Hz H-21) 502 (1H ddJ = 152 85Hz H-20) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 084 (3Ht J = 76Hz H-24) 081 (3H d J = 68Hz H-26) 080 (3Hd J = 68Hz H-27) 068 (3H s H-28) 13C NMR (125 MHz
Evidence-Based Complementary and Alternative Medicine 5
CDCl3) 120575 14092 (C-5) 1385 (C-22) 1294 (C-23) 12186 (C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5139 (C-24) 5031(C-9) 4248 (C-4) 4238 (C-13) 4064 (C-20) 3985 (C-12)3742 (C-1) 3667 (C-10) 3208 (C-7 C-8 C-25) 3184 (C-2)2901 (C-16) 2446 (C-15) 2556 (C-28) 2137 (C-11) 2036 (C-26) 1956 (C-27) 1894 (C-21) 1894 (C-19) 1210 (C-29) 1202(C-18)
120573-Sitosterol (5) White powder ESI-MS ion at mz 415[M+H]+ for C29H50O
1H NMR (500 MHz CDCl3) 120575 535(1H d J = 53Hz H-5) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 083 (3Hd J = 68Hz H-24) 081 (3H d J = 68Hz H-26) 069 (3Hs H-28) 13C NMR (125 MHz CDCl3) 120575 14092 (C-5) 12186(C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5031 (C-9) 4601(C-24) 4248 (C-13) 4238 (C-4) 3985 (C-12) 3742 (C-1)3667 (C-10) 3631 (C-20) 3412 (C-22) 3208 (C-2) 3184 (C-7 C-8) 2933 (C-25) 2841 (C-16) 2626 (C-23) 2446 (C-15)2324 (C-28) 2137 (C-11) 1998 (C-26) 1914 (C-27) 1913 (C-19) 1894 (C-21) 1221 (C-29) 1202 (C-18)
1-O-Feruloyl-3-O-p-coumaroylglycerol (6) Colorless oil ESI-MS ion at mz 4374 [M+Na]+ for C22H22O8
1H NMR(400MHz CD3OD) 120575 766 (2H d J = 160Hz H-71015840 H-710158401015840)744 (2H d J = 87Hz H-21015840 H-61015840) 718 (1H d J = 20Hz H-210158401015840) 707 (1H dd J = 83 21 Hz H-610158401015840) 680 (2H d J = 82HzH-31015840 H-51015840) 679 (1H d J = 86Hz H-510158401015840) 639 (1H d J =142Hz H-810158401015840) 635 (1H d J = 143Hz H-81015840) 428 (4H dd J= 53 19Hz H-1 H-3) 416 (1H m H-2) 387 (3H s OCH3-310158401015840) 13CNMR (100MHz CD3OD) 120575 16901 (C-9
10158401015840) 16899 (C-91015840) 16134 (C-41015840) 15071 (C-310158401015840) 14938 (C-410158401015840) 14725 (C-710158401015840)14697 (C-71015840) 13121 (C-21015840 C-61015840) 12766 (C-110158401015840) 12711 (C-11015840)12419 (C-610158401015840) 11684 (C-31015840 C-51015840) 11648 (C-510158401015840) 11510 (C-810158401015840)11480 (C-81015840) 11175 (C-210158401015840) 6861 (C-2) 6639 (C-3) 6637 (C-1) 5644 (OCH3-3
10158401015840)
120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) Colorlessamorphous solid ESI-MS ion at mz 8852 [M+Na]+ forC40H46O21
1H NMR (500MHz CD3OD) 120575 772 (1H d J =159Hz H-710158401015840) 768 (1H d J = 159Hz H-7101584010158401015840) 728 (2H d J= 20Hz H-210158401015840) 722 (2H d J = 20Hz H-2101584010158401015840) 713 (1H dd J= 85 201Hz H-610158401015840) 711 (1H dd J = 85 19Hz H-6101584010158401015840) 683(2H d J = 82Hz H-510158401015840 H-5101584010158401015840) 646 (1H d J = 1442HzH-810158401015840) 643 (1H d J = 144Hz H-8101584010158401015840) 570 (1H d J = 38HzH-11015840) 539 (1H d J = 77Hz H-3) 480 (1H d J = 102HzH-41015840) 468 (1H dd J = 102 38Hz H-21015840) 450 (1H dd J =121 327Hz H-6a) 445 (1H m H-6b) 446 (1H t J = 79HzH-4) 426 (d J = 115Hz H-1a) 424 (1H m H-51015840) 421(d br J = 114Hz H-61015840a) 410 (1H dd J = 116 35Hz H-61015840b)409 (1H d J = 115Hz H-1b) 391 (3H s OCH3-3
101584010158401015840) 391(3H s OCH3-3
10158401015840) 388 (1H d J = 76Hz H-31015840) 213 (3H sOAc-1) 210 (3H s OAc-21015840) 203 (3H s OAc-61015840) 187 (3Hs OAc-41015840) 13C NMR (125 MHz CD3OD) 120575 17260 (OAc-6
1015840)17214 (OAc-21015840) 17202 (OAc-1) 17172 (OAc-41015840) 16877(C-9101584010158401015840) 16798 (C-910158401015840) 15112 (C-410158401015840) 15076 (C-4101584010158401015840) 14951(C-310158401015840) 14943 (C-3101584010158401015840) 14823 (C-710158401015840) 14725 (C-7101584010158401015840) 12774(C-110158401015840) 12750 (C-1101584010158401015840) 12462 (C-610158401015840) 12431 (C-6101584010158401015840) 11657(C-510158401015840) 11652 (C-5101584010158401015840) 11515 (C-8101584010158401015840) 11430 (C-810158401015840) 11183
(C-210158401015840) 11168 (C-2101584010158401015840) 10391 (C-2) 9046 (C-11015840) 8152 (C-5)7955 (C-3) 7395 (C-21015840) 7379 (C-4) 7225 (C-41015840) 7006(C-5) 6982 (C-31015840) 6661 (C-1) 6453 (C-6) 6409 (C-61015840)5656 (OCH3-3
10158401015840 OCH3-3101584010158401015840) 2092 (OAc-CH3-2
1015840) 2079(OAc-CH3-1) 2068 (OAc-CH3-6
1015840) 2062 (OAc-CH3-41015840)
Assignments of the acetate and feruloyl units at positions1 21015840 41015840 61015840 (for acetyl) and 3 6 (for feruloyl) respectivelyin the sucrose molecule were corroborated by the HMBCtwo-dimensional spectrum this showed a correlationbetween methine and methylene protons at 1 21015840 41015840 and 61015840of the sucrose with methyl protons and the carbonyl carbonof the acetyl groups (1H13C1H 426 40917202213for 1 46817214210 for 21015840 48017172187 for 41015840 42141017260203 for 61015840) and between methine and methyleneprotons at 3 and 6 of the fructose with the protons at 810158401015840and 8101584010158401015840 of the feruloyl moiety and the carbonyl carbons at910158401015840 and 9101584010158401015840 (1H13C1H 64616798539 for C-3 feruloyl64316877450 445 for C-3 feruloyl)
1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) Colorless oil ESI-MS ion at mz 3998 [M-H]minus for C21H20O8
1H NMR(400MHz CD3OD) 120575 766 (1H d J = 160Hz H-71015840) 760 (1Hd J = 159Hz H-710158401015840) 744 (2H dd J = 89 23Hz H-21015840 H-61015840)705 (1H d J = 21 Hz H-210158401015840) 694 (1H dd J = 83 20Hz H-610158401015840) 679 (2H dd J = 91 25Hz H31015840 H-51015840) 677 (1H d J =84Hz H-510158401015840) 635 (1H d J = 159Hz H-81015840) 630 (1H d J =159Hz H-810158401015840) 427 (4H d J = 54Hz H-1 H-3) 416 (1H qJ = 52Hz H-2) 13C NMR (100MHz CD3OD) d 16901 (C-91015840 C-910158401015840) 16131 (C-41015840) 14965 (C-310158401015840) 14736 (C-710158401015840) 14697(C-71015840) 14681 (C-410158401015840) 13122 (C-21015840 C-61015840) 12770 (C-110158401015840) 12713(C-11015840) 12302 (C-610158401015840) 11683 (C-31015840 C-51015840) 11651 (C-510158401015840) 11520(C-210158401015840) 11482 (C-81015840) 11476 (C-810158401015840) 6865 (C-2) 6635 (C-1C-3)
2-Propyl-120573-glucopyranoside (9) Colorless amorphous solidESI-MS ion at mz 2452 [M+Na]+ for C9H18O6
1H NMR(400 MHz CD3OD) 120575 434 (1H d J = 78Hz H-1) 404 (1Hhept J = 61 Hz H-21015840) 385 (1H dd J = 119 20Hz H-6b)366 (1H dd J = 119 54Hz H-6a) 335 (1H m H-3) 327(1H m H-4) 325 (1H m H-5) 314 (1H dd J = 91 78HzH-2) 123 (3H d J = 62Hz CH3-1
1015840) 119 (3H d J = 61HzCH3-3
1015840) 13C NMR (100MHz CD3OD) 120575 10255 (C-1) 7811(C-3) 7786 (C-5) 7514 (C-2) 7258 (C-21015840) 7170 (C-4) 6279(C-6) 2381 (C-11015840) 2204 (C-31015840)
33 Chromatographic Profiles In the HPLC profiles of theWE and the OE of B karatas (Figure 1) the majority ofcomponents showed maximum absorption between 280 and320 nmThe two chromatograms were qualitatively differenthowever the main components of WE [TR 133 (3) 142 and219 (2)min] were also observed in the OE [TR 133 (3) 142and 219 (2)min] but in different proportion Compounds 45 and 9 were not observed in any of the chromatographicprofiles Figure 2
Compound 1 was not observed as a major compound inthe profile of the WE (Figure 2(a)) because it precipitatedduring the process of sample preparation before the samplewas injected into the HPLC equipment its remnant was
