Overview · 2013-08-29 · 8/29/2013 1 Aplastic Anemia: Current Thinking on the Disease, Diagnosis...
-
Upload
nguyenthuy -
Category
Documents
-
view
214 -
download
0
Transcript of Overview · 2013-08-29 · 8/29/2013 1 Aplastic Anemia: Current Thinking on the Disease, Diagnosis...
8/29/2013
1
Aplastic Anemia:
Current Thinking on the Disease,
Diagnosis and Non-Transplant
Treatment
Akiko Shimamura MD PhD
Fred Hutchinson Cancer Research Center
Seattle Children’s Hospital
University of Washington
AA&MDS International Foundation
September 7, 2013
Overview
• What is aplastic anemia?
• Causes of aplastic anemia
• Important lab tests
• Non-transplant treatment options
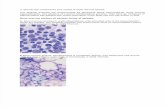
• Supportive care options healthy bone marrow Aplastic anemia
Images courtesy of Dr. Min Xu, Dept of PathologySeattle Children’s Hospital, Seattle, WA
Neutrophils
• Also called: granulocyte, “poly”, PMN, “seg”
• Function: Fights infection (bacteria and fungus) by engulfing/eating them
• ANC: Absolute Neutrophil Count– White blood cell count (WBC) X % neutrophils
Neutrophils
• “neutropenia”: ANC <1,500
• Generally do not start to see increased infection risk until ANC falls below 1,000
• Generally, the lower the ANC, the higher the potential risk of infection.
• Mild: 500-1000
• Moderate: 200-500
• Severe: <200
8/29/2013
2
Platelets
• Function: Help the blood to clot
Platelets
• Thrombocytopenia: platelet count <150,000
• Bleeding risk generally starts to increase when platelet counts fall below 100,000.
Bleeding risk
100,000-50,000: Mildly increased
50,000-20,000: Moderately increased
<10,000-20,000 Significantly increased
• Need to assess bleeding risk within clinical context (eg: surgery, risk of trauma, location of bleed)
• Function: Carry oxygen from the lungs to other organs/tissues
• “Hematocrit” and “Hemoglobin” provide a measure of the red blood cells. Normal levels vary with age and gender.
• “Reticulocytes” are young, newly produced red blood cells.
Red blood cells Aplastic anemia
Primary marrowdisease
Secondary- infection
-medications
-toxins-other disease
Acquired-autoimmune
-idiopathic
-post-hepatitis
Inherited-Fanconi anemia
-Dyskeratosis congenita
-Shwachman-Diamond syndrome-Amegakaryocytic thrombocytopenia
-Diamond-Blackfan anemia
-GATA-2
Severe Aplastic Anemia
(Camitta criteria)
• Two of the following:
– ANC<500 (<200=“very severe”)
– Platelets <20,000
– Low reticulocyte count (corrected for hemoglobin)
• Marrow cellularity <25%
• Moderate aplastic anemia: variable
definitions
Laboratory tests
• Complete blood count (CBC) with
differential
• Bone marrow biopsy
– Cellularity
• Bone marrow aspirate
– Morphology
– ?culture
– Cytogenetics
8/29/2013
3
Cytogenetic clones
x
x
x
x
x
x
x
x
x
x
x
Cytogenetics
Monosomy 7 Fluorescent in-situ
hybridization (FISH)
Normal (2 copies) Abnormal (one copy)
Paroxysmal nocturnal
hemoglobinuria (PNH)
• Clinical features:
– hemolytic anemia (red blood cell
destruction -> anemia)
– thrombosis (blood clots)
– aplastic anemia
PNH (cont)
• GPI anchors other proteins to the cell surface
• PNH cells are deficient in all GPI-anchored
cell surface proteins (eg: CD59 and CD55,
which protect cells from complement)
CD59G
P
I
G
P
I
CD55
8/29/2013
4
PNH
• Small clone size typically asymptomatic
• Unclear whether presence of small PNH
clone affects outcomes
• Treatment approach for AA with
asymptomatic PNH clone is generally the
same
+ DEB or MMC
Courtesy of Lisa Moreau, Dana Farber Cancer Institute, Boston, MA
Fanconi Anemia: Diagnostic Testing
Diagnostic Workup
• Why should we test for inherited bone marrow failure syndromes?
– Poor response to ATG/Cyclosporine (CyA)
– Standard transplant regimens may be highly toxic � Require reduced intensity conditioning regimen
– Inform donor choice
– Family counseling (eg: cord blood banking)
Diagnostic Workup
• Look for clues to inherited bone marrow failure syndrome:
– Clinical history
– Family history
• Low blood counts, malignancies at a young age, excessive toxicity with chemo/radiation, physical disorders
– Physical exam
• Shimamura and Alter, Blood Reviews, 2010. 24(3): 101-122.
