Pendular activity of human upper limbs during slow and normal walking
-
Upload
david-webb -
Category
Documents
-
view
212 -
download
0
Transcript of Pendular activity of human upper limbs during slow and normal walking
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 93:477489 (1994)
Pendular Activity of Human Upper Limbs During Slow and Normal Walking
DAVID WEBB, RUSSELL H. TUTTLE, AND MICHAEL BAKSH Department of AnthropologylSociology, Kutztown University, Kutztown, Pennsylvania 19530 (D. WJ; Department of Anthropology, University of Chicago, Chicago, Illinois 60637 (R.H.T.); Los Angeles, California 90067 (M.B.)
KEY WORDS Human walking, Natural pendular frequency, Upper limb swinging
ABSTRACT When walking at normal and fast speeds, humans swing their upper limbs in alternation, each upper limb swinging in phase with the contralateral lower limb. However, at slow and very slow speeds, the upper limbs swing forward and back in unison, at twice the stride frequency of the lower limbs. The change from “single swinging” (in alternation) t o “double swinging” (in unison) occurs consistently at a certain stride frequency for agiven individual, though different individuals may change at different stride frequencies. To explain this change in the way we use our upper limbs and individual variations in the occurrence of the change, the upper limb is mod- elled as a compound pendulum. Based on the kinematic properties of pendu- lums, we hypothesize that the stride frequency at which the change from “single swinging” to “double swinging” occurs will be at or slightly below the natural pendular frequency (NPF) of the upper limbs.
Twenty-seven subjects were measured and then filmed while walking at various speeds. The mathematically derived NPF of each subject’s upper limbs was compared to the stride frequency at which the subject changed from “single swinging” to “double swinging.” The results of the study conform very closely to the hypothesis, even when the NPF is artificially altered by adding weights to the subjects’ hands. These results indicate that the pendulum model of the upper limb will be useful in further investigations of the function of the upper limbs in human walking. o 1994 Wiley-Liss, Inc.
An old and very common observation about human walking is that the upper limbs swing in alternation, with each one swinging forward and back in phase with the contralateral lower limb (Carlet, 1872). But the upper limbs are not simply allowed to swing on their own; they are, in fact, ac- celerated by contracting some of the muscles of the shoulder and arm (Elftman, 1939; Fernandez Ballesteros et al., 1965; Jackson et al., 1978). In that way, when walking more quickly, the synchrony between the upper and lower limbs can be maintained by contracting these muscles more sharply and more often. Alternatively, when walking more slowly, the muscles contract less vigor-
ously and less frequently (Hogue, 1969; Craik et al., 1976). There comes a point, when walking very slowly, at which the up- per limbs swing forward and back, not alter- nately, but in unison and at twice the stride frequency of the lower limbs (Craik et al., 1976; Webb, 1989). This change from what Webb (1989) called “single swinging” (in al- ternation) to “double swinging” (in unison) occurs quite consistently at a certain stride
Received June 2,1992; accepted November 5, 1993 Address reprint requests to David Webb, Department of
Anthropology/Sociology, Kutztown University, Kutztown, PA 19530.
8 1994 WILEY-LISS, INC.
478 D. WEBB ET AL.
frequency for a given individual, although different individuals may change at differ- ent stride frequencies. In order to explain this change in the way the upper limbs are used and the individual variations in the occurrence of the change, Webb (1989) mod- elled the upper limb as a compound pendu- lum, keeping in mind that it is not a pas- sively swinging pendulum, but one that is actively propelled by muscular contraction.
Considerable evidence for muscular con- trol of the upper limbs during walking comes from the literature. Elftman (1939) studied upper limb mechanics in normal walking and concluded that upper limb movement could not be explained by the movement of the shoulder joint, and that other forces, presumably muscular, must be involved.