6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

Evidence-Based Complementary and Alternative Medicine 3
solvent A and acetonitrile (MeCN) as solvent B using agradient elution of 1000 (AB) for 1min 955 (AB) at 15min8515 (AB) at 15-12min 7030 (AB) at 12-20min 7030 (AB)maintained during 1min 6040 (AB) at 21-23min 2080(AB) at 23-27min 1090 (AB) at 27-28min 1090 (AB)maintained during 1min and 1000 (AB) at 29-31min Theflow rate was set to 025mLmin and the temperature of thecolumn was maintained at 35∘C System control data collec-tion and data processing were accomplished usingOpenLABLC 1260 chromatography software Working solutions ofsamples (WE OE fractions and isolated compounds) of Bkaratas were prepared by dissolving 50mg of the sample in1mL of the appropriate solvent (MeOH MeCN or H2O) andinjected (2 120583L) using an autosampler For UV detection thewavelength program was set at an acquisition of 120582 230 254280 320 and 365 nm 320 nm was selected as the optimumwavelength The equipment data acquisition processingand management of the chromatographic information werecontrolled with the OpenLab CDS ChemStatiob Edition(2001-2013) software The separation was carried out usinga Phenomenex Luna Polar C18 (50 x 21mm id 16 120583m)reverse phase column all solvents were purchased from JTBaker as HPLC grade
25 Isolation Compounds WE (1 g) was dissolved in a mix-ture ofMeOHH2O (11) from this solution 241mg of 1 spon-taneously precipitated The remainder of the WE was furtherseparated on Sephadex (500mL MeOHH2O 11) yielding 14primary fractions (WE1-WE14) WE9 (43mg) was resolvedby HPLC (Nucleosil 250 times 10mm id 5 120583m C18 Macherey-Nagel) using a 15-min gradient of MeCNMeOHH2O(60535) (23mlmin 254 and 320 nm) yielding 6 mg ofa solid yellow amorphous powder (2) with RT 953 minWE13 (465mg) was subjected to preparative TLC (EtOAcn-hexaneacetone 721) to yield compound 3 (12mg)
The OE (842 g) was partitioned by column chromatog-raphy (CC) on 336 g of silica gel (70-230mesh Merck)usingmixtures of n-hexaneCH2Cl2MeOH as eluent Elutionwas started with 100 n-hexane the polarity of the eluentwas then increased by the addition of increasing amountsof CH2Cl2 (to 100) and then MeOH This yielded 28primary fractions (OE1-OE28) Fraction OE11 (10 g) wassubjected to silica gel CC (40 g) using mixtures of n-hexaneCH2Cl2MeOH as eluent Elution was started with100 n-hexane and the polarity of the eluent was increasedby the gradual addition of CH2Cl2 (to 100) and thenMeOH This resulted in 10 subfractions (OE111-OE1110)OE1110 (40 mg) yielded a mixture of 4 and 5 OE16 (279mg)was processed with CC on silica gel (11 g) using mixturesof EtOAcMeOH as eluent starting with 100 EtOAc andincreasing the polarity with MeOH to 100 this processyielded 9 subfractions (OE161-OE169) Preparative TLC ofOE164 (32mg eluted with n-hexaneEtOAc 19) and OE17(76mg eluted with n-hexaneEtOAc 19) resulted in theisolation of 6 (10mg) and 7 (15mg) respectively FractionOE19 (347mg) was subjected to CC on silica gel (15 g)elution was started with 100 EtOAc and the polarity ofthe solvent was increased by the addition of methanol up to100 using this process it was possible to isolate compound
8 (18mg) Preparative TLC (EtOAcMeOHH2O 81505)of fraction OE22 (97mg) resulted in the isolation of 9(12mg) Finally EO23 (122mg) and EO24 (434mg) weredissolved in methanol and compounds 2 (25mg) and 1(10mg) respectively were obtained as precipitates
26 Identification of the Isolated Compounds in the Chromato-graphic Profile ofWE Isolated compounds (1-9) and extractsprepared from B karatas (WE andOE) were injected into theHPLC for identification of the compounds in the chromato-graphic profiles Peak assignments were made on the basis ofthe previously developed structure-diagnostic and supportedby examination of the UV spectrum and relative retentiontime using OpenLAB LC 1260 chromatography software
3 Results
31 Ethnobotanical Results In the field we confirmed theprevious finding that the healer ldquoIsabel Escalanterdquo and thediabetic patients of the town of Tamala use a decoction of 30 gof the dry leaves of B karatas boiled in 1 liter of water Theinfusion is cooled and drunk over the day as ldquoAgua de UsordquoThe common name of the plant in this region is ldquotimbiricherdquoAlternatively the juice of the fruit is used
32 Phytochemical Analysis The chromatographic profileswere developed to monitor the major components of WEand OE from B karatas and to make it possible to iso-late and identify them by means of various conventionalchromatographic and spectroscopic techniques The anal-ysis led to the isolation of compounds 1-9 which wereidentified based on comparison of their 1H and 13C-NMR spectral data including data obtained in 2D experi-ments (COSY HSQC HMBC NOESY TOCSY) and theirmass spectral data with those of previously describedcompounds The major compounds were identified as asteroidal glycoside [120573-sitosterol-3-O-120573-D-glucopyranoside(1)] [9] and a phenolic acid [120588-coumaric acid (3)] [10]both compounds were isolated from the traditional extract(WE) The flavonoid [cirsiliol 41015840-O-120573-D-glucopyranoside(2)] [11] from WE along with two steroidal aglycones[stigmasterol (4) and 120573-sitosterol (5)] [12] two phenyl-propanoid glycerols [1-O-feruloyl-3-O-120588-coumaroylglycerol(6) and 1-O-120588-coumaroyl-3-O-caffeoylglycerol (8)] [13]a phenylpropanoid glycoside [120573-D-(1-O-acetyl-36-O-trans-diferuloyl)fructofuranosyl-120572-D-210158404101584061015840-O triacetylglucopy-ranoside (7)] [14] and 2-propyl-D-glucopyranoside (9) [15]from OE were some of the minor compounds Figure 1 Thecomplete spectroscopic data of the isolated compounds arepresented below
120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Colorless powderFAB-MSmz 577 [M+H]+ for C35H60O6 EI-MSmz 414 [M-C6H10O5]
+ 1H NMR (400MHz Pyridine-d5) 120575 536 (1H tJ = 26Hz H-6) 507 (1H d J = 78Hz H-11015840) 458 (1H dd J= 118 25Hz H-61015840) 443 (1H dd J = 118 51 Hz H-61015840) 430(2H m H-31015840 and H-41015840) 408 (1H t J = 82Hz H-21015840) 399(2H m H-3 and H-51015840) 275 (1H ddd J = 135 48 22HzH-12) 250 (1H m H-12) 215 (2H m H-2) 199 (1H dd J =126 35Hz H-4) 186 (1Hm H-4) 172 (1H dtd J = 180 79
4 Evidence-Based Complementary and Alternative Medicine
HO
OH
O
O
OHR
OOHO
OHOH
HO
OOH
O
OOH
OOHO
O
O
OHOHHO
OH
HO HO
HO
O
O
1 23
54
6 R = OH8 R = OMe
OO
OH
OH
OHHO
9
OOO
OOH
O
O
O
OH
OCH3O
HOOCH3
O
O
O
HO
O
O
O
O
7
Figure 1 Isolated compounds from Bromelia karatas 120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Cirsiliol 41015840-O-glucoside (2) 120588-Coumaric acid (3) Stigmasterol (4) 120573-Sitosterol (5) 1-O-Feruloyl-3-O-p-coumaroylglycerol (6) 120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) 1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) 2-Propyl-120573-glucopyranoside (9)
