Olivocochlear neurons sending axon collaterals into the ventral cochlear nucleus of the rat
-
Upload
robert-benjamin -
Category
Documents
-
view
212 -
download
0
Transcript of Olivocochlear neurons sending axon collaterals into the ventral cochlear nucleus of the rat
Olivocochlear Neurons Sending AxonCollaterals Into the Ventral Cochlear
Nucleus of the Rat
MIKLOS HORVATH, K. SUZANNE KRAUS, AND ROBERT-BENJAMIN ILLING*
Neurobiological Research Laboratory, Department of Otorhinolaryngology, University ofFreiburg, D-79106 Freiburg, Germany
ABSTRACTThe olivocochlear projection constitutes the last stage of the descending auditory system
in the mammalian brain. Its neurons reside in the superior olivary complex (SOC) and projectto the inner and outer hair cell receptors in the cochlea. Olivocochlear neurons were alsoreported to send axon collaterals into the cochlear nucleus, but controversies about theirnumber and about species differences persist. By injecting the fluorescent retrograde axonaltracers diamidino yellow and fast blue into the cochlea and the ventral cochlear nucleus(VCN), we studied the distribution and number of olivocochlear neurons with and withoutaxon collaterals into the VCN of the rat. We found that olivocochlear neurons residing in thelateral superior olive (LSO), the intrinsic lateral olivocochlear cells (intrinsic LOCs), do notsend axon collaterals into the VCN. By contrast, a majority, and possibly all, olivocochlearneurons residing in the ventral nucleus of the trapezoid body (VNTB), the medial olivoco-chlear cells (MOCs), do have such axon collaterals. These cells may thus affect processing inthe ascending auditory pathway at the level of the receptors and concurrently at the level ofthe secondary sensory neurons in the cochlear nucleus. Belonging to the lateral olivocochlearsystem, shell neurons reside around the LSO and form a third group of olivocochlear cells(shell LOCs). Like intrinsic LOCs, they innervate the inner hair cells, but like MOCs they do,by means of axon collaterals, project into the VCN. These findings have implications forunderstanding both auditory signal processing and the plasticity responses that occur fol-lowing loss of cochlear function. J. Comp. Neurol. 422:95–105, 2000. © 2000 Wiley-Liss, Inc.
Indexing terms: auditory system; cochlear efferents; descending auditory system; superior olivary
complex
Conduction and integration of sensory signals in theauditory system is affected by extensive countercurrent,or descending, projections on all levels (Spangler andWarr, 1991). Compared to other sensory systems of themammalian brain, this is a unique architecture servingsensory processing. On the lowest level of the descendingauditory system, a massive projection originates in thesuperior olivary complex (SOC) of the brainstem to inner-vate outer (OHC) and inner hair cells (IHC), or theirimmediate afferent synapses, in the cochlea. This olivoco-chlear system shares its developmental origin with facialbranchial motor neurons (Bruce et al., 1997) and may beconsidered to be of the special visceral efferent type.
The olivocochlear system was considered to consist oftwo main populations of cells in the SOC (White and Warr,1983; Aschoff and Ostwald, 1987). The medial olivoco-chlear cells (MOCs) reside in the ventral nucleus of thetrapezoid body (VNTB) of both sides of the brain and
innervate OHCs in the cochlea (Warr et al., 1986). Lateralolivocochlear cells (LOCs) are associated to the lateralsuperior olive (LSO) and innervate predominantly the ip-silateral cochlea and terminate beneath the IHCs (Warr etal., 1986, 1997). According to recent reports by Vetter etal. (1991) and Warr et al. (1997), the second group ofefferent auditory neurons, the LOCs of the rat, should be
Grant sponsor: Deutsche Forschungsgemeinschaft; Grant numbers: Il18/6-2 and Il 18/9-1; Grant sponsor: Research Commission of KlinikumFreiburg.
K.S.K. is a graduate student at the Faculty of Biology, University ofFreiburg, Germany.
*Correspondence to: Dr. R.-B. Illing, Neurobiological Research Labora-tory, Univ.-HNO-Klinik, Killianstr.5, D-79106 Freiburg, Germany.E-mail: [email protected]
Received 9 August 1999; Revised 11 February 2000; Accepted 11 Febru-ary 2000
THE JOURNAL OF COMPARATIVE NEUROLOGY 422:95–105 (2000)
© 2000 WILEY-LISS, INC.
further divided into two subgroups. Small and fusiformcells are located within the ipsilateral LSO and are termedintrinsic LOCs; larger olivocochlear neurons are found inthe periolivary regions around the LSO of both sides ex-tending dendrites into the LSO (Vetter and Mugnaini,1992) and may be called shell neurons or shell LOCs.
The collateral projections of olivocochlear cells were ex-tensively studied in the gerbil, mouse, and guinea pig, butseveral controversies still exist about their pattern. Ryanet al. (1990) reported that the unmyelinated intrinsicLOCs innervate mainly the central part of the ventralcochlear nucleus (VCN) and the myelinated MOCs theperipheral VCN in the gerbil. By contrast, Brown et al.(1988), Brown and Benson (1992), and Brown (1993) foundno cochlear nucleus collaterals of LOCs in gerbil, mouse,and cat. However, a majority of MOC axons (60–100%,depending on species) was found to give off collaterals togranule cell-containing regions of the VCN after incom-plete filling of the VCN with the retrograde tracer sub-stance. This gave rise to the conjecture that possibly up to100% of these cells provide axon collaterals into VCN.Winter et al. (1989) described a similar projection patternin guinea pig. However, they found that only 3.5–9.9% ofMOCs give off collaterals into the VCN. White and Warr(1983) showed, by injecting horseradish peroxidase (HRP)into the cochlea, that axons of olivocochlear cells leave theolivocochlear bundle (OCB) at several points and enter thedorsal cochlear nucleus (DCN) and VCN in rat. The posi-tion and number of cell bodies that belong to these collat-erals have not been evaluated in detail.