Fernandez Ballesteros et al. (1965) exam- ined muscular activity of the arm and shoul- der during normal walking for 21 men and 2 women ranging in age from 16 to over 50. The subjects were instructed to walk “with natural posture, stride and speed along a corridor 2.5 m wide and 16 m long.” The researchers found that, during protraction of the upper limb, there was some activity of the internal rotators of the humerus (upper latissimus dorsi and teres major), while re- traction of the limb was associated with ac- tivity of posterior deltoid, middle deltoid, upper latissimus dorsi, teres major, su- praspinatus and upper trapezius. Fernan- dez Ballesteros et al. (1965) concluded that posterior deltoid was the primary extensor of the arm at the shoulder during normal walking. In order to make sure that the muscles were required and that upper limb swinging was not a purely passive phenome- non, they loosely bound the forearms of five subjects, preventing them from swinging their upper limbs. Even under these condi- tions, the subjects used teres major, latissi- mus dorsi and posterior deltoid during the gait, with timing similar to that associated with unrestrained walking, indicating “that the innervation of the muscles during the arm swing is part of a centrally determined pattern of locomotion” (Fernandez Balle- steros et al., 1965:308). However, because protraction of the arm is not always associ- ated with muscular activity, Fernandez Bal- lesteros et al. (1965) also concluded that
gravity and passive elastic forces were par- tially responsible for the observed pattern of movement.
Hogue (1969) studied 15 college students walking at three different speeds and on both a level and an inclined treadmill. He reported on quite a few muscles but noted significant activity in only three: teres ma- jor, middle deltoid, posterior deltoid. In each set of conditions (three speeds; two inclina- tions), posterior deltoid was the most active, followed by middle deltoid and then teres major. Hogue (1969) interpreted the action of teres major to be retraction of the arm. Middle deltoid apparently kept the upper limb slightly abducted so it would not bump the torso as it swung. Posterior deltoid was active at the end of protraction, decelerating the upper limb, and during retraction. It is difficult to interpret Hogue’s results in terms of double swinging, because he had his subjects walk at “cadences” of 70,90 and 120 “steps”/min. If he meant a “step” to be the movement from heel strike of one foot to the next heel strike of the opposite foot, as is the current convention, then his subjects’ stride frequencies were .58 Hz, .75 Hz and 1.0 Hz, respectively. It is hard to imagine that subjects who were walking naturally at .58 Hz were not double swinging, since none of Webb‘s (1989) or Craik et al.’s (1976) sub- jects did so. Yet, Hogue makes no mention of double swinging in his article. On the other hand, if he meant a “step” to be the move- ment from heel strike of one foot to the next heel strike of the same foot (what we now call a “stride”), his subjects would have been walking with stride frequencies of 1.2 Hz, 1.5 Hz and 2.0 Hz. Since it is difficult to walk with a stride frequency of 2.0 Hz, this too is hard t o imagine. Hogue (1969) does not define “step” in his article.
Craik et al. (1976) noted that of the mus- cles they surveyed (posterior deltoid, ante- rior deltoid, biceps, and triceps), only poste- rior deltoid was consistently active during a wide range of walking speeds, including those slow speeds associated with double swinging of the upper limbs. In their (five) subjects, posterior deltoid was active while walking very slowly in two short bursts cor- responding to the two times during each stride when the upper limbs were retracted.
PENDULAR ACTIVITY OF UPPER LIMBS DURING WALKING 479
This suggests that even during double swinging, the movements of upper limbs are kept in phase with the lower limbs (at a 2:l ratio) by the muscles of the shoulder joint.
Jackson et al. (1978) observed significant activity of all three parts of deltoid, latissi- mus dorsi and triceps brachiii at a variety of stride frequency ranges. The lowest stride frequency category (<90 stepslmin, or .75 Hz) is near the border between single swing- ing and double swing for most people in the studies of Craik et al. (1976) and Webb (1989), and at that speed, posterior deltoid showed the most noticeable action. Unfortu- nately, Jackson et al. (1978) did not deal with very slow walking and double swing- ing.
Natural pendular frequency and entrainment
Searle (1915) noted that every pendulum has a natural frequency of oscillation which depends on the acceleration of gravity and the distribution of the pendulum’s mass rel- ative to its fulcrum. This frequency is re- ferred to as the natural pendular frequency (NPF), and McMahon (1984) points out that a pendulum can be caused to swing by an external force which is applied with a fre- quency equal to the pendulum’s NPF, even if the external force is quite small. The pendu- lum will even swing at a frequency slightly different from its NPF, either above or below it, if the external force is applied with a fre- quency slightly different from the NPF. The pendulum, when swinging in this fashion, is said to be entrained by the external force. If the external force is removed and the pendu- lum is allowed to swing freely, it will quickly adopt its own natural frequency. If the fre- quency of the external force is changed so that it is quite different from the NPF of the pendulum, the pendulum will stop swing- ing. However, as the frequency of the exter- nal force is moved farther and farther from the NPF of the pendulum, it may once again entrain the pendulum when its frequency approaches a simple multiple of the NPF. At this point, about two times the NPF, the pendulum will begin to swing at one-half the frequency of the external force, and there- fore at approximately its own NPF. This phenomenon of entrainment can occur with
external forces having frequencies which are greater than the NPF (two times, three times, etc.) or less than the NPF (one-half, one-third, etc.).
