OF Vol. No. 36, of pp. by for and in U. SA. Guanine ...sion to xanthosine 5’-monophosphate. This...
Transcript of OF Vol. No. 36, of pp. by for and in U. SA. Guanine ...sion to xanthosine 5’-monophosphate. This...

THE JOURNAL OF BIOLOGICAL CHEMISTRY 0 1991 by The American Society for Biochemistry and Molecular Biology, Inc
Vol. 266, No. 36, Issue of December 25, pp. 24815-24828,1991 Printed in U. S A .
Guanine Nucleotide Biosynthesis Is Regulated by the Cellular p53 Concentration*
(Received for publication, May 24, 1991)
James L. SherleyS From the Department of Molecular Biology, Lewis Thomas Laboratory, Princeton University, Princeton, New Jersey 08544
As an approach to defining the role of p53 in cellular proliferation, murine cell lines were derived which contain a stably transfected temperature-inducible p53 expression system. Cell lines derived with the system exhibited a 3-6-fold physiologic elevation in the cel- lular p53 concentration when grown at 32.5 “C. A p53 induction phenotype was defined by examination of the growth properties of these lines at 32.5 “C. The induction phenotype had three main features: 1) a 2- 4-fold increase in doubling time and biphasic growth kinetics; 2) delayed early S phase transit; and 3) com- plete reversibility either by growth at 37 “C or by growth in the presence of added hypoxanthine or xan- thosine. The reversal of the induction phenotype by these purine salvage precursors implicated the purine nucleotide biosynthetic pathway as the cellular target for the antiproliferative action of p53. Subsequent ge- netic and biochemical analyses identified a p53 induc- tion-related purine pathway defect which was local- ized to the step of inosine Ci’-monophosphate conver- sion to xanthosine 5’-monophosphate. This enzymatic step catalyzed by inosine 5’-monophosphate dehydro- genase (EC 1.2.1.14) is the rate-limiting step for GTP synthesis. Extracts from p53-inducible cells growing at the induction temperature show a specific reduction in inosine 5’-monophosphate dehydrogenase enzy- matic activity. These findings define p53 as a cellular regulator of the synthesis of GTP, a key regulatory nucleotide for many important cellular processes. Moreover, observations of the growth behavior of p53- inducible cells suggest that by regulating the produc- tion of GTP, p53 can control cellular quiescence.
One of the major goals of cancer research is the elucidation of mechanisms of neoplastic transformation. A precise under- standing of the molecular details of such processes is likely to lead to the identification of new therapeutic targets and the consequent design of new diagnostic and therapeutic agents t o combat human cancer. A great deal of the present knowl- edge of neoplastic pathways has been derived from the study of viral and cellular oncogenes (for recent reviews see Bishop, 1991; Cantley et al., 1991; Cross and Dexter, 1991; Hunter, 1991; Lewin, 1991; Sawyers et al., 1991). By and large, onco- genes are genes whose encoded proteins are components of
*This work was carried out in the laboratory of Dr. Arnold J. Levine with the support, in part, of National Institutes of Health Grant CA41086. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
2 Supported by National Research Service Award CA08562-02. Present address: Fox Chase Cancer Ctr., 7701 Burholme Ave., Phil- adelphia, PA 19111.
signal transduction pathways that translate a variety of spe- cific environmental stimuli into gene expression and subse- quent cell growth. Activation of such genes by mutation in the protein-coding portion or by inappropriate expression of the normal gene product can lead to cellular transformation. In addition to these typical oncogenes, for which aberrant expression is the hallmark of transformation, recently a novel class of genes has emerged that is also involved in the trans- formation process. These new transformation-related genes have been termed variably antioncogenes or tumor suppressor genes due to the striking frequency of their inactivation in human tumors (for reviews see Hansen and Cavenee, 1988; Green, 1989; Sager, 1989; Haber and Housman, 1991; Mar- shall, 1991).