Unilateral removal of the cochlea in adult rats causes asubstantial reemergence of the growth-associatedprotein-43 (GAP-43) in the neuropil of the VCN on the sideof the lesion (Illing and Horvath, 1995). This protein istightly related to axonal growth and synaptic plasticity(Benowitz et al., 1990; Benowitz and Routtenberg, 1997).Thus, the increased expression of GAP-43 in the VCN mayserve as an indicator for axonal sprouting and remodelingof synapses in the cochlear nucleus following the loss ofinput from the spiral ganglion. However, following co-chlear removal, neuronal cell bodies of the cochlear nu-cleus do not reexpress GAP-43; the few cells in the co-chlear nucleus that express GAP-43 mRNA at low levelsdo not change this expression (Illing et al., 1997, 1999). Ittherefore appears that the GAP-43-immunoreactive fibersin the VCN belong to neurons that do not reside in thecochlear nucleus itself, but in other regions of the brain-stem. These neurons may synthesize GAP-43 in response
to the cochlear lesion and export the protein to the co-chlear nucleus. In addition to afferents from the cochleaby the axons of spiral ganglion cells (which degenerateupon cochlear ablation), the VCN also receives major pro-jections from noncochlear sources. These include the DCN(Adams and Warr, 1976; Wickesberg et al., 1991), the SOC(Adams, 1983; Covey et al., 1984; Spangler et al., 1987;Shore et al., 1991; Schofield, 1991, 1994; Warr and Beck,1996; Ostapoff et al., 1997), the contralateral cochlearnucleus (Cant and Gaston, 1982; Wenthold, 1987; Shore etal., 1992; Alibardi, 1998), the inferior colliculus (Shore etal., 1991; Caicedo and Herbert, 1993), the auditory cortex(Weedman and Ryugo, 1996a,b), as well as some nonau-ditory projections from the locus coeruleus (Klepper andHerbert, 1991), the dorsal raphe nucleus (Klepper andHerbert, 1991), the dorsal column nuclei, and the spinaltrigeminal nuclei (Itoh et al., 1987; Wright and Ryugo1996). Among these regions, the only population of neu-rons showing a reemergence of GAP-43 in their cell bodyfollowing cochleotomy was found in the ipsilateral LSO(Illing et al., 1997, 1999). These cells appear to be intrinsicLOC neurons and are possible candidates for the emer-gence of GAP-43 and the concomitant plastic reaction inthe neuropil of the VCN. However, the distribution ofolivocochlear cells having collaterals to the VCN has notyet been described in the rat.
The present study was done to determine the distribu-tion and number of olivocochlear cells that give off collat-erals into the VCN en route to the cochlea in the rat. Theretrograde axonal tracers Diamidino Yellow (DY) and FastBlue (FB; Conde, 1987) were used to label cell bodies ofindividual olivocochlear neurons and to visualize cellsthat project to the cochlear nucleus. These tracers havebeen successfully employed to label olivocochlear neuronsin previous studies (Robertson et al., 1987a,b; Aschoff andOstwald, 1988; Robertson and Winter, 1988; Winter et al.,1989).
MATERIALS AND METHODS
Sixteen adult Wistar rats of both sexes were used in thisstudy. The fluorescent neuronal tracers DY (Sigma-Aldrich, Germany) or FB (Sigma-Aldrich) were introducedinto the inner ear or injected into the VCN in order toidentify those olivocochlear cells that give off collateralsinto the cochlear nucleus. Animals were anesthetized byintraperitoneal (i.p.) injection of a mixture of Ketanest S
Abbreviations
AChE acetylcholinesteraseAVCN anteroventral cochlear nucleusChAT choline acetyltransferaseDCN dorsal cochlear nucleusDY diamidino yellowFB fast blueGAP-43 growth-associated protein-43HRP horseradish peroxidaseIHC inner hair celllfp longitudinal fasciculus of ponsLOCs lateral olivocochlear cellsLSO lateral superior oliveMNTB medial nucleus of the trapezoid bodyMOCs medial olivocochlear cellsMSO medial superior olive
n5sp spinal trigeminal tractn5sr sensory root of trigeminal nerven7 facial nerveN7 facial nucleusn8 vestibulocochlear nerveOCB olivocochlear bundleOHC outer hair cellPn pontine nucleipt pyramidal tractPVCN posteroventral cochlear nucleusSOC superior olivary complexSPO superior periolivary nucleustb trapezoid bodyVCN ventral cochlear nucleusVNTB ventral nucleus of the trapezoid body
96 HORVATH ET AL.
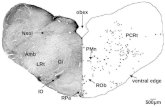
Fig. 1. Drawings of serial frontal sections through the brainstemof a representative animal showing the distribution of retrogradelylabeled cells following FB application into the left cochlea. Trianglesindicate the predominantly ipsilateral distribution of LOCs in theSOC. Squares show the bilateral distribution of MOCs in the VNTB.
Each drawing integrates counts from three neighboring sections. Onesymbol represents one labeled neuron outside the LSO. In the LSO,one triangle represents five labeled neurons. Section numbers werecounted from caudal to rostral, starting at the caudal end of the DCN.For abbreviations, see list. Scale bar 5 1 mm.
97AXON COLLATERALS OF OLIVOCOCHLEAR NEURONS
(50 mg/kg, Parke-Davis, Ann Arbor, MI) and Rompun (5mg/kg, Bayer Leverkusen, Germany).
Two kinds of operations were done. In the first group(n 5 6), the left cochlea was approached by a lateralopening of the tympanic bulla. To expose the cochlea, thefacial nerve (n7) was cut at its exit from the skull. A smallhole was drilled into the cochlear wall, centered over themiddle of its long axis (cp. Voelker et al., 1980), and partof the perilymph was soaked off using small tissue wicks.FB (n 5 4) or DY (n 5 2) was tamped into the cochlea andthe opening closed with surgical bone wax. The bulla wassubsequently filled with Gelfoam (Gelita, B. Braun, Ger-many) and the wound surgically closed.