According to Webb‘s (1989) model, the hu- man upper limb can be viewed as a pendu- lum which is entrained by the actions of the shoulder muscles and the movement of the torso during walking. One prediction based on the pendular model is that people will swing their upper limbs near or above their NPFs, but not much below (except as they are entrained by the movements of the torso and by muscular contractions). Using the muscles of the shoulder, it should be possi- ble to keep the upper limbs moving in syn- chrony with the contralateral lower limbs at almost any frequency, even while running. However, if the subject walks with a stride frequency below the NPF of his upper limbs, a different and less efficient neuromuscular mechanism would have to be used to main- tain the synchrony. Specifically, the upper limb would have to be retracted during pro- traction of the contralateral lower limb, but would not be allowed to swing freely for- ward, since that would be too fast to remain in phase. Instead, the extensors of the shoul- der would have to slow the forward swing of the upper limb, thereby robbing it of the nec- essary momentum to carry it forward during retraction of the lower limb. Then, flexors of the shoulder would be needed for the for- ward swing of the upper limb, and again to slow the first half of the backward swing. This is clearly much less efficient than the mechanisms for normal walking already de- scribed by previous researchers (Craik et al., 1976; Jackson et al., 1978; Fernandez Bal- lesteros et al., 1965; Hogue, 1969), which call for active retraction of the upper limb followed by nearly passive forward swing. It is therefore unlikely that subjects will sin- gle-swing when walking with stride fre- quencies significantly below their upper limb NPFs, and that they will be forced to double swing, using the shoulder muscles in much the same way as described by other authors.
The aforementioned predictions can be tested in two ways: (1) by observing subjects who are walking at or above the NPFs of their upper limbs, noting whether they are
480
F
D. WEBB ET AL.
F
\
Fig. 1. A simple pendulum (a) is one in which all the mass is concentrated in a single bob at the distal end. The human upper limb clearly does not fit this definition, and must instead be modelled as a compound pendulum (b) which has its mass distributed unevenly about its vertical axis. For (a) and (b), F is the fulcrum, m is the striped area representing the mass, and c is the center of mass.
indeed always single swinging; ( 2 ) by ob- serving subjects who are walking below their upper limb NPFs, to see if they stop swinging at stride frequencies slightly below the NPF and if they double-swing at stride frequencies significantly below the NPF.
Modelling the upper limbs as pendulums The human upper limb must be modelled
as a compound pendulum, one that has its mass distributed (perhaps unevenly) along its vertical axis (Fig. 1). Orear (1979) gives the period of oscillation of a compound pen- dulum as:
I Eq. 1
where T (tau) is the period, v is the circular constant (=3.1416), I is the moment of iner- tia, m is the mass of the pendulum, L is the distance from the fulcrum to the center of mass, and g is the acceleration due to grav- ity ( ~ 9 . 8 m/sec2). The quantities I and L must be determined experimentally for each pendulum for which the period is desired. The moment of inertia (I) is given by the formula
I = Crj2mj Eq. 2
where j indicates the jth segment of a com- pound pendulum, r is the distance from the fulcrum to that segment, and m is the mass of the segment (Orear, 1979). The distance to the center of mass of the pendulum (L) is taken as the weighted average of the dis- tances to each of the segments
Cmjrj L = - Cmj Eq. 3
where m, r and j are as in Equation 2. Hav- ing found the period of a pendulum by this method, it is easy to calculate the NPF since it is the reciprocal of the period.
MATERIALS AND METHODS Subjects
A total of 35 subjects were studied, 27 of whom provided useable results. Of these 27, 18 are Peruvian Indians and 9 are Euro- Americans from North America. There were 19 males and 8 females, with a total age range of 8-66 years. Their statures ranged from 1.155 to 1.792 m.