Previous studies on oncogenes have given a sound founda- tion to the signal transduction paradigm as a powerful theo- retical construct for the scientific discussion of cellular trans- formation. Typical oncogenes are easily fit into one of four categories: 1) growth factors; 2) growth factor receptors; 3) cytoplasmic transducers; and 4) transcriptional factors. Pres- ently, it is not clear where most antioncogenes fit in this paradigm. There are both structural and biochemical data to suggest that the NF1 antioncogene, which is mutated in von Recklinghausen neurofibromatosis, is a regulator of cyto- plasmic transducers like ras (Buchberg et al., 1990; Xu et al., 1990a, 1990b). However, similarly informative experimental data on other antioncogenes are not available. Recently, some investigators have suggested that the products of some an- tioncogenes are involved in transcriptional activation (Call et al., 1990; Fields and Jang, 1990; Gessler et al., 1990; Haber et al., 1990; Raycroft et al., 1990; Robbins et al., 1990; O’Rourke et al., 1990). Despite this headway, progress has been difficult in determining the precise actions of these important gene products.
This article describes studies with the goal of elucidating the cellular function of an important cellular antioncogene, p53. Although a number of earlier studies characterized p53 as a nuclear proto-oncogene (for review, see Lane and Ben- chimol, 1990; Levine and Momand, 1990), recent studies have refined its categorization to the growing class of antionco- genes. The p53 gene is altered in many human cancers (Baker et al., 1989; Nigro et al., 1989; Takahasi et al., 1989; Chiba et al., 1990; Malkin et al., 1990; Srivastava et al., 1990); and, in cell culture, expression of the wild type p53 gene suppresses both the transformation of primary cells by other oncogenes (Finlay et at., 1989; Eliyahu et ab, 1989; Mercer et al., 1990a) and the growth of transformed and tumor-derived cell lines (Baker et al., 1990; Chen et al., 1990; Diller, et al., 1990; Michalovitz et al., 1990; Martinez et al., 1991). Previous studies have demonstrated the growth suppressive effects of wild type p53 when overexpressed in transformed cells. How- ever, to date, the precise role of the antioncogene in cellular proliferation remains obscure.
24815

24816 p53 Concentration Regulates GTP Synthesis
This report details the use of an inducible p53 expression system to examine from a mechanistic viewpoint the cellular and biochemical effects of wild type p53 expression in non- transformed proliferating cells. Cell lines derived with induc- ible oncogenes or antioncogenes have been previously de- scribed (Holt et al., 1986; Nishikura and Murray, 1987; Schweinfest et al., 1988; Eilers et al., 1989; Johansen et al., 1989; Mercer et al., 199Ob; Mercer et al., 1991; McClinton et al., 1990). However, these cell lines have been used mostly to confirm ideas about the general properties of transformation- related genes and have not been exploited as unique tools to precisely define molecular mechanisms. This experimental approach was used to define p53 as a cellular regulator of GTP synthesis, suggesting a role for the protein as a novel regulator in the signal transduction paradigm.
EXPERIMENTAL PROCEDURES
Materials-Hypoxanthine, uridine, xanthosine, deoxyribonucleo- sides, and thin-layer chromatography nucleotide markers were pur- chased from Sigma. ~-[~~S]Methionine (1200 Ci/mmol), [8-14C]ino- sine (50 mCi/mmol), and [8-'4C]inosine 5'-monophosphate (55 mCi/ mmol) were supplied by Amersham. [5'-3H]Orotic acid (20 Ci/mmol) was purchased from Du Pont-New England Nuclear. ['4C]Sodium formate was supplied by either Amersham (55 mCi/mmol) or by Du Pont-New England Nuclear (58 mCi/mmol). Polyethyleneimine-cel- lulose thin-layered chromatography plates were purchased from EM- Science.
Cell Culture"C127 cells were supplied by the American Type Culture Collection. All cells were maintained in monolayer culture with Dulbecco's modified minimal Eagle's medium (DMEM)' supple- mented with 10% non-heat-inactivated fetal bovine serum (GIBCO) at 37 "C in a humidified incubator with 5% COz. Dialyzed fetal bovine serum was purchased from Hasleton Biologics, Inc.