In the second group (n 5 10), FB was injected into theleft VCN, immediately followed by application of DY intothe cochlea of the same side. To approach the VCN, asmall craniotomy was made in the left occipital bone andthe overlying cerebellar flocculus and paraflocculus wereaspirated to expose the cochlear nucleus. FB was injectedthrough a glass micropipette with a tip diameter of about50 mm. Using pressure, 0.15 ml saturated solution of FBwere slowly injected; the pipette was allowed to remain inplace for 10 minutes before being withdrawn. The appli-cation of DY into the cochlea was done as described for thefirst group of animals. The bulla and the cavity of theflocculus were filled with Gelfoam and the wound surgi-cally closed. At completion of the operations, the rats weregiven the analgesic Novalgin (10 mg/kg intramuscularly[i.m.], Hoechst AG, Frankfurt am Main, Germany). Theanimals were allowed to recover for a period of 5 or 6 days.They were then deeply anesthetized with an overdose ofNembutal (300 mg/kg i.p., Sanofi, Paris) and fixed bytranscardial perfusion with 4% paraformaldehyde inphosphate buffer (0.1 M, pH 7.4, 4°C). Thirty micrometer-thick frozen sections of the brainstem were cut in thefrontal plane and mounted on gelatin-subbed slides. Sec-tions were viewed with a Zeiss microscope using epi-illumination. The exciting filter used had a peak at 405nm and the barrier filter cutoff was 455 nm. To localize theinjection site and the labeled cells within the variousbrainstem nuclei, maps were made based on camera lu-cida drawings. Individual olivocochlear neurons were ex-amined using a 1003 oil-immersion objective in order todistinguish single from double-labeled cells. Care and useof the animals as reported here were approved byRegierungsprasidium Freiburg, permission number 37/9185.81/1/267.2.
The number of labeled cells was determined using pro-file counts. For neurons labeled by FB, cell bodies recog-nizable as such were counted; for neurons labeled withDY, cell nuclei were counted. In each case, raw countswere corrected for numerical overestimation resultingfrom double counts due to sectioning using the equation
provided by Abercrombie (1946; cp. Guillery and Herrup,1997). This was done based on the following measure-ments: cell body diameter as apparent in FB staining ofneurons in LSO: 9.9 6 0.4 (n 5 13), in VNTB: 15.5 6 0.4(n 5 39), of shell neurons: 13.9 6 0.5 (n 5 14); nucleusdiameter as apparent in DY staining in neurons of LSO:8.4 6 0.2 (n 5 26), of VNTB: 10.960.3 (n 5 30), in shellneurons: 10.0 6 0.4 (n 5 12). All counting data in thisstudy went through this kind of correction.
RESULTS
Olivocochlear neurons
Following FB or DY application into the cochlea, retro-gradely labeled cells were encountered bilaterally in theSOC as described earlier (White and Warr, 1983; Aschoffand Ostwald, 1988). Labeled cells were found in the facial,vestibular, and reticular nuclei, but these will not be dealtwith any further in this study. Small (diameter approxi-mately 10 mm) and fusiform neurons inside the LSO (in-trinsic LOCs) were labeled virtually exclusively on theipsilateral side. The majority of larger (approximately 15mm) multipolar cells of the VNTB (MOCs) resided on thecontralateral side. Additionally, larger (about 15 mm) neu-rons in the periolivary region around the LSO were la-beled with an ipsilateral dominance. These neurons werelocated mainly dorsal, caudal, and rostral to the LSO, andtheir dendrites were seen to extend almost always into theLSO. This group of olivocochlear cells may be called shellneurons (Vetter and Mugnaini, 1992), or shell LOCs. Fig-ure 1 shows the distribution of neurons in the SOC thatwere retrogradely labeled following FB application intothe left cochlea of one representative animal. There wasno difference in the distribution of labeled cells after theintracochlear application of DY or FB. The total number oflabeled olivocochlear cells was 901.0 6 17.7 SEM aftertracing with FB and 1,129.0 6 13.0 SEM after DY appli-cation. Averaging over six cases, 509.2 6 19.8 SEM cellswere labeled in the ipsilateral LSO, 54.2 6 5.0 SEM cellsin the ipsilateral shell, and 170.0 6 13.7 SEM cells in theipsilateral VNTB. Contralaterally, no labeled neuronswere found in the LSO in three cases, but up to two labeledcells were seen in others. There were 14.3 6 1.3 SEMlabeled contralateral shell neurons and 228.3 6 17.7 SEMlabeled cells in the contralateral VNTB. DY-labeled cellscould be classified only according to the location of cellsand the size of the nucleus as the dendritic morphology ofthe labeled cells was not revealed by the tracer. Table 1summarizes the counts of retrogradely labeled olivoco-chlear neurons over all six experiments.
TABLE 1. Numbers of Retrogradely Labeled Olivocochlear Neurons Following DY or FB Application Into the Cochlea
Experiment no.
LSO Shell VNTB
TotalIpsilateral Contralateral Ipsilateral Contralateral Ipsilateral Contralateral
M441 (FB) 519 2 40 11 154 200 926M314 (FB) 497 2 56 14 166 201 936M341 (FB) 491 2 55 20 121 189 878M398 (FB) 430 0 41 14 163 216 864M417 (DY) 559 0 61 15 211 296 1,142M421 (DY) 559 0 72 12 205 268 1,116Mean 6 SEM 509.2 6 19.8 1.0 6 0.4 54.2 6 5.0 14.3 6 1.3 170.0 6 13.7 228.3 6 17.7 977.0 6 49.5
98 HORVATH ET AL.
Fig. 2. Distribution of neurons onthe level of SOC projecting to the VCN.A: Injection site into the cochlear nu-cleus, representative for this experi-mental series in both position and size.Dark gray area indicates peak concen-tration of FB and the gray surroundshows diffusion range of the tracer.B: Representative distribution of retro-gradely labeled neurons following FBinjection into the left VCN togetherwith DY application into the left co-chlea. Neurons labeled from cochleaonly are not shown. Open circles indi-cate FB-labeled cells that project to theVCN. Filled circles represent double-labeled cells, i.e., olivocochlear cellssending an axon collateral into theVCN. Each drawing shows counts fromone section only. Note that there aremany more cells in the SOC projectingto the VCN as compared to those pro-jecting to the cochlea (see Fig. 1). Sec-tion numbering as for Figure 1. For ab-breviations, see list. Scale bars 5 1 mmin A,B.