69 38Hz H-1) 168 (1Hm H-25) 141 (1H ddt J = 158 9843Hz H-20) 136 (1H m H-8) 110 (1H m H-17) 102 (1Hm H-24) 100 (3H d J = 65Hz H-21) 095 (3H s H-19)095 (1H m H-14) 092(3H d J = 73Hz H-26) 089 (3H t J= 74Hz H-29) 088 (3H d J = 69Hz H-27) 067 (3H s H-18) 13C NMR (100MHz Pyridine-d5) 120575 14091 (C-5) 12192(C-6) 10257 (C-11015840) 7859 (C-3) 7847 (C-31015840) 7812 (C-51015840)7530 (C-21015840) 7168 (C-41015840) 6283 (C-61015840) 5684 (C-14) 5625(C-17) 5035 (C-9) 4605 (C-24) 4249 (C-13) 3996 (C-4)3934 (C-12) 3749 (C-1) 3693 (C-10) 3640 (C-20) 3421 (C-22) 3219 (C-7) 3206 (C-8) 3026 (C-2) 2947 (C-25) 2856(C-16) 2639 (C-23) 2452 (C-15) 2340 (C-28) 2130 (C-11)2000 (C-27) 1943 (C-19) 1922 (C-26) 1903 (C-21) 1217 (C-29) 1199 (C-18)
Cirsiliol 41015840-O-glucoside (2) Yellow prisms HR-ESIMS ion atmz 5151204 [M+Na]+ for C23H24O12
1H NMR (400MHzPyridine-d5) 120575 796 (1H d J = 22Hz H-21015840) 772 (1H d J =86Hz H-51015840) 752 (1H dd J = 86 23Hz H-61015840) 703 (1H sH-3) 673 (1H s H-8) 580 (1H d J = 77Hz H-110158401015840) 461 (1Hdd J = 120 21 Hz H-6a
10158401015840) 444 (1H dd J = 121 53Hz H-6b10158401015840) 438 (1H t J = 89Hz H-310158401015840) 434 (1H d J = 89Hz H-
410158401015840) 426 (1H t J = 89Hz H-210158401015840) 418 (1H ddd J = 90 52
21 Hz H-510158401015840) 402 (3H s OCH3-6) 391 (s OCH3-7)13C
NMR (100MHz Pyridine-d5) 120575 18355 (C-4) 16476 (C-2)15983 (C-7) 15402 (C-9) 15391 (C-5) 15054 (C-41015840) 14900(C-31015840) 13357 (C-6) 12689 (C-11015840) 1197 (C-61015840) 11825 (C-51015840)11583 (C-21015840) 10687 (C-10) 10537 (C-3) 10354 (C-110158401015840) 9197(C-8) 7968 (C-510158401015840) 7891 (C-310158401015840) 7518 (C-210158401015840) 7157 (C-410158401015840)6269 (C-610158401015840) 6096 (6-OCH3) 5684 (7-OCH3)
120588-Coumaric Acid (3) Colorless amorphous powder ESI-MSion at mz 163 [M-H]minus for C9H8O3
1H NMR (400MHzCD3OD) 120575 750 (1H d J = 157Hz H-7) 741 (2H d J =86Hz H-2 H-6) 678 (2H d J= 86Hz H-3 H-5) 633 (1Hd J = 158Hz H-8) 13C NMR (100MHz CD3OD) 120575 17520(C-9) 15842 (C-4) 14418 (C-7) 13054 (C-2 C-6) 12801 (C-1) 11666 (C-3 C-5) 11576 (C-7)
Stigmasterol (4) White powder ESI-MS ion at mz 413[M+H]+ for C29H48O
1H NMR (500MHz CDCl3) 120575 535(1HmH-6) 515 (1H dd J = 152 80Hz H-21) 502 (1H ddJ = 152 85Hz H-20) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 084 (3Ht J = 76Hz H-24) 081 (3H d J = 68Hz H-26) 080 (3Hd J = 68Hz H-27) 068 (3H s H-28) 13C NMR (125 MHz
Evidence-Based Complementary and Alternative Medicine 5
CDCl3) 120575 14092 (C-5) 1385 (C-22) 1294 (C-23) 12186 (C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5139 (C-24) 5031(C-9) 4248 (C-4) 4238 (C-13) 4064 (C-20) 3985 (C-12)3742 (C-1) 3667 (C-10) 3208 (C-7 C-8 C-25) 3184 (C-2)2901 (C-16) 2446 (C-15) 2556 (C-28) 2137 (C-11) 2036 (C-26) 1956 (C-27) 1894 (C-21) 1894 (C-19) 1210 (C-29) 1202(C-18)
120573-Sitosterol (5) White powder ESI-MS ion at mz 415[M+H]+ for C29H50O
1H NMR (500 MHz CDCl3) 120575 535(1H d J = 53Hz H-5) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 083 (3Hd J = 68Hz H-24) 081 (3H d J = 68Hz H-26) 069 (3Hs H-28) 13C NMR (125 MHz CDCl3) 120575 14092 (C-5) 12186(C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5031 (C-9) 4601(C-24) 4248 (C-13) 4238 (C-4) 3985 (C-12) 3742 (C-1)3667 (C-10) 3631 (C-20) 3412 (C-22) 3208 (C-2) 3184 (C-7 C-8) 2933 (C-25) 2841 (C-16) 2626 (C-23) 2446 (C-15)2324 (C-28) 2137 (C-11) 1998 (C-26) 1914 (C-27) 1913 (C-19) 1894 (C-21) 1221 (C-29) 1202 (C-18)
1-O-Feruloyl-3-O-p-coumaroylglycerol (6) Colorless oil ESI-MS ion at mz 4374 [M+Na]+ for C22H22O8
1H NMR(400MHz CD3OD) 120575 766 (2H d J = 160Hz H-71015840 H-710158401015840)744 (2H d J = 87Hz H-21015840 H-61015840) 718 (1H d J = 20Hz H-210158401015840) 707 (1H dd J = 83 21 Hz H-610158401015840) 680 (2H d J = 82HzH-31015840 H-51015840) 679 (1H d J = 86Hz H-510158401015840) 639 (1H d J =142Hz H-810158401015840) 635 (1H d J = 143Hz H-81015840) 428 (4H dd J= 53 19Hz H-1 H-3) 416 (1H m H-2) 387 (3H s OCH3-310158401015840) 13CNMR (100MHz CD3OD) 120575 16901 (C-9
10158401015840) 16899 (C-91015840) 16134 (C-41015840) 15071 (C-310158401015840) 14938 (C-410158401015840) 14725 (C-710158401015840)14697 (C-71015840) 13121 (C-21015840 C-61015840) 12766 (C-110158401015840) 12711 (C-11015840)12419 (C-610158401015840) 11684 (C-31015840 C-51015840) 11648 (C-510158401015840) 11510 (C-810158401015840)11480 (C-81015840) 11175 (C-210158401015840) 6861 (C-2) 6639 (C-3) 6637 (C-1) 5644 (OCH3-3
10158401015840)
120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) Colorlessamorphous solid ESI-MS ion at mz 8852 [M+Na]+ forC40H46O21
1H NMR (500MHz CD3OD) 120575 772 (1H d J =159Hz H-710158401015840) 768 (1H d J = 159Hz H-7101584010158401015840) 728 (2H d J= 20Hz H-210158401015840) 722 (2H d J = 20Hz H-2101584010158401015840) 713 (1H dd J= 85 201Hz H-610158401015840) 711 (1H dd J = 85 19Hz H-6101584010158401015840) 683(2H d J = 82Hz H-510158401015840 H-5101584010158401015840) 646 (1H d J = 1442HzH-810158401015840) 643 (1H d J = 144Hz H-8101584010158401015840) 570 (1H d J = 38HzH-11015840) 539 (1H d J = 77Hz H-3) 480 (1H d J = 102HzH-41015840) 468 (1H dd J = 102 38Hz H-21015840) 450 (1H dd J =121 327Hz H-6a) 445 (1H m H-6b) 446 (1H t J = 79HzH-4) 426 (d J = 115Hz H-1a) 424 (1H m H-51015840) 421(d br J = 114Hz H-61015840a) 410 (1H dd J = 116 35Hz H-61015840b)409 (1H d J = 115Hz H-1b) 391 (3H s OCH3-3
101584010158401015840) 391(3H s OCH3-3
10158401015840) 388 (1H d J = 76Hz H-31015840) 213 (3H sOAc-1) 210 (3H s OAc-21015840) 203 (3H s OAc-61015840) 187 (3Hs OAc-41015840) 13C NMR (125 MHz CD3OD) 120575 17260 (OAc-6
1015840)17214 (OAc-21015840) 17202 (OAc-1) 17172 (OAc-41015840) 16877(C-9101584010158401015840) 16798 (C-910158401015840) 15112 (C-410158401015840) 15076 (C-4101584010158401015840) 14951(C-310158401015840) 14943 (C-3101584010158401015840) 14823 (C-710158401015840) 14725 (C-7101584010158401015840) 12774(C-110158401015840) 12750 (C-1101584010158401015840) 12462 (C-610158401015840) 12431 (C-6101584010158401015840) 11657(C-510158401015840) 11652 (C-5101584010158401015840) 11515 (C-8101584010158401015840) 11430 (C-810158401015840) 11183
(C-210158401015840) 11168 (C-2101584010158401015840) 10391 (C-2) 9046 (C-11015840) 8152 (C-5)7955 (C-3) 7395 (C-21015840) 7379 (C-4) 7225 (C-41015840) 7006(C-5) 6982 (C-31015840) 6661 (C-1) 6453 (C-6) 6409 (C-61015840)5656 (OCH3-3
10158401015840 OCH3-3101584010158401015840) 2092 (OAc-CH3-2
1015840) 2079(OAc-CH3-1) 2068 (OAc-CH3-6
1015840) 2062 (OAc-CH3-41015840)
Assignments of the acetate and feruloyl units at positions1 21015840 41015840 61015840 (for acetyl) and 3 6 (for feruloyl) respectivelyin the sucrose molecule were corroborated by the HMBCtwo-dimensional spectrum this showed a correlationbetween methine and methylene protons at 1 21015840 41015840 and 61015840of the sucrose with methyl protons and the carbonyl carbonof the acetyl groups (1H13C1H 426 40917202213for 1 46817214210 for 21015840 48017172187 for 41015840 42141017260203 for 61015840) and between methine and methyleneprotons at 3 and 6 of the fructose with the protons at 810158401015840and 8101584010158401015840 of the feruloyl moiety and the carbonyl carbons at910158401015840 and 9101584010158401015840 (1H13C1H 64616798539 for C-3 feruloyl64316877450 445 for C-3 feruloyl)
1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) Colorless oil ESI-MS ion at mz 3998 [M-H]minus for C21H20O8