Plasmid Constructions-The target plasmid pLCRcala, containing the wild type murine p53 cDNA under the control of the previously described BPV LCR-SV40 hybrid promoter (Spaholz et al., 19851, was constructed as follows. Plasmid p407-1 (Spaholz et ai., 1985) was digested with EcoRI and HindIII, and the 5010-base pair fragment containing the LCR-SV40 hybrid promoter was isolated and filled in with the Klenow fragment of Escherichia coli DNA polymerase I. After the ligation of BamHI linkers to the ends of the fragment, regenerating the terminal EcoRI site, the fragment was digested with BamHI and ligated to the 2334-base pair BamHI fragment from the p53 cDNA expression vector pll-4 (Tan et al., 1986). This BamHI fragment contained the entire wild type murine p53 coding region, 50 base pairs of 5"untranslated sequence, 287 base pairs of 3"untrans- lated sequence, and a 3"located SV40 mRNA splice site and poly- adenylation signal. For incomplete systems, plasmid pLCRO was constructed by circularizing the final modified 5010-base pair frag- ment without a p53 gene insert.
The two tsE2 expression vectors, pJSla and pJSld, were derived from plasmid C59-451. This plasmid, generously provided by D. DiMaio (Yale University School of Medicine, New Haven, CT), was constructed by the transfer of the tsE2 allele of plasmid pE2-tsl (DiMaio and Settleman, 1988) into the BPV E2 expression vector C59 (Yang et al., 1985a). Plasmid C59 contained the region of the BPV genome from position 2360 to 4451 under the control of the SV40 early region. Plasmid C59-451 was first amplified in E. coli strain GM48 (dam, dcm) to prevent methylation of BclI sites. It was subsequently digested to completion with BamHI and then partial digested with BclI. The 4632-base pair partial fragment deleted for BPV sequences from position 3838 to 4451 was isolated by agarose gel purification. This procedure removed the coding region of the BPV-transforming protein E5, position 3878-4010 (Honvitz et al., 1988). Plasmid pJSld was constructed by circularization of the iso- lated BcfI partial fragment with an inserted EcoRI linker. To con- struct plasmid pJSla, the 1321-base pair HindIII/EcoRI fragment containing the Tn5 neomycin resistance gene from plasmid DOL- (Korman et al., 1987) was isolated, filled in with the Klenow fragment,
The abbreviations used are: DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; DFBS, dialyzed fetal bovine serum; sAMP, adenylosuccinic acid; XMP, xanthosine 5'-monophosphate; REF, rat embryo fibroblast; BPV, bovine papilloma virus.
and ligated to BamHI linkers. After digestion with BanHI, the fragment was ligated to the 4632-base pair BclI partial fragment from plasmid (259-451. The resultant plasmid, pJSla, contained the in- serted neomycin resistance gene downstream and tandem to the tsE2 gene driven by the SV40 early region. In transfection experiments, this plasmid forms G418-resistant colonies as efficiently as a deriva- tive plasmid in which all of the upstream BPV translational start sites have been deleted (data not shown).
Plasmid pSLneo is a derivative pSVZneo that confers G418 sulfate resistance to animal cells. Its construction has been described (Lupton and Levine, 1985).
Construction of p53-inducible Cell Lines and Noninducible Control Cell Lines-To derive the series of cell lines (see Table I) the following indicated quantities of CsC1-gradient purified plasmids were com- bined If: 1 pg of pSLneo, 10 pg of pLCRcala; lg: 1 pg of pSLneo, 5 pg of pJSld, 5 pg of pLCRO; Ih: 1 pg of pSLneo, 5 pg of pJSld, 5 pg of pLCRcala (trans configuration for the neomycin resistance gene); lm: 1 pg of pJSla, 10 pg of pLCRO; In: 1 pg of pJSla, 10 pg of pLCRcala (cis configuration for the neomycin resistance gene). Each plasmid mixture was used to transfect C127 cells by calcium phos- phate precipitation (Kingston, 1987) at 37 "C. Resistant colonies were selected in media containing 1 mg/ml G418 sulfate (GIBCO) and expanded at 37 "C for further analysis. All lines were maintained in culture at 37 "C in medium containing 1 mg/ml G418 sulfate. p53- inducible lines maintained under these conditions for 150 doublings exhibited no detectable phenotypic changes when compared to early passage cells.