99AXON COLLATERALS OF OLIVOCOCHLEAR NEURONS
Double-labeling experiments
In the second series of experiments, we combined theretrograde labeling of olivocochlear cells with the visual-ization of cells that project to the VCN. DY was applied tothe left cochlea to trace olivocochlear cells and FB wasinjected into the VCN of the same side. The average num-ber of labeled olivocochlear cells (929.0 6 42.6 SEM) wasindistinguishable to the counts obtained after a singleapplication of FB or DY into the cochlea as detailed above(977.0 6 49.5 SEM). In five experiments, the injection siteof FB, including their diffusion range, was restricted tothe VCN and included both the posteroventral (PVCN)and anteroventral cochlear nucleus (AVCN). Figures 2and 4 document the injection sites of FB in the VCN of tworepresentative cases. The most medial and rostral parts ofthe VCN were not filled with the tracer.
Following the FB injection into the VCN, a large num-ber of periolivary cells were labeled, showing an ipsilat-eral dominance (ipsilateral vs. contralateral 1.5:1). Prin-cipal neurons of the ipsilateral medial nucleus of thetrapezoid body (MNTB) were also labeled. The ipsilateralLSO had a few (8.8 6 2.3 SEM), the contralateral had nolabeled neurons. Additionally, multipolar neurons of thecontralateral VCN, some cells in the deeper layers of thecontralateral DCN, and neurons of all three subnuclei ofthe inferior colliculus were also labeled. Figure 2 providesdata of one representative experiment, showing the dis-tribution of neurons in the SOC that were retrogradelylabeled after injection of FB into the VCN. In five otherexperimental cases, the injection site extended beyond theVCN and covered part of the white matter immediatelymedial of it. As axons of the olivocochlear neurons run inthis bundle, tracer uptake by its fibers resulted in retro-grade labeling of cells in several regions of the brainstem,including the ipsilateral LSO. These experiments were notconsidered for our quantitative evaluations.
Olivocochlear neurons with collateralsto the VCN
The combination of DY and FB allowed us to identifyolivocochlear cells giving off collaterals into the VCN. Thecombined application of these tracers resulted in partlyintermingled populations of blue and/or yellow-labeledneurons (Figs. 3A,B). Single-labeled neurons showed onlyyellow fluorescence of the nucleus (Fig. 3C) or blue stain-
ing of the cytoplasm (Fig. 3D). Double-labeled neuronswere clearly distinguishable by the intense yellow fluores-cence of their nucleus and their blue cytoplasm (Figs.3E–G). We found double-labeled cells in the periolivaryregion around the LSO (shell LOCs) and in the VNTB(MOCs) of both sides of the brain. Across five experiments,only six double-labeled neurons were found in the ipsilat-eral LSO. Figure 4 shows the distribution of single anddouble-labeled olivocochlear cells in the SOC. Fifty-sixpercent of the MOCs in the VNTB and 39% of shell neu-rons were seen by virtue of their double labeling to sendcollaterals into the VCN. These percentages resulted fromretrograde transport originating in a restricted area of theVCN. They are expected to grow, possibly up to 100%, witha larger or even complete coverage of the VCN with tracer,which is difficult to achieve for practical reasons. Table 2summarizes counts and SEMs of single and double-labeled olivocochlear neurons in the LSO, shell region,and the VNTB.
DISCUSSION
The major results of the present study are that (1) theintrinsic lateral olivocochlear neurons in the LSO of therat do not have axon collaterals reaching into VCN; (2) asignificant number, and possibly a majority, of shell neu-rons around the LSO of both sides give off collaterals intoVCN and, by virtue of their specific connectivity, consti-tute a distinct population of olivocochlear cells; and (3) amajority of MOCs, but possibly even all of them, residingin the VNTB of both sides send axon collaterals into theVCN. They are therefore in a position to massively influ-ence the ascending auditory pathway, not only at the levelof the hair cells, but also on the level of the cochlearnucleus.
Single labeling of olivocochlear cells
In the present study, we could identify and quantify twogroups of LOCs — one in the ipsilateral LSO (intrinsicLOCs) and another in the periolivary region around theLSO of both sides (shell LOCs). MOCs were found in theVNTB of both sides. The distribution and number of olivo-cochlear cells are in general agreement with previousstudies. White and Warr (1983) described the dual (i.e.,lateral and medial) origin of the OCB in the rat by usingaxonal transport of HRP. Injection of free HRP into thecochlea resulted in the retrograde labeling of 350 cells inthe LSO and 300 cells in the VNTB. The numbers oflabeled olivocochlear cells in the present study are signif-icantly larger than these counts (509.2 6 19.8 cells in theLSO and 398.3 6 30.8 cells in the VNTB), but somewhatsmaller than found previously with the same fluorescenttracers (540 cells in the LSO and 556 in the VNTB, Aschoffand Ostwald, 1988; cp. Robertson et al., 1989). In addition,we also recognized labeled shell neurons first described byVetter et al. (1991). Shell neurons were also traced byintracochlear injection of cholera toxin tracers. It wasshown that their dendrites penetrate into the LSO andsurrounding areas (Vetter and Mugnaini, 1992). In sixcases, we counted 68.5 6 5.3 SEM shell neurons, 54.2 65.0 SEM cells around the ipsilateral, and 14.3 6 1.3 SEMcells around the contralateral LSO. Injection of choleratoxins resulted in 111 labeled shell neurons, 105 ipsilat-eral, and 6 contralateral (Vetter and Mugnaini, 1992).