1H NMR(400MHz CD3OD) 120575 766 (1H d J = 160Hz H-71015840) 760 (1Hd J = 159Hz H-710158401015840) 744 (2H dd J = 89 23Hz H-21015840 H-61015840)705 (1H d J = 21 Hz H-210158401015840) 694 (1H dd J = 83 20Hz H-610158401015840) 679 (2H dd J = 91 25Hz H31015840 H-51015840) 677 (1H d J =84Hz H-510158401015840) 635 (1H d J = 159Hz H-81015840) 630 (1H d J =159Hz H-810158401015840) 427 (4H d J = 54Hz H-1 H-3) 416 (1H qJ = 52Hz H-2) 13C NMR (100MHz CD3OD) d 16901 (C-91015840 C-910158401015840) 16131 (C-41015840) 14965 (C-310158401015840) 14736 (C-710158401015840) 14697(C-71015840) 14681 (C-410158401015840) 13122 (C-21015840 C-61015840) 12770 (C-110158401015840) 12713(C-11015840) 12302 (C-610158401015840) 11683 (C-31015840 C-51015840) 11651 (C-510158401015840) 11520(C-210158401015840) 11482 (C-81015840) 11476 (C-810158401015840) 6865 (C-2) 6635 (C-1C-3)
2-Propyl-120573-glucopyranoside (9) Colorless amorphous solidESI-MS ion at mz 2452 [M+Na]+ for C9H18O6
1H NMR(400 MHz CD3OD) 120575 434 (1H d J = 78Hz H-1) 404 (1Hhept J = 61 Hz H-21015840) 385 (1H dd J = 119 20Hz H-6b)366 (1H dd J = 119 54Hz H-6a) 335 (1H m H-3) 327(1H m H-4) 325 (1H m H-5) 314 (1H dd J = 91 78HzH-2) 123 (3H d J = 62Hz CH3-1
1015840) 119 (3H d J = 61HzCH3-3
1015840) 13C NMR (100MHz CD3OD) 120575 10255 (C-1) 7811(C-3) 7786 (C-5) 7514 (C-2) 7258 (C-21015840) 7170 (C-4) 6279(C-6) 2381 (C-11015840) 2204 (C-31015840)
33 Chromatographic Profiles In the HPLC profiles of theWE and the OE of B karatas (Figure 1) the majority ofcomponents showed maximum absorption between 280 and320 nmThe two chromatograms were qualitatively differenthowever the main components of WE [TR 133 (3) 142 and219 (2)min] were also observed in the OE [TR 133 (3) 142and 219 (2)min] but in different proportion Compounds 45 and 9 were not observed in any of the chromatographicprofiles Figure 2
Compound 1 was not observed as a major compound inthe profile of the WE (Figure 2(a)) because it precipitatedduring the process of sample preparation before the samplewas injected into the HPLC equipment its remnant was
6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

4 Evidence-Based Complementary and Alternative Medicine
HO
OH
O
O
OHR
OOHO
OHOH
HO
OOH
O
OOH
OOHO
O
O
OHOHHO
OH
HO HO
HO
O
O
1 23
54
6 R = OH8 R = OMe
OO
OH
OH
OHHO
9
OOO
OOH
O
O
O
OH
OCH3O
HOOCH3
O
O
O
HO
O
O
O
O
7
Figure 1 Isolated compounds from Bromelia karatas 120573-Sitosterol-3-O-120573-D-glucopyranoside (1) Cirsiliol 41015840-O-glucoside (2) 120588-Coumaric acid (3) Stigmasterol (4) 120573-Sitosterol (5) 1-O-Feruloyl-3-O-p-coumaroylglycerol (6) 120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) 1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) 2-Propyl-120573-glucopyranoside (9)
69 38Hz H-1) 168 (1Hm H-25) 141 (1H ddt J = 158 9843Hz H-20) 136 (1H m H-8) 110 (1H m H-17) 102 (1Hm H-24) 100 (3H d J = 65Hz H-21) 095 (3H s H-19)095 (1H m H-14) 092(3H d J = 73Hz H-26) 089 (3H t J= 74Hz H-29) 088 (3H d J = 69Hz H-27) 067 (3H s H-18) 13C NMR (100MHz Pyridine-d5) 120575 14091 (C-5) 12192(C-6) 10257 (C-11015840) 7859 (C-3) 7847 (C-31015840) 7812 (C-51015840)7530 (C-21015840) 7168 (C-41015840) 6283 (C-61015840) 5684 (C-14) 5625(C-17) 5035 (C-9) 4605 (C-24) 4249 (C-13) 3996 (C-4)3934 (C-12) 3749 (C-1) 3693 (C-10) 3640 (C-20) 3421 (C-22) 3219 (C-7) 3206 (C-8) 3026 (C-2) 2947 (C-25) 2856(C-16) 2639 (C-23) 2452 (C-15) 2340 (C-28) 2130 (C-11)2000 (C-27) 1943 (C-19) 1922 (C-26) 1903 (C-21) 1217 (C-29) 1199 (C-18)
Cirsiliol 41015840-O-glucoside (2) Yellow prisms HR-ESIMS ion atmz 5151204 [M+Na]+ for C23H24O12
1H NMR (400MHzPyridine-d5) 120575 796 (1H d J = 22Hz H-21015840) 772 (1H d J =86Hz H-51015840) 752 (1H dd J = 86 23Hz H-61015840) 703 (1H sH-3) 673 (1H s H-8) 580 (1H d J = 77Hz H-110158401015840) 461 (1Hdd J = 120 21 Hz H-6a
10158401015840) 444 (1H dd J = 121 53Hz H-6b10158401015840) 438 (1H t J = 89Hz H-310158401015840) 434 (1H d J = 89Hz H-
410158401015840) 426 (1H t J = 89Hz H-210158401015840) 418 (1H ddd J = 90 52
21 Hz H-510158401015840) 402 (3H s OCH3-6) 391 (s OCH3-7)13C
NMR (100MHz Pyridine-d5) 120575 18355 (C-4) 16476 (C-2)15983 (C-7) 15402 (C-9) 15391 (C-5) 15054 (C-41015840) 14900(C-31015840) 13357 (C-6) 12689 (C-11015840) 1197 (C-61015840) 11825 (C-51015840)11583 (C-21015840) 10687 (C-10) 10537 (C-3) 10354 (C-110158401015840) 9197(C-8) 7968 (C-510158401015840) 7891 (C-310158401015840) 7518 (C-210158401015840) 7157 (C-410158401015840)6269 (C-610158401015840) 6096 (6-OCH3) 5684 (7-OCH3)
120588-Coumaric Acid (3) Colorless amorphous powder ESI-MSion at mz 163 [M-H]minus for C9H8O3
1H NMR (400MHzCD3OD) 120575 750 (1H d J = 157Hz H-7) 741 (2H d J =86Hz H-2 H-6) 678 (2H d J= 86Hz H-3 H-5) 633 (1Hd J = 158Hz H-8) 13C NMR (100MHz CD3OD) 120575 17520(C-9) 15842 (C-4) 14418 (C-7) 13054 (C-2 C-6) 12801 (C-1) 11666 (C-3 C-5) 11576 (C-7)
Stigmasterol (4) White powder ESI-MS ion at mz 413[M+H]+ for C29H48O
1H NMR (500MHz CDCl3) 120575 535(1HmH-6) 515 (1H dd J = 152 80Hz H-21) 502 (1H ddJ = 152 85Hz H-20) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 084 (3Ht J = 76Hz H-24) 081 (3H d J = 68Hz H-26) 080 (3Hd J = 68Hz H-27) 068 (3H s H-28) 13C NMR (125 MHz
Evidence-Based Complementary and Alternative Medicine 5
CDCl3) 120575 14092 (C-5) 1385 (C-22) 1294 (C-23) 12186 (C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5139 (C-24) 5031(C-9) 4248 (C-4) 4238 (C-13) 4064 (C-20) 3985 (C-12)3742 (C-1) 3667 (C-10) 3208 (C-7 C-8 C-25) 3184 (C-2)2901 (C-16) 2446 (C-15) 2556 (C-28) 2137 (C-11) 2036 (C-26) 1956 (C-27) 1894 (C-21) 1894 (C-19) 1210 (C-29) 1202(C-18)
120573-Sitosterol (5) White powder ESI-MS ion at mz 415[M+H]+ for C29H50O
1H NMR (500 MHz CDCl3) 120575 535(1H d J = 53Hz H-5) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 083 (3Hd J = 68Hz H-24) 081 (3H d J = 68Hz H-26) 069 (3Hs H-28) 13C NMR (125 MHz CDCl3) 120575 14092 (C-5) 12186(C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5031 (C-9) 4601(C-24) 4248 (C-13) 4238 (C-4) 3985 (C-12) 3742 (C-1)3667 (C-10) 3631 (C-20) 3412 (C-22) 3208 (C-2) 3184 (C-7 C-8) 2933 (C-25) 2841 (C-16) 2626 (C-23) 2446 (C-15)2324 (C-28) 2137 (C-11) 1998 (C-26) 1914 (C-27) 1913 (C-19) 1894 (C-21) 1221 (C-29) 1202 (C-18)
1-O-Feruloyl-3-O-p-coumaroylglycerol (6) Colorless oil ESI-MS ion at mz 4374 [M+Na]+ for C22H22O8
1H NMR(400MHz CD3OD) 120575 766 (2H d J = 160Hz H-71015840 H-710158401015840)744 (2H d J = 87Hz H-21015840 H-61015840) 718 (1H d J = 20Hz H-210158401015840) 707 (1H dd J = 83 21 Hz H-610158401015840) 680 (2H d J = 82HzH-31015840 H-51015840) 679 (1H d J = 86Hz H-510158401015840) 639 (1H d J =142Hz H-810158401015840) 635 (1H d J = 143Hz H-81015840) 428 (4H dd J= 53 19Hz H-1 H-3) 416 (1H m H-2) 387 (3H s OCH3-310158401015840) 13CNMR (100MHz CD3OD) 120575 16901 (C-9
10158401015840) 16899 (C-91015840) 16134 (C-41015840) 15071 (C-310158401015840) 14938 (C-410158401015840) 14725 (C-710158401015840)14697 (C-71015840) 13121 (C-21015840 C-61015840) 12766 (C-110158401015840) 12711 (C-11015840)12419 (C-610158401015840) 11684 (C-31015840 C-51015840) 11648 (C-510158401015840) 11510 (C-810158401015840)11480 (C-81015840) 11175 (C-210158401015840) 6861 (C-2) 6639 (C-3) 6637 (C-1) 5644 (OCH3-3