Segmentation and calculations To determine the NPFs of the subjects’
upper limbs, it was necessary to divide their
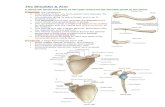
PENDULAR ACTIVITY OF UPPER LIMBS DURING WALKING 48 1 acromion
maximum arm
elbow
wrist
knuckle 111 fingertip
Fig. 2. To calculate the natural pendular frequency of a subject’s upper limb, 6 segments were marked on the skin with an ink pen. The fulcrum was taken to be coincident with the acromion process, when viewed lat- erally. The distance of each segment from the fulcrum was calculated as the average of the distances to the proximal and distal boundaries of each segment as marked on the skin. In the drawing shown here, the midpoint of the proximal forearm segment is taken as (D, - D,)/2.
limbs into several segments and, as in Equa- tions 2 and 3, to determine the distance from the fulcrum (r) and the mass (m) of each segment (Fig. 2). The mass of each segment was estimated by measuring its volume and multiplying by its density as determined ex- perimentally with sectioned cadaveral limbs (Webb, 1989). The volume was measured by water displacement as indicated in Figure 3.
For each subject, the NPFs of both limbs were estimated using the methods described above and the following densities of upper limb segments: 1.035 glcc for arm segments; 1.064 glcc for forearm segments; 1.118 glcc for hand segments. These values are the weighted averages of values calculated from cadavers for this experiment and those ob- tained from the literature (Chandler et al., 1975; Clauser et al., 1969; Drillis and Con- tini, 1966).
Observations of walking trials The subjects were asked to walk back and
forth, on level ground, at several different speeds. Each walking trial was recorded on videotape so that the subject’s stride fre- quency could easily be determined, and it
Fig. 3. The volume of the upper limb segments is measured by filling a cylinder (manufactured for this purpose) to the point where water begins to run from the spout. Once the water stops running out, the subject’s limb is submerged to the distalmost mark, causing more water to run out. This run-off is collected in a graduated cylinder and measured. The limb is then submerged to the next mark, the overflow is collected and the accumu- lated volume measured. This procedure is continued un- til the entire limb is submerged and the cumulative vol- ume at each mark is determined. The volume of each segment is then calculated by subtracting the value taken at its lower limit from that taken at its upper limit.
was noted whether he or she was single swinging or double swinging. The stride fre- quency at which that subject changed from single- to double swinging was then com- pared to the volumetrically derived natural pendular frequency of each subject’s upper limbs. Again, it was predicted that the stride frequency at which the change occurred would be below the NPF of the upper limbs.
In order to be sure that it was the physical properties of the upper limbs, and not neuro- logical differences among individuals, which determined the point at which each subject
482 D. WEBB ET AL.
acromion
maximum arm
4
5 fingertip 6
knuckle 111
Fig. 4. When calculating the NPF of a weighted up- per limb, the original six segments are used, but the mass of the weight is added to the distalmost segment. Measurements of the weighted hand indicate that the midpoint of the distal segment is best approximated by averaging the distances to the proximal interphalangeal joint of the grasping hand (ray 111) and (as in Fig. 3) to the metacarpophalangeal joint of ray 111.
changed from single- to double swinging, the NPFs of some subjects’ upper limbs were changed and they were asked to repeat the experiment. The natural frequencies of their limbs were changed by having them carry weights in their hands (Fig. 4). Knowing the mass of the weights and their distance from the subject’s shoulders, it is possible to cal- culate the new, altered pendular frequency by adding that information to the computer model. The artificially produced pendular frequency was then compared to the stride frequency at which the subjects changed to double swinging while carrying the weights.
RESULTS Figure 5 is typical of the results for indi-
viduals. For most subjects, single swinging occurred when stride frequency was near or above the NPF; double swinging occurred when stride frequency was significantly be- low the NPF. Some subjects did not swing their upper limbs in any regular fashion when walking with stride frequencies slightly below their NPFs, and these trials (having no discernible upper limb swinging frequency) could not be shown on a graph of the type seen in Figure 5.