Fig. 3. Retrogradely labeled cells in the SOC following DY appli-cation into the left cochlea and FB injection into the left VCN.A: Labeled cells in and around the ipsilateral LSO. Outline of LSO isindicated by dashed line. Arrowheads point to shell neurons. B: La-beled neurons in the VNTB on the side of the injection; MOC neuronmay be recognized by their yellow nucleus. Double-labeled cells can-not easily be identified at this magnification. tb, trapezoid body.C: Single-labeled cells in the ipsilateral LSO showing only yellownuclear staining through axonal transport of the tracer DY. D: Single-labeled neuron in the ipsilateral VNTB, showing only blue staining ofthe cytoplasm through axonal transport of FB. E–G: Double-labeledneurons in the ipsilateral (E) and contralateral (F, G) VNTB showingyellow fluorescence of the nucleus and blue staining of the cytoplasm.These cells prove, by virtue of their double labeling, that they sendaxon collaterals to both the cochlea and the cochlear nucleus. Arrowpoints to FB labeling of the cytoplasm of a neuron; arrowhead pointsto DY labeling of its nucleus. Scale bar 5 100 mm for A,B; 10 mm forC–G.
101AXON COLLATERALS OF OLIVOCOCHLEAR NEURONS
Fig. 4. Olivocochlear neurons in the SOC labeled after DY appli-cation into the left cochlea and FB injection into the left VCN.A: Injection site into the cochlear nucleus, representative for thisexperimental series in both position and size. Dark gray area indi-cates peak concentration of FB and the gray surround shows diffusionrange of the tracer. B: Drawings of frontal sections with single anddouble-labeled neurons through the SOC. Each drawing representsdata from three neighboring sections. Neurons single labeled from the
cochlear nucleus are not shown (see Fig. 2). Triangles indicate LOCs,squares indicate MOCs. In the LSO, each triangle represents fivelabeled neurons; outside the LSO each symbol marks one cell. Opensymbols represent neurons single labeled by DY, filled symbols rep-resent double-labeled neurons. Filled symbols indicate olivocochlearcells that also project to VCN. Section numbering as in Figure 2. Forabbreviations, see list. Scale bars 5 1 mm in A,B.
These differences may be explained by a difference inuptake efficiency of the different tracers.
Following tracer application into the cochlea, marginalto low levels of FB remained in the middle ear cavity. Thismay have resulted in labeling of a comparatively sparsepopulation of motor neurons of middle ear muscles (Span-gler et al., 1982; Keller et al., 1983; Rouiller et al., 1989)and salivatory glands (Hiura, 1977). However, areas ofoverlap with olivocochlear cells only exist for stapedialmotor neurons and are marginal even there (Rouiller etal., 1989). Should this labeling have occurred, our count-ing error would not exceed 5%.
Double-labeling experiments
In our second experimental group, we have combinedthe retrograde tracing of the olivocochlear cells with DYand the injection of FB into the VCN. This tracer combi-nation has been proven useful in numerous neuroana-tomical studies dealing with virtually all projectional sys-tems in the mammalian brain. Double-labeled cells can beeasily identified by a yellow nucleus and blue cytoplasm(Figs. 3E–G). This combination of tracers has also beenused to study the collateral projections of olivocochlearcells in the guinea pig (Robertson and Winter 1988; Win-ter et al., 1989).
Quantitative evaluation was based only on those exper-iments in which the injection and local diffusion of FBwere confined to the VCN and included both the PVCNand the AVCN (Figs. 2, 4) but not adjacent white matter.Regions that were found to project to the VCN includedthe ipsilateral DCN, the ipsilateral and contralateralSOC, the contralateral DCN and VCN, and the inferiorcolliculus of both sides. These projections are known andwere analyzed in previous studies in the cat (Adams andWarr, 1976; Cant and Gaston, 1982; Adams, 1983; Span-gler et al., 1987), guinea pig (Wenthold, 1987; Schofield,1991, 1994; Shore et al., 1991, 1992; Ostapoff et al., 1997),and rat (Weedman and Ryugo, 1996a,b; Warr and Beck,1996; Alibardi, 1998). Our study specifically focuses onolivocochlear cells projecting to the VCN.
MOCs with collaterals to the VCN
Our results show that the majority of MOCs, located inthe VNTB of both sides, send collaterals into the VCN(Fig. 4, Table 2). Double-labeled cells were found along thewhole rostrocaudal extent of the VNTB and the percent-age of double-labeled cells was almost the same on the twosides (ipsilateral 58%, contralateral 54%). Because thetracer injection into the VCN could not cover the wholenucleus, the counts we obtained must necessarily be min-imal estimates. We suggest that in fact 80–100% of MOCssend collaterals into VCN. This estimate would be consis-tent with previous observations in some other species.Using HRP injection into the cochlea, 67–100% (depend-ing on the species) of thick MOC axons were found to giveoff collaterals into the VCN in the cat (Brown at al., 1988),mouse (Brown et al., 1988; Brown and Benson, 1992), andgerbil (Brown et al., 1988; Ryan et al. 1990). In the rat,HRP injection into the cochlea showed that collateralsleave the OCB and enter the VCN at two points: at thejunction between the DCN and PVCN and from the mainOCB as it descends on the medial side of the vestibularnerve, entering the AVCN (White and Warr, 1983). Thesecollaterals are also apparent with stains for acetylcho-linesterase (AChE; White and Warr, 1983). Injection of
TA
BL
E2.
Num
bers
and
Loc
aliz
atio
nof
Oliv
ococ
hlea
rN
euro
nsF
ollo
win
gD
YA
pplic
atio
nIn
toth
eC
ochl
eaan
dF
BIn
ject
ion
Into
the
VC
Nof
the
Sam
eS
ide1
Exp
erim
ent
no.