10158401015840)
120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) Colorlessamorphous solid ESI-MS ion at mz 8852 [M+Na]+ forC40H46O21
1H NMR (500MHz CD3OD) 120575 772 (1H d J =159Hz H-710158401015840) 768 (1H d J = 159Hz H-7101584010158401015840) 728 (2H d J= 20Hz H-210158401015840) 722 (2H d J = 20Hz H-2101584010158401015840) 713 (1H dd J= 85 201Hz H-610158401015840) 711 (1H dd J = 85 19Hz H-6101584010158401015840) 683(2H d J = 82Hz H-510158401015840 H-5101584010158401015840) 646 (1H d J = 1442HzH-810158401015840) 643 (1H d J = 144Hz H-8101584010158401015840) 570 (1H d J = 38HzH-11015840) 539 (1H d J = 77Hz H-3) 480 (1H d J = 102HzH-41015840) 468 (1H dd J = 102 38Hz H-21015840) 450 (1H dd J =121 327Hz H-6a) 445 (1H m H-6b) 446 (1H t J = 79HzH-4) 426 (d J = 115Hz H-1a) 424 (1H m H-51015840) 421(d br J = 114Hz H-61015840a) 410 (1H dd J = 116 35Hz H-61015840b)409 (1H d J = 115Hz H-1b) 391 (3H s OCH3-3
101584010158401015840) 391(3H s OCH3-3
10158401015840) 388 (1H d J = 76Hz H-31015840) 213 (3H sOAc-1) 210 (3H s OAc-21015840) 203 (3H s OAc-61015840) 187 (3Hs OAc-41015840) 13C NMR (125 MHz CD3OD) 120575 17260 (OAc-6
1015840)17214 (OAc-21015840) 17202 (OAc-1) 17172 (OAc-41015840) 16877(C-9101584010158401015840) 16798 (C-910158401015840) 15112 (C-410158401015840) 15076 (C-4101584010158401015840) 14951(C-310158401015840) 14943 (C-3101584010158401015840) 14823 (C-710158401015840) 14725 (C-7101584010158401015840) 12774(C-110158401015840) 12750 (C-1101584010158401015840) 12462 (C-610158401015840) 12431 (C-6101584010158401015840) 11657(C-510158401015840) 11652 (C-5101584010158401015840) 11515 (C-8101584010158401015840) 11430 (C-810158401015840) 11183
(C-210158401015840) 11168 (C-2101584010158401015840) 10391 (C-2) 9046 (C-11015840) 8152 (C-5)7955 (C-3) 7395 (C-21015840) 7379 (C-4) 7225 (C-41015840) 7006(C-5) 6982 (C-31015840) 6661 (C-1) 6453 (C-6) 6409 (C-61015840)5656 (OCH3-3
10158401015840 OCH3-3101584010158401015840) 2092 (OAc-CH3-2
1015840) 2079(OAc-CH3-1) 2068 (OAc-CH3-6
1015840) 2062 (OAc-CH3-41015840)
Assignments of the acetate and feruloyl units at positions1 21015840 41015840 61015840 (for acetyl) and 3 6 (for feruloyl) respectivelyin the sucrose molecule were corroborated by the HMBCtwo-dimensional spectrum this showed a correlationbetween methine and methylene protons at 1 21015840 41015840 and 61015840of the sucrose with methyl protons and the carbonyl carbonof the acetyl groups (1H13C1H 426 40917202213for 1 46817214210 for 21015840 48017172187 for 41015840 42141017260203 for 61015840) and between methine and methyleneprotons at 3 and 6 of the fructose with the protons at 810158401015840and 8101584010158401015840 of the feruloyl moiety and the carbonyl carbons at910158401015840 and 9101584010158401015840 (1H13C1H 64616798539 for C-3 feruloyl64316877450 445 for C-3 feruloyl)
1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) Colorless oil ESI-MS ion at mz 3998 [M-H]minus for C21H20O8
1H NMR(400MHz CD3OD) 120575 766 (1H d J = 160Hz H-71015840) 760 (1Hd J = 159Hz H-710158401015840) 744 (2H dd J = 89 23Hz H-21015840 H-61015840)705 (1H d J = 21 Hz H-210158401015840) 694 (1H dd J = 83 20Hz H-610158401015840) 679 (2H dd J = 91 25Hz H31015840 H-51015840) 677 (1H d J =84Hz H-510158401015840) 635 (1H d J = 159Hz H-81015840) 630 (1H d J =159Hz H-810158401015840) 427 (4H d J = 54Hz H-1 H-3) 416 (1H qJ = 52Hz H-2) 13C NMR (100MHz CD3OD) d 16901 (C-91015840 C-910158401015840) 16131 (C-41015840) 14965 (C-310158401015840) 14736 (C-710158401015840) 14697(C-71015840) 14681 (C-410158401015840) 13122 (C-21015840 C-61015840) 12770 (C-110158401015840) 12713(C-11015840) 12302 (C-610158401015840) 11683 (C-31015840 C-51015840) 11651 (C-510158401015840) 11520(C-210158401015840) 11482 (C-81015840) 11476 (C-810158401015840) 6865 (C-2) 6635 (C-1C-3)
2-Propyl-120573-glucopyranoside (9) Colorless amorphous solidESI-MS ion at mz 2452 [M+Na]+ for C9H18O6
1H NMR(400 MHz CD3OD) 120575 434 (1H d J = 78Hz H-1) 404 (1Hhept J = 61 Hz H-21015840) 385 (1H dd J = 119 20Hz H-6b)366 (1H dd J = 119 54Hz H-6a) 335 (1H m H-3) 327(1H m H-4) 325 (1H m H-5) 314 (1H dd J = 91 78HzH-2) 123 (3H d J = 62Hz CH3-1
1015840) 119 (3H d J = 61HzCH3-3
1015840) 13C NMR (100MHz CD3OD) 120575 10255 (C-1) 7811(C-3) 7786 (C-5) 7514 (C-2) 7258 (C-21015840) 7170 (C-4) 6279(C-6) 2381 (C-11015840) 2204 (C-31015840)
33 Chromatographic Profiles In the HPLC profiles of theWE and the OE of B karatas (Figure 1) the majority ofcomponents showed maximum absorption between 280 and320 nmThe two chromatograms were qualitatively differenthowever the main components of WE [TR 133 (3) 142 and219 (2)min] were also observed in the OE [TR 133 (3) 142and 219 (2)min] but in different proportion Compounds 45 and 9 were not observed in any of the chromatographicprofiles Figure 2
Compound 1 was not observed as a major compound inthe profile of the WE (Figure 2(a)) because it precipitatedduring the process of sample preparation before the samplewas injected into the HPLC equipment its remnant was
6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

Evidence-Based Complementary and Alternative Medicine 5
CDCl3) 120575 14092 (C-5) 1385 (C-22) 1294 (C-23) 12186 (C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5139 (C-24) 5031(C-9) 4248 (C-4) 4238 (C-13) 4064 (C-20) 3985 (C-12)3742 (C-1) 3667 (C-10) 3208 (C-7 C-8 C-25) 3184 (C-2)2901 (C-16) 2446 (C-15) 2556 (C-28) 2137 (C-11) 2036 (C-26) 1956 (C-27) 1894 (C-21) 1894 (C-19) 1210 (C-29) 1202(C-18)
120573-Sitosterol (5) White powder ESI-MS ion at mz 415[M+H]+ for C29H50O
1H NMR (500 MHz CDCl3) 120575 535(1H d J = 53Hz H-5) 352 (1H tdd J = 109 55 41 Hz H-3) 101 (3H s H-29) 092 (3H d J = 66Hz H-19) 083 (3Hd J = 68Hz H-24) 081 (3H d J = 68Hz H-26) 069 (3Hs H-28) 13C NMR (125 MHz CDCl3) 120575 14092 (C-5) 12186(C-6) 7197 (C-3) 5703 (C-14) 5613 (C-17) 5031 (C-9) 4601(C-24) 4248 (C-13) 4238 (C-4) 3985 (C-12) 3742 (C-1)3667 (C-10) 3631 (C-20) 3412 (C-22) 3208 (C-2) 3184 (C-7 C-8) 2933 (C-25) 2841 (C-16) 2626 (C-23) 2446 (C-15)2324 (C-28) 2137 (C-11) 1998 (C-26) 1914 (C-27) 1913 (C-19) 1894 (C-21) 1221 (C-29) 1202 (C-18)
1-O-Feruloyl-3-O-p-coumaroylglycerol (6) Colorless oil ESI-MS ion at mz 4374 [M+Na]+ for C22H22O8
1H NMR(400MHz CD3OD) 120575 766 (2H d J = 160Hz H-71015840 H-710158401015840)744 (2H d J = 87Hz H-21015840 H-61015840) 718 (1H d J = 20Hz H-210158401015840) 707 (1H dd J = 83 21 Hz H-610158401015840) 680 (2H d J = 82HzH-31015840 H-51015840) 679 (1H d J = 86Hz H-510158401015840) 639 (1H d J =142Hz H-810158401015840) 635 (1H d J = 143Hz H-81015840) 428 (4H dd J= 53 19Hz H-1 H-3) 416 (1H m H-2) 387 (3H s OCH3-310158401015840) 13CNMR (100MHz CD3OD) 120575 16901 (C-9
10158401015840) 16899 (C-91015840) 16134 (C-41015840) 15071 (C-310158401015840) 14938 (C-410158401015840) 14725 (C-710158401015840)14697 (C-71015840) 13121 (C-21015840 C-61015840) 12766 (C-110158401015840) 12711 (C-11015840)12419 (C-610158401015840) 11684 (C-31015840 C-51015840) 11648 (C-510158401015840) 11510 (C-810158401015840)11480 (C-81015840) 11175 (C-210158401015840) 6861 (C-2) 6639 (C-3) 6637 (C-1) 5644 (OCH3-3