Laboratory tests
• Paroxysmal nocturnal hemoglobinuria
• Fanconi anemia (children, young adults)
• AST, ALT, GGT, bilirubin (liver)
• BUN, creatinine, electrolytes (kidney)
• Tests for infections
• Tests for immune/rheumatologic
disorders
• HLA testing of patient and siblings
• Telomere length testing?
TTAGGG n
AATCCC n
5’3’
X-linke
dominant
dominant &
recessive
recessive
DKC1TERC
TERT NOP10
NHP2
TIN2
TIN2
TIN2
TIN2
T
dominantShelterin
Slide courtesy of Dr. Blanche AlterAnd Dr. Sharon Savage, NCI.Published in Shimamura and Alter, Blood Reviews, 2010. Vol 24: pp 101-122
Telomeres
8/29/2013
5
Telomeres shorten with age Telomere length and AA
• Very short telomeres are associated
with an inherited bone marrow failure syndrome (dyskeratosis congenita)
• Shorter telomere length:
– Decreased response to ATG/CyA
– Increased risk of relapse
– Increased risk of clonal progression (MDS)
Scheinberg et al. JAMA 2010; 304: 1358-1364
When to treat?
• Severe or very severe aplastic anemia
• Transfusion-dependent cytopenias
• Note: Limited data on whether it is
advantageous to treat patients with moderate
AA. Some are stable and some improve
spontaneously. Generally work up and
observe.
BMT vs ATG/CsA?
• Survival
• Short-term versus long-term complications
• BMT is curative
ATG: Anti-thymocyte globulin
Higher response rates to horse ATG over rabbit ATG.
Scheinberg et al. NEJM 2011; 365: 430-8.
ATG: toxicities
1. During infusion: Fevers, shaking chills, rash/hives, low blood pressure, difficulty breathing
2. 2-3 weeks post-infusion: serum sickness. Fever, rash, pain in muscles/joints, abdomenalpain/diarrhea, neurological symptoms
3. Blood counts typically fall for the first few weeks (require intensive transfusion support)
4. Immunosuppression
8/29/2013
6
Cyclosporine (CsA): toxicities
-High blood pressure
-Kidney toxicity
-Low magnesium
-Increased body hair/facial hair
-Swollen gums
-Neurologic toxicities (eg: seizure)
-Immunosuppression
Major life-threatening complications of ATG/CsA
-Infection
Fungus
Bacteria
-Bleeding (eg: stroke)
Goal of ATG/CsA
• Blood counts often fail to return to normal
• Goals:
• transfusion-independence
• low risk of severe, life-threatening infections
• (Responses typically seen after ≈3-6 months after initiation of treatment. Some improvement may continue even 1 year post-therapy.)
Pt #: 119 146 51 42 77
*
Pediatric aplastic anemia: >70% response to ATG/CsA(Adults: 60-70% response rate)
Response a
t 6 m
onth
s (
%)
Yrs F/U: 3yr 5yr 10yr 10yr
Pediatric aplastic anemia: survival after ATG/CsA(Adults: 70-80%) Complications after ATG/CsA
• Failure to respond (refractory disease):
25-30%
• Relapse after initial response: 30%
• Clonal evolution (10-15% at 10 years)
– Prognosis varies with specific cytogenetic clones
8/29/2013
7
Maciejewski, J. P. et al. Blood 2002;99:3129-3135
Upfront treatment
Other immunosuppressive agents failed to improve response, relapse or clonal evolution:
MMF (Scheinberg et al. Br J Haematol. 2006: 133: 606-
611)
Sirolimus (Scheinberg et al. Haematologica. 2009; 94:
348-354)
Alemtuzumab (Scheinberg et al. Blood. 2012; 119: 345-354)
Small case reports/series suggest that tacrolimusmight be an alternative to CyA (Alsultan et al. Pediatr
Blood Cancer. 2009; 52: 626-630; Macartney et al, Pediatr Blood Cancer.
2009; 52:525-527)
G-CSF
No benefit for hematologic response or survival in randomized prospective trial
Association with clonal evolution in some retrospective studies
Consider in patients with active infection, persistent fever, or persistently low ANC
Cyclosporin taper
Gradual taper may minimize risk of relapse. Br J Haematol. 2008 Jan;140(2):197-205.