LS
OS
hel
lV
NT
BT
otal
Ipsi
late
ral
all
dlC
ontr
alat
eral
all
Ipsi
late
ral
all
dlC
ontr
alat
eral
all
dlIp
sila
tera
lal
ldl
Con
tral
ater
alal
ldl
All
dl
M42
249
70
051
2415
917
610
619
712
993
626
8M
424
410
00
459
127
191
113
305
170
963
299
M43
637
71
030
107
615
870
205
9477
718
0M
437
486
40
5722
129
160
106
216
128
931
270
M43
954
51
066
189
421
312
620
589
1038
237
Mea
n6
SE
M46
3.0
630
.51.
26
0.7
049
.86
6.0
16.6
63.
111
.06
1.4
7.0
60.
917
9.6
610
.310
4.2
69.
322
5.6
620
.112
2.0
614
.692
9.0
642
.625
0.8
620
.2
1 Dou
ble-
labe
led
(dl)
cell
sse
ndi
ng
coll
ater
als
into
the
VC
N.
103AXON COLLATERALS OF OLIVOCOCHLEAR NEURONS
3H-leucine and biotinylated dextran amine into the VNTBof the rat resulted in strong anterograde labeling in thecochlear nuclei and identification of the OCB (Warr andBeck, 1996). However, indirect evidence from other stud-ies suggested that collateral projections from the OCB tothe VCN are very sparse in the rat. Cutting the entireOCB causes only slight reductions in levels of AChE in thecochlear nucleus (Osen et al., 1984). In microassays forcholine acetyltransferase (ChAT), a reduction of onlyabout 15% was observed in the cochlear nucleus granulecell region of the rat (Godfrey et al., 1987); a reduction of70% was found in the cat (Godfrey et al., 1990).
Injecting FB into the cochlear nucleus and DY into thecochlea of the guinea pig, Winter et al. (1989) saw only3.5–9.9% of MOCs projecting to the cochlear nucleus. Thisdifference to the data obtained in the present study couldeither be an interspecies difference between guinea pigand rat or the result of methodological differences. Inthree experimental cases, Winter et al. (1989) injected a2% aqueous solution of FB into the cochlear nucleus (in-cluding the DCN). However, the 9.9% double labeling ofMOCs was reported in only one experiment, whereas theothers showed even fewer double-labeled cells.
The local targets of the collateral projections fromMOCs could not be determined in the present study as ourinjection site occupied both the peripheral and centralVCN (Figs. 2, 4). Previous ultrastructural investigationsshowed that cochlear nucleus collaterals of MOCs termi-nate on varicose dendrites of small cells and on largedendrites of multipolar cells in mice (Benson and Brown1990; Benson et al., 1996). The massive collateral projec-tion of MOCs found in the present study indicates thatMOCs must play a crucial role in feedback control ofauditory processing. These cells can directly influence themotility of OHCs (Dallos et al., 1990; Liberman et al.,1990) as well as the secondary sensory neurons in thecochlear nucleus. They are themselves under the influenceof an excitatory input from the contralateral cochlea byway of the cochlear nucleus, thus serving to link bothcochlea and both cochlear nuclei (Robertson and Winter,1988; Thompson and Thompson, 1991). This regional feed-back loop, together with descending pathways from theinferior colliculus (Faye-Lund, 1986; Caicedo and Herbert,1993; Vetter et al., 1993) and auditory cortex (Feliciano etal., 1995; Weedman and Ryugo, 1996a,b), constitutes theanatomical basis for the efferent control of auditory pro-cessing in the lower brainstem.
LOCs with collaterals to the VCN
The second main finding of the present study is that thetwo types of lateral olivocochlear neurons differ in theircollateral projection pattern. Although intrinsic LOCs inthe LSO, apart from extremely rare single cells, do notsend collaterals into the VCN, a majority of shell neuronsaround the LSO may send axon branches into the VCN.Previous studies have shown that the projections from theLSO to the cochlear nucleus are very sparse in the guineapig (Winter et al., 1989; Schofield, 1994; Ostapoff et al.,1997) and cat (Spangler et al., 1987). Weedman and Ryugo(1996a) did not observe any labeled cells in the LSO fol-lowing FB injection into the rat cochlear nucleus. Onaverage, we found only 8.8 6 2.3 SEM LSO cells thatproject to the VCN. Across all five experiments, only six ofthese neurons (1.2 6 0.7 SEM) proved, by virtue of theirdouble labeling, to be olivocochlear cells. When our injec-tion extended medially to the VCN and touched the OCB,
nearly all LOCs in the LSO were double labeled. This isevidence that the thin unmyelinated axons are capable oftransporting either tracer. The failure of labeling thesecells by tracer injections strictly confined to the VCN doesimply the absence of axon terminals there.
There is virtually no projection from intrinsic LOCs tothe VCN in the normal adult rat. Therefore, we mustconclude that the cochleotomy-induced reemergence ofGAP-43 in the neuropil of the VCN and in cell bodies ofintrinsic LOCs reported earlier (Illing et al., 1997, 1999)are independent consequences of the cochlear lesion —unless there occurs a cochleotomy-dependent sprouting ofthe axons of these olivocochlear neurons into the cochlearnucleus, a possibility currently investigated in this labo-ratory.
Our results support the evidence that LOCs are dividedinto two subclasses in the rat: the small (diameter approx-imately 10 mm) and fusiform intrinsic neurons in the LSOand the larger (diameter approximately 15 mm) and mul-tipolar shell neurons around the LSO (Vetter and Mug-naini, 1992). It has already been reported that thesegroups of cells have different terminal arborizations in thecochlea (Warr et al., 1997). Here we show that the axonalprojection pattern of these neurons is also different: manyshell LOCs send collaterals into the VCN, whereas theintrinsic LOCs in the LSO project only to the cochlea.Apparently, shell neurons share size, dendritic morphol-ogy, and the collateral projection pattern including thepossibility to project to both cochleae, with MOCs (Aschoffand Ostwald, 1988; Robertson et al., 1989), but their lo-calization close to the LSO, and their innervation of IHCsin the cochlea with the LOCs.