10158401015840)
120573-D-(1-O-Acetyl-36-O-trans-diferuloyl)-fructofuranosyl-120572-D-210158404101584061015840-O- triacetylglucopyranoside (7) Colorlessamorphous solid ESI-MS ion at mz 8852 [M+Na]+ forC40H46O21
1H NMR (500MHz CD3OD) 120575 772 (1H d J =159Hz H-710158401015840) 768 (1H d J = 159Hz H-7101584010158401015840) 728 (2H d J= 20Hz H-210158401015840) 722 (2H d J = 20Hz H-2101584010158401015840) 713 (1H dd J= 85 201Hz H-610158401015840) 711 (1H dd J = 85 19Hz H-6101584010158401015840) 683(2H d J = 82Hz H-510158401015840 H-5101584010158401015840) 646 (1H d J = 1442HzH-810158401015840) 643 (1H d J = 144Hz H-8101584010158401015840) 570 (1H d J = 38HzH-11015840) 539 (1H d J = 77Hz H-3) 480 (1H d J = 102HzH-41015840) 468 (1H dd J = 102 38Hz H-21015840) 450 (1H dd J =121 327Hz H-6a) 445 (1H m H-6b) 446 (1H t J = 79HzH-4) 426 (d J = 115Hz H-1a) 424 (1H m H-51015840) 421(d br J = 114Hz H-61015840a) 410 (1H dd J = 116 35Hz H-61015840b)409 (1H d J = 115Hz H-1b) 391 (3H s OCH3-3
101584010158401015840) 391(3H s OCH3-3
10158401015840) 388 (1H d J = 76Hz H-31015840) 213 (3H sOAc-1) 210 (3H s OAc-21015840) 203 (3H s OAc-61015840) 187 (3Hs OAc-41015840) 13C NMR (125 MHz CD3OD) 120575 17260 (OAc-6
1015840)17214 (OAc-21015840) 17202 (OAc-1) 17172 (OAc-41015840) 16877(C-9101584010158401015840) 16798 (C-910158401015840) 15112 (C-410158401015840) 15076 (C-4101584010158401015840) 14951(C-310158401015840) 14943 (C-3101584010158401015840) 14823 (C-710158401015840) 14725 (C-7101584010158401015840) 12774(C-110158401015840) 12750 (C-1101584010158401015840) 12462 (C-610158401015840) 12431 (C-6101584010158401015840) 11657(C-510158401015840) 11652 (C-5101584010158401015840) 11515 (C-8101584010158401015840) 11430 (C-810158401015840) 11183
(C-210158401015840) 11168 (C-2101584010158401015840) 10391 (C-2) 9046 (C-11015840) 8152 (C-5)7955 (C-3) 7395 (C-21015840) 7379 (C-4) 7225 (C-41015840) 7006(C-5) 6982 (C-31015840) 6661 (C-1) 6453 (C-6) 6409 (C-61015840)5656 (OCH3-3
10158401015840 OCH3-3101584010158401015840) 2092 (OAc-CH3-2
1015840) 2079(OAc-CH3-1) 2068 (OAc-CH3-6
1015840) 2062 (OAc-CH3-41015840)
Assignments of the acetate and feruloyl units at positions1 21015840 41015840 61015840 (for acetyl) and 3 6 (for feruloyl) respectivelyin the sucrose molecule were corroborated by the HMBCtwo-dimensional spectrum this showed a correlationbetween methine and methylene protons at 1 21015840 41015840 and 61015840of the sucrose with methyl protons and the carbonyl carbonof the acetyl groups (1H13C1H 426 40917202213for 1 46817214210 for 21015840 48017172187 for 41015840 42141017260203 for 61015840) and between methine and methyleneprotons at 3 and 6 of the fructose with the protons at 810158401015840and 8101584010158401015840 of the feruloyl moiety and the carbonyl carbons at910158401015840 and 9101584010158401015840 (1H13C1H 64616798539 for C-3 feruloyl64316877450 445 for C-3 feruloyl)
1-O-p-Coumaroyl-3-O-caffeoylglycerol (8) Colorless oil ESI-MS ion at mz 3998 [M-H]minus for C21H20O8
1H NMR(400MHz CD3OD) 120575 766 (1H d J = 160Hz H-71015840) 760 (1Hd J = 159Hz H-710158401015840) 744 (2H dd J = 89 23Hz H-21015840 H-61015840)705 (1H d J = 21 Hz H-210158401015840) 694 (1H dd J = 83 20Hz H-610158401015840) 679 (2H dd J = 91 25Hz H31015840 H-51015840) 677 (1H d J =84Hz H-510158401015840) 635 (1H d J = 159Hz H-81015840) 630 (1H d J =159Hz H-810158401015840) 427 (4H d J = 54Hz H-1 H-3) 416 (1H qJ = 52Hz H-2) 13C NMR (100MHz CD3OD) d 16901 (C-91015840 C-910158401015840) 16131 (C-41015840) 14965 (C-310158401015840) 14736 (C-710158401015840) 14697(C-71015840) 14681 (C-410158401015840) 13122 (C-21015840 C-61015840) 12770 (C-110158401015840) 12713(C-11015840) 12302 (C-610158401015840) 11683 (C-31015840 C-51015840) 11651 (C-510158401015840) 11520(C-210158401015840) 11482 (C-81015840) 11476 (C-810158401015840) 6865 (C-2) 6635 (C-1C-3)
2-Propyl-120573-glucopyranoside (9) Colorless amorphous solidESI-MS ion at mz 2452 [M+Na]+ for C9H18O6
1H NMR(400 MHz CD3OD) 120575 434 (1H d J = 78Hz H-1) 404 (1Hhept J = 61 Hz H-21015840) 385 (1H dd J = 119 20Hz H-6b)366 (1H dd J = 119 54Hz H-6a) 335 (1H m H-3) 327(1H m H-4) 325 (1H m H-5) 314 (1H dd J = 91 78HzH-2) 123 (3H d J = 62Hz CH3-1
1015840) 119 (3H d J = 61HzCH3-3
1015840) 13C NMR (100MHz CD3OD) 120575 10255 (C-1) 7811(C-3) 7786 (C-5) 7514 (C-2) 7258 (C-21015840) 7170 (C-4) 6279(C-6) 2381 (C-11015840) 2204 (C-31015840)
33 Chromatographic Profiles In the HPLC profiles of theWE and the OE of B karatas (Figure 1) the majority ofcomponents showed maximum absorption between 280 and320 nmThe two chromatograms were qualitatively differenthowever the main components of WE [TR 133 (3) 142 and219 (2)min] were also observed in the OE [TR 133 (3) 142and 219 (2)min] but in different proportion Compounds 45 and 9 were not observed in any of the chromatographicprofiles Figure 2
Compound 1 was not observed as a major compound inthe profile of the WE (Figure 2(a)) because it precipitatedduring the process of sample preparation before the samplewas injected into the HPLC equipment its remnant was
6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

6 Evidence-Based Complementary and Alternative Medicine
DAD1 D Sig=3204 Ref=off
2827262524232221201918171615 29 301312111098765432 1410
Time [min]
0
50
100
150
200
250
300
350[m
AU]
(a)
2 3 4 5 6 7 8 9 10 11 12 13 1410 16 17 18 19 20 21 22 23 24 25 26 27 28 29 3015
Time [min]
DAD1 D Sig=3204 Ref=off
[mAU
]
0
100
200
300
400
500
(b)
Figure 2 HPLC-DAD profiles of the WE and the OE (a) Water extract (b) organic extract
observed at 254 nm at a retention time of 3065min (data notshown)
34 Hypoglycemic Effect of the Water Extract in a ChronicTrial for 42 Days After the induction of experimental hyper-glycemia the HC group presented higher glucose values thanthe NC group throughout the 42-day duration of the studywhile in the GB group a hypoglycemic effect was observedfrom days 7 to 42 this effect was statistically significantcompared with the HG group and with the time 0 valuesof the GB group A similar hypoglycemic effect beginningat day 7 and continuing until day 42 was observed afterthe oral administration of BK-WE confirming that both theplant extract and the drug glibenclamide exert a chronichypoglycemic effect (see Table 1)
The levels of glycated hemoglobinwere elevated in theHCgroup compared to the NC CG and BK-WE groups Bothglibenclamide and the extract controlled the increase in thevalues of HB1Ac but the effect was not statistically significantcompared to the HG or to the animalsrsquo own time 0 valuesThe cholesterol values were similar in all the groups (data notshown) and vLDL levels were not modified by the controldrug or by the extract (see Table 2)
35 Hypoglycemic Effect of the Isolated Compounds Afterthe induction of experimental hyperglycemia the hypergly-caemic group (HC) presented higher glucose values than