1.2
1.1
1.0
0.9
0.8
0.7 7 0.4 0.6 0.8 1.0 1.2
stride frequency
Fig. 5. Upper limb swinging frequency versus stride frequency. This graph, for subject US28, shows upper limb frequency versus stride frequency for 10 trials (solid squares represent double-swinging trials; open squares represent single-swinging trials). The dotted line indicates the natural pendular frequency for US28’s upper limbs (0.805). On the right of the graph, (i.e., near or above the natural frequency of his upper limbs), US28 swung his upper limbs in time with his lower limbs so that, a t a stride frequency of 0.8 Hz he swung his upper limbs at 0.8 Hz; at a stride frequency of 1.0 Hz, he swung his upper limbs at 1.0 Hz, and so on. Hence, the regres- sion line of upper limb frequency on stride frequency has a slope of 1. To the left of the dotted line, when his stride frequency was much less than the natural frequency of his upper limbs, he swung them at twice the stride fre- quency. Therefore, at a stride frequency of 0.5 Hz, he swung his upper limbs at 1 Hz, and so on. The slope of this regression line is 2.
Figure 6 shows all trials for all subjects, including those trials during which the sub- jects swung their upper limbs irregularly. Double swinging occurred only when sub- jects walked with stride frequencies below their upper limb NPFs, while single swing- ing occurred near and above the NPF, and irregular swinging occurred near the NPF though mostly below it.
Figure 7 shows all trials for 5 subjects who were tested under two conditons: normal walking at various speeds, and walking with hand weights at various speeds. The weighted trials show the same general rela- tionship to upper limb pendular frequency as do the unweighted trials.
DISCUSSION Research on walking subjects shows a
clear relationship between upper limb me- chanics and interlimb coordination. The up- per limbs generally swing in alternation,
PENDULAR ACTIVITY OF UPPER LIMBS DURING WALKING 483
0.3 1 0.7 0
X
0.85 0.9 0.95 Natural Pendular Frequency (Hz)
Fig. 6. Stride frequency of each trial versus the up- per limb NPF of the subject making that trial. There- fore, all the trials of one individual lie in a vertical col- umn whose position is determined by the NPF of the subject's upper limbs. (It is possible for two subjects to have the same NPF and, hence, their trials would ap- pear in the same column on the graph.) If NPFs were calculated for both upper limbs (as was usually the case), the lower NPF was taken as the appropriate one
for that individual. This was done to ensure that each subject was categorized by an NPF below which both upper limbs must be entrained. The dashed line (with a slope of one) indicates where stride frequency is equal to the calculated NF'F and, hence, marks a hypothetical boundary below which we would expect double-swinging to occur. X s represent single swinging trials; solid dots represent double swinging trials; open squares repre- sent indeterminate trials (see text).
D. WEBB ET AL.
X
X x a r(
8'
a
+ +
+ - +
f / +
+ +
A
A
,I
- 5 (
Natural Pendular Frequency (Hz)
X X
X
X
X
X
El ., x / F
X
0
0
15
Fig. 7. Stride frequency versus upper limb NPF for all trials of 5 subjects who were tested under two condi- tions: normal walking at various speeds, and walking with hand weights at various speeds. As in Figure 6, the dashed line indicates where stride frequency equals NPF, and each vertical column of symbols indicates one subject's trials under one set of conditions. Hence, each subject is represented twice: once with weighted upper
limbs and once without weights. This graph shows that lowering the NPF ofthe upper limbs (by adding weights) lowers the stride frequency at which subjects change from single- to double swinging. Unweighted trials are represented by the same symbols as in Figure 6; weighted trials are represented by crosses, circles and triangles, for single swinging, indeterminate and double swinging, respectively.
PENDULAR ACTIVITY OF UPPER LIMBS DURING WALKING 485
each one moving forward and back in time with the contralateral lower limb, but be- cause they are active pendulums bound by the laws of pendulum physics, they do not always swing in this familiar way. When we walk with a stride frequency below the natu- ral pendular frequency of our upper limbs, our upper limbs no longer swing in alterna- tion, but begin to swing in unison, and at twice the stride frequency of the lower limbs. This is true even when the upper limbs’ NPF is altered by artificial means, such as by adding weights to the hands, so that the change to double swinging occurs below the new, artificially produced NPF.
Thus, the mechanical properties of the up- per limbs affect the way we coordinate their movements with those of the lower limbs, but not without regard to neural mecha- nisms which control the way we walk. That we maintain neural control of our upper limbs is proven by the fact that we use mus- cular force to keep our upper limbs moving in synchrony with our lower limbs at all times, whether at a 1 : l ratio or a 2:l ratio (Craik et al., 1976). Thus, when we walk with a cadence below the natural pendular frequency of our upper limbs, we accelerate them to an average frequency exactly double the stride frequency, maintaining some form of synchrony between upper and lower limbs, and this requires coordinated muscu- lar effort. Therefore, in contrast to some ear- lier studies (Inman et al., 1981), in the con- text of human walking, we must consider the upper limbs not as passively swinging pendulums, but as pendulums that are ac- tively controlled by the neuromuscular sys- tem.