ACKNOWLEDGMENTS
We thank Mrs. B. Hobmaier, Mr. W. Kuhn, and Mrs. G.Wittmann for technical assistance. M.H. was supported byOTKA, Hungary (grant T 025231), and received a fellow-ship from the Deutsche Akademische Austauschdienst(DAAD).
LITERATURE CITED
Abercrombie M. 1946. Estimation of nuclear population from microtomesections. Anat Rec 94:239–247.
Adams JC. 1983. Cytology of periolivary cells and the organization of theirprojections in the cat. J Comp Neurol 215:275–289.
Adams JC, Warr WB. 1976. Origins of axons in the cat’s acoustic striaedetermined by injection of horseradish peroxidase into severed tracts.J Comp Neurol 170:107–122.
Alibardi L. 1998. Ultrastructural and immunocytochemical characteriza-tion of commissural neurons in the ventral cochlear nucleus of the rat.Anat Anz 180:427–438.
Aschoff A, Ostwald J. 1987. Different origin of cochlear efferents in somebat species, rats and guinea pigs. J Comp Neurol 264:56–72.
Aschoff A, Ostwald J. 1988. Distribution of cochlear efferents and olivo-collicular neurons in the brainstem of rat and guinea pig. Exp BrainRes 71:241–251.
Benowitz LI, Routtenberg A. 1997. GAP-43: an intrinsic determinant ofneuronal development and plasticity. Trends Neurosci 20:84–91.
Benowitz LI, Perrone-Bizzozerd NI, Neve RL, Rodriguez W. 1990. GAP-43as a marker for structural plasticity in the mature CNS. Prog BrainRes 86:309–320.
Benson TE, Brown MC. 1990. Synapses formed by olivocochlear axonbranches in the mouse cochlear nucleus. J Comp Neurol 295:52–70.
Benson TE, Berglund AM, Brown MC. 1996. Synaptic input to cochlearnucleus dendrites that receive medial olivocochlear synapses. J CompNeurol 365:27–41.
104 HORVATH ET AL.
Brown MC. 1993. Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brainstem. J Comp Neurol337:600–613.
Brown MC, Benson TE. 1992. Transneuronal labeling of cochlear nucleusneurons by HRP-labeled auditory nerve fibers and olivocochlearbranches in mice. J Comp Neurol 321:645–665.
Brown MC, Liberman MC, Benson TE, Ryugo DK. 1988. Brainstembranches from olivocochlear axons in cats and rodents. J Comp Neurol278:591–603.
Bruce LL, Kingsley J, Nichols DH, Fritzsch B. 1997. The development ofvestibulocochlear efferents and cochlear afferents in mice. Int J DevNeurosci 15:671–692.
Caicedo A, Herbert H. 1993. Topography of descending projections from theinferior colliculus to auditory brainstem nuclei in the rat. J CompNeurol 328:377–392.
Cant NB, Gaston KC. 1982. Pathways connecting the right and left co-chlear nuclei. J Comp Neurol 212:313–326.
Conde F. 1987. Further studies on the use of the fluorescent tracers fastblue and diamidino yellow: effective uptake area and cellular storagesites. J Neurosci Methods 21:31–43.
Covey E, Jones DR, Casseday JH. 1984. Projections from the superiorolivary complex to the cochlear nucleus in the tree shrew. J CompNeurol 226:289–305.
Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR. 1990. Themechanisms and biophysics of hearing. New York: Springer. 418 p.
Faye-Lund H. 1986. Projection from the inferior colliculus to the superiorolivary complex in the albino rat. Anat Embryol 175:35–52.
Feliciano M, Saldana E, Mugnaini E. 1995. Direct projections from the ratprimary auditory neocortex to nucleus sagulum, paralemniscal region,superior olivary complex and cochlear nuclei. Aud Neurosci 1:287–308.
Godfrey DA, Park-Hellendall JL, Dunn JD, Ross CD. 1987. Effect of olivo-cochlear bundle transection on choline acetyltransferase activity in therat cochlear nucleus. Hear Res 28:237–251.
Godfrey DA, Beranek KL, Carlson L, Parli JA, Dunn JD, Ross CD. 1990.Contribution of centrifugal innervation to choline acetyltransferaseactivity in the cat cochlear nucleus. Hear Res 49:259–280.
Guillery RW, Herrup K. 1997. Quantification without pontification: choos-ing a method for counting objects in sectioned tissues. J Comp Neurol386:2–7.
Hiura T. 1977. Salivatory neurons innervate the submandibular and sub-lingual glands in the rat: horseradish peroxidase study. Brain Res137:145–149.
Illing RB, Horvath M. 1995. Re-emergence of GAP-43 in cochlear nucleusand superior olive following cochlear ablation in the rat. Neurosci Lett194:9–12.
Illing RB, Horvath M, Laszig R. 1997. Plasticity of the auditory brainstem:effects of cochlear ablation on GAP-43 immunoreactivity in the rat.J Comp Neurol 382:116–138.
Illing RB, Cao QL, Forster CR, Laszig R. 1999. Auditory brainstem: devel-opment and plasticity of GAP-43 mRNA expression in the rat. J CompNeurol 412:353–372.
Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. 1987. Directprojections from the dorsal column nuclei and the spinal trigeminalnuclei to the cochlear nuclei in the cat. Brain Res 400:145–150.
Keller JT, Saunders MC, Ongkiko CM, Johnson J, Frank E, Van LoverenH, Tew JM Jr. 1983. Identification of motoneurons innervating thetensor tympani and tensor veli palatini muscles in the cat. Brain Res270:209–215.
Klepper A, Herbert H. 1991. Distribution and origin of noradrenergic andserotoninergic fibers in the cochlear nucleus and inferior colliculus ofthe rat. Brain Res 557:190–201.
Liberman MC, Dodds LW, Pierce S. 1990. Afferent and efferent innerva-tion of the cat cochlea: quantitative analysis with light and electronmicroscopy. J Comp Neurol 301:443–460.