the normoglycemic group (NC) Through the 180 minutesboth groups present a stable glycemia the NC around100mgdl and the HC around 190mgdl with no statisticaldifference between their own time 0 However HC presentshigher glucose levels compared to NC When the hypo-glycemic agent glibenclamide (5mgkg) was administrated(GC) a statistically significant hypoglycemic effect from 60to 180 min compared against the HC and their own time 0could be observedThe compound (1) 120573-Sitosterol-3-O-120573-D-glucopyranoside exerts a hypoglycemic effect but it is onlystatistically significant at 180minThe compound (2) Cirsiliol41015840-O-glucoside exerts a statistically significant hypoglycemiceffect from 60 to 180min like glibenclamide The compound(3) 120588-Coumaric acid also produces a hypoglycemic effectsince 120min but it is only statistically significant at time180min (see Table 3)
4 Discussion
The results of the present work support the traditional useof B karatas in the treatment of type 2 diabetes The extracttested here which is similar to the traditionally used infusionpossesses a chronic hypoglycemic effect and the observedeffect was sustained throughout a 42-day period The plantextract was also able to control the elevation in glycatedhemoglobin with no effect on cholesterol or vLDL levels
Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

Evidence-Based Complementary and Alternative Medicine 7
Table 1 Chronic hypoglycemic effect of Bromelia karatas on STZ-NA induced diabetic rats
GlucoseGroups
(mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl) (mgdl)T0 T7 T14 T21 T28 T35 T42
NC 124 plusmn 32 129 plusmn 17 124 plusmn 24 127 plusmn 11 125 plusmn 43 118 plusmn 64 131 plusmn 27HC 174 plusmn 1061 171 plusmn 151 172 plusmn 971 153 plusmn 381 168 plusmn 731 162 plusmn 381 169 plusmn 871
CG 180 plusmn 251 128 plusmn 49 a1 147 plusmn 94 a1 133 plusmn 62 a1 150 plusmn 9 a1 134 plusmn 114 a1 152 plusmn 108 a1
5mgkgBk-WE 186 plusmn 311 138 plusmn 33 a1 146 plusmn 28 a1 138 plusmn 31 a1 141 plusmn 51 a1 1328 plusmn 42 1198861 1402 plusmn 78 a1
218mgKgThe values represent the mean plusmn SEM Superscripted numbers in the same column indicate statistically significant differences compared with the respectivecontrol group Superscripted letters in the same row indicate statistically significant differences compared with time 0 a1 (p lt 005)
Table 2 Chronic hypoglycemic effects of Bromelia karatas on STZ-NA induced diabetic rats
T0 T14 T28 T42
Groups HbA1c vLDL HbA1c vLDL HbA1c vLDL HbA1c vLDL() (mgdl) () (mgdl) () (mgdl) () (mgdl)
NC 36 plusmn 01 142 plusmn 8 36 plusmn 01 158 plusmn 3 35 plusmn 01 126 plusmn 8 36 plusmn 01 122 plusmn 2HC 37 plusmn 01 104 plusmn 1 41 plusmn 2 15 plusmn 27 42 plusmn 01 1a 238 plusmn 31a 43 plusmn 011a 226 plusmn 5 a
CG 38 plusmn 02 138 plusmn 2 42 plusmn 02 1a 178 plusmn 3 39 plusmn 02 20 plusmn 3 39 plusmn 02 23 plusmn 33 a
5mgkgBk-WE 36 plusmn 01 20 plusmn 21 41 plusmn 02 21 plusmn 46 40 plusmn 01 172 plusmn 3 41 plusmn 01 25 plusmn 6218mgKgThe values represent the mean plusmn SEM Superscripted letters in the same row indicate statistically significant differences compared with time 0 Superscriptednumbers in the same column indicate statistically significant differences compared with the respective control group a1 (p lt 00) VLDL was calculated usingthe following VLDL = 02 x TG
The chromatographic profiles and the absorption spectraof the extracts ofB karatas indicated the presence of phenoliccompounds and flavonoids The organic extract was used toincrease the chemical profile of the plant making it possibleto isolate several phenolic compounds including glycosidesand glycerides of phenylpropanoids flavonoids and phytos-terols 1 4 and 5 are themajor phytosterols in higher plants 2is part of the structural group of polymethoxylated flavoneswhich are distributed mainly in the Rutaceae and have beenshown to have a broad spectrum of biological activity thatincludes anti-inflammatory anticancer and antiatherogenicproperties [16] 3 6 and 8 have been previously described inthe family Bromeliaceae [17] 7 was isolated for the first timefrom Sparganium stoloniferum [14] and 9 is not commonlyisolated from natural products Sharma et al (1989) [18]isolated the isopropyl-120572-D-glucopyranoside from the coralSclerophytum capitalis they assumed that this compound isnot an artifact produced during the investigation becausesimilar to us they did not use propanol in the extraction andpurification process It should be noted that 1-9 have not beenreported previously for B karatas 1 and 3 were consideredthe major compounds in the infusion (WE) Several studiesshow that saponins regulate blood glucose levels and preventdiabetic complications due to their antioxidant activity [19]sitosterol-3-O-120573-D-glucopyranoside (1) was shown to havehypoglycemic and antihyperglycemic effects in STZ-NA ratstreated with doses of 025 and 050mgkg for 21 days using
glibenclamide as a positive control and to improve biochem-ical and hematological parameters such as total cholesteroltriglycerides high-density lipoprotein (HDL) low-densitylipoprotein (LDL) blood urea nitrogen creatinine red bloodcells platelets and white blood cells [20] On the otherhand Amalam et al (2016) [21] demonstrated the ldquoantidi-abeticrdquo potential of 120588-coumaric acid (3) by showing that itexerts a protective role in pancreatic b-cells of diabetic ratsby reducing ROS-induced oxidative stress and improvingantioxidant status and by providing evidence for the par-ticipation of GLUT-2 in controlling glucose homeostasis Amore recent trial showed the powerful antihyperglycemic andantihyperlipidemic efficacy of 120588-coumaric acid in STZ-NArats treated orally with 40mgkg body mass for six weeksthis process may be mediated via modulation of TNF-120572 andadipocytokine secretion as well as by upregulation of PPAR120574mRNA expression [22] The effect of the WE from Bromeliakaratas in this test was similar to that of glibenclamideconsequently we were able to confirm that 1 and 3 are theactive principles of the plant
The previously reported hypoglycemic effect of com-pounds 1 and 3 is here supported by our own data for 120573-Sitosterol-3-O-120573-D-glucopyranoside we observe nearly 80of glucose reduction after 180 min a similar result waspreviously described in a chronic experiment [20] and alsothe previous observation about the hypoglycemic effect of120588-Coumaric acid observed by [21] was corroborated here
8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

8 Evidence-Based Complementary and Alternative Medicine
Table 3 Acute hypoglycemic effect of the isolated compounds
Glucose levels in the maltose curve [mgdl]GroupTime (min) T0 T60 T120 T180Normoglycemic control 106 plusmn 5 b 114 plusmn 9b 110 plusmn 7b 105 plusmn 5b
Hyperglycaemic control 196 plusmn 7 191 plusmn 7 187 plusmn 3 194 plusmn 6Hyperglycemic + glibenclamide 192 plusmn 8 118 plusmn 10ab 107 plusmn 7ab 106 plusmn 9ab5mgkg120573-Sitosterol-3-O-120573-D-glucopyranoside 72mgkg 186 plusmn 6 203 plusmn 5 172 plusmn 2 156 plusmn 7b
Cirsiliol 41015840-O-glucoside 194 plusmn 5 179 plusmn 8a 160 plusmn 10ab 133 plusmn 14ab18mgkg120588-Coumaric acid 187 plusmn 8 198 plusmn 7 180 plusmn 7a 166 plusmn 9a363mgkgThe values represent the mean plusmn SEM In the same row a indicates statistically significant differences compared with time 0 In the same column b indicatesstatistically significant differences compared with the diabetic control group p lt 005 n = 3