The distribution of stride frequencies about each subject’s upper limb NPF rein- forces the conclusions drawn above; namely, that when we walk with a cadence near to or greater than our upper limb NPF, we single swing our upper limbs; when we walk with a slower cadence we double swing them. But a close look at the distribution of relative stride frequency (Figs. 8 and 9) shows some- thing not visible in the graph of stride fre- quency against individual NPF (Fig. 6). From Figures 8 and 9, it is apparent that the subjects avoided walking at a cadence ex- actly equal to their NPFs. Instead, despite
attempts to make them walk as close to their NPFs as possible, they preferred to walk at cadences above or below their own natural pendular frequencies.
The most reasonable explanation for this avoidance is that walking in the transition zone between single- and double swinging is uncomfortable or confusing. As noted above, when the subjects walked with cadences near their upper limb NPFs, they appeared uncoordinated and uncomfortable, and it was frequently impossible to assign them to either the single swinging or the double swinging category. Since the upper limbs are active pendulums, being propelled by the movements of the torso and the contrac- tion of shoulder muscles (Elftman, 1939; Fernandez-Ballesteros et al., 1965; Jackson et al., 19781, swinging at precisely the NPF would be difficult since it would require that the shoulder muscles not be used at all, which is apparently not normal (Craik et al., 1976). Furthermore, when intermediate tri- als could be assigned to one category, it was always to single swinging, which may indi- cate a preference for single swinging as op- posed to double swinging.
If humans have a predilection for single swinging, it should come as no surprise. Sin- gle swinging is comparable, in terms of in- terlimb coordination, to the diagonal walk- ing pattern of virtually all other primates, and hence it may reflect a neurological ten- dency which we share with other members of our order. Another reason to assume that we have a preference for single swinging is the fact that we normally walk with a ca- dence higher than our upper limb natural pendular frequency. In fact, Ralston (1976) showed that the most efficient walking speed, in energy expenditure per unit dis- tance, among “normal” men is about 80 d m i n . From a regression of men’s walking speed versus stride frequency (Webb, 1989), 80 d m i n corresponds to a stride frequency of about 0.88 Hz which should be compared, for a man of about 1.75 m stature, with an NPF of about 0.77 Hz. A stride frequency of 0.88 Hz for a man with an upper limb NPF of 0.77 Hz gives a relative stride frequency of 1.14. With respect to the distribution of rela- tive stride frequency in Figure 8, the value 1.14 lies above the category containing the
486 D. WEBB ET AL.
Fig. 8. This histogram shows the distribution of rel- ative stride frequency for all trials of all subjects. Rela- tive stride frequency is taken as the stride frequency of each walking trial divided by the NPF of the subject who made it. This ratio (stride frequencyiNPFf was then plotted, with single swinging trials shown by stipling, indeterminate (uncoordinated) swinging trials shown in dark grey, and doubleswinging trials shown by cross-
value 1.00, and it therefore indicates single swinging. In Figure 9a, which shows the dis- tribution of single-swinging trials about in- dividual NPFs, 1.14 lies in the category which contains the mean for all single swinging trials (1.13). It is therefore proba- bly the most natural stride frequency, which is in keeping with Ralston’s (1976) finding that relative stride frequencies near 1.14 are probably the most energy efficient.
Even if single swinging is not preferred, the phenomenon of pendulum “entrain- ment” may account for the fact that many subjects single swung even while walking with stride frequencies somewhat below their upper limb NPFs. Entrainment, as de- scribed by McMahon (1984), is the process by which an external force acting on a pen- dulum can cause it to swing at a frequency other than its natural frequency. Entrain- ment occurs when an external mechanism imposes its own frequency on a pendulum. Given a 1:l entrainment, where the outside agency is moving the pendulum at a fre-
hatching. The bars are 0.05 wide, and a relative stride frequency of 1.00 indicates that the subject was walking with a stride frequency equal to the NPF of his upper limbs. Note that a relative stride frequency of 1.00 ap- pears to be avoided, whereas single swinging at a stride frequency slightly above the subjects’ NPF s and double swinging at a stride frequency well below NPF seem to be preferred.