Osen KK, Mugnaini E, Dahl AL, Christiansen AH. 1984. Histochemicallocalization of acetylcholinesterase in the cochlear and superior olivarynuclei. A reappraisal with emphasis on the cochlear granule cellssystem. Arch Ital Biol 122:169–212.
Ostapoff EM, Benson CG, Saint Marie RL. 1997. GABA and glycine-immunoreactive projections from the superior olivary complex to thecochlear nucleus in guinea pig. J Comp Neurol 381:500–512.
Robertson D, Winter IM. 1988. Cochlear nucleus inputs to olivocochlearneurones revealed by combined anterograde and retrograde labellingin the guinea pig. Brain Res 462:47–55.
Robertson DK, Cole S, Corbett K. 1987a. Quantitative estimate of bilater-
ally projecting medial olivocochlear neurones in the guinea pig brain-stem. Hear Res 27:177–181.
Robertson D, Cole KS, Harvey AR 1987b. Brainstem organization of effer-ent projections to the guinea pig cochlea studied using the fluorescenttracers fast blue and diamidino yellow. Exp Brain Res 66:449–457.
Robertson D, Harvey AR, Cole KS. 1989. Postnatal development of theefferent innervation of the rat cochlea. Brain Res Dev Brain Res 47:197–207.
Rouiller EM, Capt M, Dolivo M, De Ribaupierre F. 1989. Neuronal orga-nization of the stapedius reflex pathways in the rat: a retrograde HRPand viral transneuronal tracing study. Brain Res 476:21–28.
Ryan AF, Keithley EM, Wang ZX, Schwartz IR. 1990. Collaterals fromlateral and medial olivocochlear efferent neurons innervate differentregions of the cochlear nucleus and adjacent brainstem. J Comp Neurol300:572–582.
Schofield BR. 1991. Superior paraolivary nucleus in the pigmented guineapig: separate classes of neurons project to the inferior colliculus and thecochlear nucleus. J Comp Neurol 312:68–76.
Schofield BR. 1994. Projections to the cochlear nuclei from principal cells inthe medial nucleus of the trapezoid body in guinea pigs. J Comp Neurol344:83–100.
Shore SE, Helfert RH, Bledsoe SC Jr, Altschuler RA, Godfrey DA. 1991.Descending projections to the dorsal and ventral divisions of the co-chlear nucleus in guinea pig. Hear Res 52:255–268.
Shore SE, Godfrey DA, Helfert RH, Altschuler RA, Bledsoe SC Jr. 1992.Connections between the cochlear nuclei in guinea pig. Hear Res 62:16–26.
Spangler KM, Warr WB. 1991. The descending auditory system. In: Alt-schuler RA, Hoffman DW, Bobbin RP, Clopton B, editors. Neurobiologyof hearing, Vol. 2. New York: Raven Press. p 27–45.
Spangler KM, Henkel CK, Miller IJ Jr. 1982. Localization of the motorneurons to the tensor tympani muscle. Neurosci Lett 32:23–27.
Spangler KM, Cant NB, Henkel CK, Farley GR, Warr WB. 1987. Descend-ing projections from the superior olivary complex to the cochlear nu-cleus of the cat. J Comp Neurol 259:452–465.
Thompson AM, Thompson GC. 1991. Posteroventral cochlear nucleus pro-jections to olivocochlear neurons. J Comp Neurol 303:267–285.
Vetter DE, Mugnaini E. 1992. Distribution and dendritic features of threegroups of rat olivocochlear neurons. Anat Embryol 185:1–16.
Vetter DE, Adams JC, Mugnaini E. 1991. Chemically distinct rat olivoco-chlear neurons. Synapse 7:21–43.
Vetter DE, Saldana E, Mugnaini E. 1993. Input from the inferior colliculusto medial olivocochlear neurons in the rat: a double label study withPHA-L and cholera toxin. Hear Res 70:173–186.
Voelker FA, Henderson CM, Macklin AW, Tucker WE. 1980. Evaluatingthe rat inner ear. A technique using scanning electron microscopy.Arch Otolaryngol 106:613–617.
Warr WB, Beck JE. 1996. Multiple projections from ventral nucleus of thetrapezoid body in the rat. Hear Res 93:83–101.
Warr WB, Guinan JJ, White JS. 1986. Organization of the efferent fibers:the lateral and medial olivocochlear systems. In: Altschuler RA, Hof-mann DW, Bobbin RP, editors. Neurobiology of hearing: The cochlea.New York: Raven Press. p 333–348.
Warr WB, Beck JE, Neely ST. 1997. Efferent innervation of the inner haircell region: origins and terminations of two lateral olivocochlear sys-tems. Hear Res 108:89–111.
Weedman DL, Ryugo DK. 1996a. Pyramidal cells in primary auditorycortex project to cochlear nucleus in rat. Brain Res 706:97–102.
Weedman DL, Ryugo DK. 1996b. Projections from auditory cortex to thecochlear nucleus in rats: synapses on granule cell dendrites. J CompNeurol 371:311–324.
Wenthold RJ. 1987. Evidence for a glycinergic pathway connecting the twocochlear nuclei: an immunocytochemical and retrograde transportstudy. Brain Res 415:183–187.
White JS, Warr WB. 1983. The dual origins of the olivocochlear bundle inthe albino rat. J Comp Neurol 219:203–214.
Wickesberg RE, Whitlon D, Oertel D. 1991. Tuberculoventral neuronsproject to the multipolar cell area but not to the octopus cell area of theposteroventral cochlear nucleus. J Comp Neurol 313:457–468.
Winter IM, Robertson D, Cole KS. 1989. Descending projections fromauditory brainstem nuclei to the cochlea and cochlear nucleus of theguinea pig. J Comp Neurol 280:143–157.
Wright DD, Ryugo DK. 1996. Mossy fiber projections from the cuneatenucleus to the cochlear nucleus in the rat. J Comp Neurol 365:159 –172.
105AXON COLLATERALS OF OLIVOCOCHLEAR NEURONS