For the first time the hypoglycemic effect of Cirsiliol 41015840-O-glucoside is reported which exerts better effect with a 30reduction in blood sugar levels after 180 min
This work supports the traditional use of the plant to treattype 2 diabetes and describes the compound Cirsiliol 41015840-O-glucoside as a novel hypoglycemic agent further investiga-tions are needed to establish the hypoglycemic mechanism ofthe plant and the compound Cirsiliol 41015840-O-glucoside
Data Availability
The data used to support the findings of this study areavailable from the corresponding author upon request
Conflicts of Interest
The authors declare that there are no conflicts of interestregarding the publication of this paper
Acknowledgments
The authors acknowledge M C Ramiro Cruz-Duran forthe plant determination and M C Christian Alan Cabello-Hernandez for handling the animals at the BioteriumThis project was partially sponsored by DGAPA PAPIITIN228216 and PAPIIT IN226719
References
[1] IDF Diabetes Atlas Country Reports Report Builder Mexico8th Edition edition 2017
[2] American Diabetes Association ldquoClassification and diagnosisof diabetesrdquoDiabetes Care vol 38 supplementary 1 pp S8ndashS162015
[3] A Espejo-Serna A R Lopez-Ferrari N Martınez-Correa andVA Pulido-Esparza ldquoBromeliad flora of Chiapas stateMexicorichness and distributionrdquo Phytotaxa vol 310 no 1 article 12017
[4] F Parada and C Duque ldquoStudies on the aroma of Pinuela fruitpulp (Bromelia plumieri) free and bound volatile composition
and characterization of some glucoconjugates as aroma precur-sorsrdquo Journal of High Resolution Chromatography vol 21 no 10pp 577ndash581 1998
[5] A Andrade-Cetto and A E Medina-Hernandez ldquoHypo-glycemic effect of Bromelia plumieri (E Morren) LB Smleaves in STZ-NA-induced diabetic ratsrdquo Frontiers in Pharma-cology vol 4 2013
[6] N Olaw ldquoCommittee for the update of the guide for the careand use of laboratory animalsrdquo in Guide for the Care and Use ofLaboratory Animals Institute for Laboratory Animal ResearchDivision on Earth and Life Studies and National ResearchCouncil 8th edition 2011
[7] P Masiello C Broca R Gross et al ldquoExperimental NIDDMdevelopment of a newmodel in adult rats administered strepto-zotocin and nicotinamiderdquo Diabetes vol 47 no 2 pp 224ndash2291998
[8] A Andrade-Cetto S M Escandon-Rivera G M Torres-Valle and L Quijano ldquoPhytochemical composition and chronichypoglycemic effect of rhizophora mangle cortex on STZ-NA-induced diabetic ratsrdquo Revista Brasileira de Farmacognosia vol27 no 6 pp 744ndash750 2017
[9] N P Rai B B Adhikari A Paudel et al ldquoPhytochemicalconstituents of the flowers of Sarcococca coriacea of Nepaleseoriginrdquo Journal of Nepal Chemical Society vol 21 pp 1ndash7 2006
[10] R Karthikeyan C Devadasu and P S Babu ldquoIsolation charac-terization and RP-HPLC estimation of P-coumaric acid frommethanolic extract of durva grass (Cynodon dactylon Linn)(Pers)rdquo International Journal of Analytical Chemistry vol 2015Article ID 201386 7 pages 2015
[11] C Lin Y Huang L Cheng S Sheu and C Chen ldquoBioactiveflavonoids from Ruellia tuberosardquoNational Research Institute ofChinese Medicine vol 17 no 3 pp 103ndash109 2006
[12] M Hassan H Mohammed A Nageeb et al ldquoPhytochemicaland pharmacological studies of Citharexylum quadrangulareJacq leavesrdquo Journal of Medicinal Plants Research vol 10 no18 pp 232ndash241 2016
[13] R H Delaporte K P Guzen A Laverde Jr A R dosSantos andMH Sarragiotto ldquoPhenylpropanoid glycerols fromTillandsia streptocarpa Baker (Bromeliaceae)rdquo Biochemical Sys-tematics and Ecology vol 34 no 7 pp 599ndash602 2006
[14] O Shirota S Sekita M Satake Y Ni and H Weiyi ldquoChemicalconstituents of Chinese folk medicine lsquoSan Lengrsquo Sparganiumstoloniferumrdquo Journal of Natural Products vol 59 no 3 pp242ndash245 1996
Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

Evidence-Based Complementary and Alternative Medicine 9
[15] W Li K Koike Y Asada T Yoshikawa and T Nikaido ldquoBio-transformation of low-molecular-weight alcohols by Coleusforskohlii hairy root culturesrdquo Carbohydrate Research vol 338no 8 pp 729ndash731 2003
[16] S Li M-H Pan C-Y Lo et al ldquoChemistry and health effects ofpolymethoxyflavones and hydroxylated polymethoxyflavonesrdquoJournal of Functional Foods vol 1 no 1 pp 2ndash12 2009
[17] L MManetti R H Delaporte and A Laverde Jr ldquoMetabolitossecundarios da famılia bromeliaceaerdquo Quımica Nova vol 32no 7 pp 1885ndash1897 2009
[18] P SharmaMAlam andMV Chari ldquoIsolation and structure ofisopropyl-a-o-glucoside from the coral Sclerophytum capitalisrdquoJournal of Natural Products vol 52 no 2 pp 395ndash397 1989
[19] A El Barky S A Hussein A-E Alm-Eldeen A Hafez and TMohamed ldquoReview diabetes management saponins and theirpotential role in diabetes mellitusrdquo 2017
[20] N Somsak P Peerawit and T Chusri ldquoHypoglycemic activ-ity in diabetic rats of stigmasterol and sitosterol-3-O-120573-D-glucopyranoside isolated from Pseuderanthemum palatiferum(Nees) Radlk leaf extractrdquo Journal of Medicinal Plants Researchvol 9 no 20 pp 629ndash635 2015
[21] V Amalan N Vijayakumar D Indumathi and A Ramakr-ishnan ldquoAntidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats role of pancreatic GLUT 2 Invivo approachrdquo Biomedicine amp Pharmacotherapy vol 84 pp230ndash236 2016
[22] A Abdel-Moneim S M A El-Twab A I Yousef E S AReheim and M B Ashour ldquoModulation of hyperglycemia anddyslipidemia in experimental type 2 diabetes by gallic acidand p-coumaric acid The role of adipocytokines and PPAR120574rdquoBiomedicine amp Pharmacotherapy vol 105 pp 1091ndash1097 2018
Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom

Stem Cells International
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
MEDIATORSINFLAMMATION
of
EndocrinologyInternational Journal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Disease Markers
Hindawiwwwhindawicom Volume 2018
BioMed Research International
OncologyJournal of
Hindawiwwwhindawicom Volume 2013
Hindawiwwwhindawicom Volume 2018
Oxidative Medicine and Cellular Longevity
Hindawiwwwhindawicom Volume 2018
PPAR Research
Hindawi Publishing Corporation httpwwwhindawicom Volume 2013Hindawiwwwhindawicom
The Scientific World Journal
Volume 2018
Immunology ResearchHindawiwwwhindawicom Volume 2018
Journal of
ObesityJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Computational and Mathematical Methods in Medicine
Hindawiwwwhindawicom Volume 2018
Behavioural Neurology
OphthalmologyJournal of
Hindawiwwwhindawicom Volume 2018
Diabetes ResearchJournal of
Hindawiwwwhindawicom Volume 2018
Hindawiwwwhindawicom Volume 2018
Research and TreatmentAIDS
Hindawiwwwhindawicom Volume 2018
Gastroenterology Research and Practice
Hindawiwwwhindawicom Volume 2018
Parkinsonrsquos Disease
Evidence-Based Complementary andAlternative Medicine
Volume 2018Hindawiwwwhindawicom
Submit your manuscripts atwwwhindawicom