quency sufficiently close to its natural fre- quency that entrainment has resulted, the frequency of the external force can be slowly decreased and the pendulum will follow (for a while). Eventually, the frequency of the external mechanism will drop below the lower bound of the zone of entrainment and the pendulum’s frequency will jump back up to its natural frequency. If the external fre- quency decreases far enough, the pendulum will become reentrained in other zones of entrainment near whole-number ratios such as 1:2,1:3, and so forth. It is therefore possi- ble for the forces generated by the lower limbs and trunk to entrain the upper limbs to a frequency slightly below their natural frequency, and only when the stride fre- quency has sufficiently decreased that it is
Fig. 9. Separate histograms of relative stride fre- quency for single swinging (a), double swinging (b) and indeterminate (c) trials. Scale and bar width are the same as for Figure 8.
488 D. WEBB ET AL.
no longer in the zone of 1:l entrainment, will the subject begin to double swing his upper limbs.
Furthermore, since the upper torso ro- tates about a vertical axis in synchrony with the flexion and extension of the upper limbs, the limbs do not hang from stationary ful- cra. Instead, their fulcra move with them, increasing their range and period of oscilla- tion and therefore decreasing their effective natural pendular frequencies. Hence, the calculated NPFs may be slightly high for walking humans.
It should also be noted that the method of estimating living subjects’ NPFs was based on cadaveral experiments which were slightly biased, because the apparatus for measuring the volume of limb segments pro- duced a slight underestimation for forearm segments but not for arm segments (Webb, 1989). This may have had an effect on the degree to which the subjects single swung below their calculated NPFs. On average, the NPF calculated by Webb‘s method was 1.9% higher than the measured NPFs of the cadaveral specimens. The cadaver limbs were suspended by a pin through the shoul- der and swung from an apparatus designed for that purpose. Their NPFs were calcu- lated as the reciprocal of the swing time av- eraged over ten full swings, and these “measured” NPFs were compared to those calculated on the basis of volumetric and lin- ear measurements, as described in Materi- als and Methods. Since the same volumetric method was used with living subjects, their NPFs were probably also overestimated by about 1.9%.
Another possible source of error in esti- mating the subjects’ upper limb NPFs comes from the fact that many of the subjects flexed and extended their elbows while they walked. This is quite natural (Suzuki, 1985) but it changes the upper limb from a com- pound pendulum to a complex (jointed) pen- dulum. Since the formula for determining the period of a complex pendulum is itself far more complex (Daish, 1972; Searle, 1915), the simpler formula for a compound pendulum may have added to our overesti- mation of the subjects’ NPFs. Even the more complex formula may not have produced more accurate estimates, because it is based
on the assumption that the pendulum is a freely swinging, passive object, an assump- tion which does not apply to the elbow joint in normal walking. The complex formula is not applicable to the cadavers, since they are generally less flexible than living upper limbs and since they have no active muscles to flex and extend their elbows.
One additional note concerning entrain- ment: the lowest bar in Figures 8 and 9c does not indicate double swinging, as might be expected. Instead, it represents a single example of indeterminate swinging, sug- gesting that the subject could not find a com- fortable ratio between upper limb move- ment and lower limb movement. A closer look at the values encompassed by that cate- gory may provide the explanation. The value 0.5 for relative stride frequency represents a theoretical boundary much like the NPF of the upper limbs, since it is one-half the NPF, and marks the lowest point at which double swinging should occur; below that triple swinging should begin. Hence, the subject may have entered the region of indetermi- nate swinging between double swinging and triple swinging, just as many other subjects did in the region between single- and double swinging.
CONCLUSION The merits of the active pendulum hy-
pothesis of upper limb motion are twofold. First, if we consider the upper limbs as ac- tive pendulums, we have good reson to in- clude them in studies of comparative pri- mate locomotion. It has been more common to concentrate on the lower limbs in human walking (Inman et al., 1981; Boccardi et al., 1981; Stein et al., 1973; Marey, 1874), but we have four limbs, not two, and their inter- actions are a clue to our evolutionary history and the mechanics of bipedal locomotion. Our initial preoccupation with the lower limbs is understandable in light of our bi- pedal posture and the fact that we use our upper limbs for so much more than walking, but there is still much that we can learn from a more holistic view of human locomo- tion.