More rapid taper if serious side effects with CsA
Counts may continue to improve over long term
Yrs F/U: 3yr 5yr 10yr 10yr
Treatment of relapse
• Often responds to re-starting or
increasing dose of cyclosporin
• May require re-induction with ATG/CsA
or consideration of bone marrow
transplant
8/29/2013
8
Refractory AA
Persistence of severe AA 6 months following initiation of ATG/CsA
Consider unrelated donor bone marrow transplant
If no suitable donor, consider:
Second round of ATG/CyA (30-50% response)
Alemtuzumab (30-40% response) Scheinberg et al. Blood. 2012 119: 345-354
High dose cyclophosphamide (on study protocol)
Alemtuzumab (Campath)
-Antibody directed against CD52 which is found on the surface of lymphocytes
-Used in autoimmune diseases, lymphoid cancers, and transplants
-Prospective trial at NIH (Scheinberg et al., Blood 2012; 119: 345-354)
Hematologic response at 6 months:
Treatment-naïve: 16 patients
Non-responders to ATG/CsA: 27 patients
Relapse after ATG/CsA: 25 patients
Responses to Alemtuzumab
% r
espondin
g
Previously treated with ATG/CsA
Eltrombopag (Promacta)• Oral medication, stimulates the thrombopoietin
receptor (c-MPL) which is on the surface of megakaryocytes (which make platelets) and blood stem cells
• Olnes et al. NEJM 2012; 367: 11-19
• Treated 25 patients refractory to ATG/CsA for 12 weeks
• 11 (44%) had significant improvement in at least one blood cell type (many multilineage)
• 9 became independent of platelet transfusions
• 3 became independent of rbc transfusions
• 9 had improved neutrophil counts (median increase 1350)
High dose cyclophosphamide
-Comparable response rates compared to ATG/CsA
-?lower relapse rates and lower rates of clonal evolution?
-High rates of fungal infection
-Prolonged neutropenia and very delayed responses requiring intensive supportive care
-Currently recommended on investigational protocols
Supportive Care
8/29/2013
9
Supportive care: anemia
• Transfusion support (red cells)
– Indications: Symptomatic anemia (fatigue, exercise intolerance, rapid
heart rate and breathing, headache,
light-headedness, poor growth)
Supportive care: anemia
• Transfusion support (red cells)
– Risks:
-allosensitization: patient develops antibodies against the transfused red cells or platelets such that transfused cells are rapidly destroyed
-iron overload secondary to red cell transfusions
-initiation of subcutaneous desferalinfusions
-oral iron chelating medication (Exjade) is now available
-transfusion reaction
-infection
Clinical Complications of Iron Overload
• Liver disease with fibrosis and cirrhosis
• Cardiac failure, arrythmias
• Hypopituitarism:• central hypogonadism
• Growth hormone deficiency
• Central hypothyroidism
• Poor growth
• Diabetes mellitus
• Primary hypothyroidism
• Primary hypogonadism
• Hypoparathyroidism
Table 5: Iron Chelation Therapies
Route Toxicities Advantages Disadvantages Monitoring
Deferoxamine
(Desferal) SQ,IV
-skin irritation
-hearing impairment
-decreased vision
-skeletal abnormalities
-infection risk
(Yersinia)
-well defined
toxicity profile
-efficacy
-treatment of
cardiac iron overload
-inconvenience
-poor compliance
-infection and bleeding
risks with SQ infusions if
neutropenic or thrombocytopenic
-annual auditory and
visual testing
- liver iron annually
- cardiac iron and
cardiac function annually (after age 10)
Deferiprone
(L1) PO
-neutropenia
-arthritis
-hepatic fibrosis
-convenience (po)
-may enhance
cardiac iron
chelation
-possible lower efficacy
-toxicity profile
-not approved in US
-regular cbc with
differential
-ALT (monthly)
- liver iron annually
- cardiac iron and
cardiac function annually
(after age 10)
Deferasirox (ICL670,
Exjade)
PO
-GI
-rash
-renal
-transaminitis
-neutropenia
-convenience (po)
-low toxicity to
date
-relatively new
-limited longterm
experience
-creatinine (monthly)
-creatinine clearance
-ALT monthly
-liver iron annually
-cardiac iron and cardiac
function annually (after
age 10)
Iron Chelation Therapies
Supportive care: thrombocytopenia
• Transfusion support (platelets)
– Indications: Symptomatic (bleeding, increased bruising)
Prophylaxis: prior to surgical procedures
history of recurrent or serious bleeding
Key points:
For bleeding, evaluate other potential causes (eg: vitamin K deficiency, liver dysfunction)
Anti-fibrinolytic agents may be helpful (eg: Amicar)
Follow-up post-treatment
• Monitor blood counts
• Post-therapy bone marrow exam
• ?Regular bone marrow exams thereafter?
• Check marrow if blood counts
progressively fall without apparent cause