A second advantage of the modified (ac- tive) pendulum hypothesis is that it pro- vides a framework to unite previous human
PENDULAR ACTIVITY OF UPPER LIMBS DURING WALKING 489
locomotion studies (e.g., Carlet, 1872; Burn- side, 1927; Elftman, 1939; Ralston, 1976; Craik et al., 1976; Alexander, 1984) and forms a basis from which to direct further research.
ACKNOWLEDGMENTS This research was supported by the Har-
vard Travellers Club and NSF grant BNS- 8504290 (R. Tuttle, principal investigator).
LITERATURE CITED Alexander RM (1984) Stride length and speed for adults,
children, and fossil hominids. Am. J . Phys. Antrhopol. 63: 23-2 7.
Boccardi S, Pedotti A, Rodano R, and Santambrogio GC (1981) Evaluation of muscular moments at the lower limb joints by an on-line processing of kinematic data and ground reaction. J . Bone Joint Surg. 14:35-45.
Burnside LH (1927) Coordination in the locomotion of infants. Genet Psycho1 Monographs 2:279-372.
Carlet G (1872) l h d e de la Marche. Annales des Sci- ences Naturelles: Zoologie et Paleontologie 16 (Juil- let):81-92.
Chandler RF, Clauser CE, McConville JT, Reynolds HM, and Young JM (1975) Investigation of Inertial Properties of the Human Body. Army Medical Re- search Laboratory Technical Report 74-137 (NTIS #AD-A016-485). Wright-Patterson Air Force Base, OH.
Clauser CE, McConville JT, and Young J M (1969) Weight, volume and center of mass of segments of the human body. Army Medical Research Laboratory technical Report 69-70 (NTIS #AD-710-622). Wright- Patterson Air Force Base, OH.
Craik R, Herman R, and Finley FR (1976) Interlimb coordination. In RM Herman, S Grillner, PSG Stein, and DG Stuart (eds.): Neural Control of Locomotion, Advances in Behavioral Biology 18. New York: Ple- num Press, pp. 51-64.
Daish CB (1972) The Physics of Ball Games. Toronto: Hodder and Stoughton.
Drillis and Contini (1966) Body Segment Parameters. Office of Vocational Rehabilitation, Department of Health, Education and Welfare, Report 116603. New York: New York University School of Engineering and Science.
Elftman H (1939) The function of the arms in walking. Human Biol. 11:529-535.
Fernandez Ballesteros ML, Buchthal F, and Rosenfalck P (1965) The Pattern of Muscular Activity During the Arm Swing of Natural Walking. Acta Physiologica Scandinavica 63t29G-310.
Hogue RE (1969) Upper-extremity muscular activity at different cadences and inclines during normal gait. Phys. Therapy 49:963-972.
Inman RT, Ralston HJ, and Todd F (1981) Human Walking. Baltimore: Williams and Wilkins Co.
Jackson Khf, Joseph J , and Wyard SJ (1978) A mathe- matical model of arm swing during human locomo- tion. Journal of Biomechanics 11 :277-289.
Marey fiJ (1874) Animal Mechanism: A Treatise on Ter- restrial and Aerial Locomotion. New York: D. Apple- ton & Co.
McMahon, TA (1984) Muscles, Reflexes and Locomo- tion. Princeton: Princeton University Press.
Orear J (1979) Physics. New York: Macmillan Publish- ing, Co.
Ralston HJ (1976) Energetics ofHuman Walking. In RM Herman, S Grillner, PSG Stein, and DG Stuart (eds.): Neural Control of Locomotion, Advances in Behav- ioral Biology 18. New York: Plenum Press, pp. 77-98.
Searle GFC (1915) Experimental Harmonic Motion: A Manual for the Laboratory. Cambridge: Cambridge University Press.
Stein RE, Pearson KG, Smith RS, and Redford J B (eds.) (1973) The Control of Posture and Locomotion. New York: Plenum Press.
Suzuki R(1985) Human adult walking. In S Kondo fed.): Primate Morphophysiology, Locomotor Analyses and Human Bipedalism. Tokyo: University of Tokyo Press, pp. 3-24.
Webb D (1989) The Function of the Upper Limbs in Human Walking. Ph.D. thesis. Chicago: University of Chicago, Dept. ofhthropology.