Growth Curue Analyses-For growth curve analyses, cells were grown to one-half confluency at 37 "C and replenished with fresh growth medium (DMEM, 10% FBS, 1 mg/ml G418 sulfate). Sixteen h later, the cells were replated at 1/40 their original density into 25- cm2 flasks and placed at either 37 or 32.5 "C. Twenty-four h later, cell counts were started using an ElzoneTM Model 112LT5NBCD/ ADC electronic particle counter (Particle Data, Incorporated).
Plating Efficiency Assays-The starting cells for plating efficiency assays were treated in one of two ways. Some assays were initiated with exponentially growing cells which were treated as described above for growth curve analyses. Other assays were initiated with quiescent cells. Quiescent cells were prepared as previously described (Sherley and Kelly, 1988b). At the start of plating assays, cells were plated in regular culture medium at 200 cells per 10-cm diameter dish or 100 cells per 6-cm diameter dish and placed at either 37 or 32.5 "C. Dishes at 37 "C were stained with crystal violet after 14 days, while dishes at 32.5 "C were stained after 21 days to compensate for the general decrease in growth rate at low temperature. For plating assays with DFBS, exponential cells were grown 2 days prior to plating in medium supplemented with 10% DFBS, and quiescent cells were prepared as previously (Sherley and Kelly, 1988b), but with the substitution of 0.5% DFBS for 0.5% FBS. Both types of cells were subsequently plated in medium supplemented with 10% DFBS. Cul- ture media containing l mg/ml G418 sulfate was used for all plating experiments with G418-resistant cell lines.
Flow Cytometry-For cell cycle analyses, cells were grown to one- half confluency at 37 "C and replenished as for growth curve analyses. Sixteen h later, the cells were replated at 1/40 their original density
TABLE I p53-inducible and control cell lines
Cell line type" :$%$ Clonability DT < 100 h'
%
p53(10) = If 20 100 (5/5) 80 (4/5)
tE2p53(5:5) = l h 14 100 (5/5) 60 (3/5) cE2-0(1:10) = l m 6 3 100 (6/6) 83 (5/6) cE2p53(1:10) = In 6, 3 100 (6/6) 17 (1/6) C127 N A ~ NA NA
tE2-0(5:5) = l g 12 100 (5/5) 100 (5/5)
"Numbers in parentheses refer to micrograms of each indicated plasmid transfected as described under "Experimental Procedures": p53, pLCRcala (with 1 pg of pSLneo when no other plasmid noted); 0, pLCRO; tE2, pJSld, and 1 pg of pSLneo (trans configuration); cE2, pJSla (cis configuration).
* G418-resistant colonies per number of transfected cells. Percentage of cloned lines with doubling times less than 100 h.
NA, not applicable. Doubling times for such lines were less than 30 h.

p53 Concentration Regulates GTP Synthesis 24817
and placed at 37 or 32.5 "C. After 4 days, cells were harvested and prepared for flow cytometry as previously described (Sherley and Kelly, 198813). Samples were analyzed with an Epics 753 fluorescence- activated cell sorter (Coulter Electronics, Inc., Hialeah, FL).
Immunoprecipitation Analyses-For pulse-labeling analyses, cells were grown to one-fifth or one-tenth confluency (depending on their doubling time) in 75-cm2 flasks. After replenishment with fresh media, one flask was placed at 32.5 "C, while the other was returned to 37 'C. Two days later, the cells were rinsed briefly with methionine- free DMEM, 10% DFBS. Cells were then labeled at their respective temperature for 2 h with 2 ml of methionine-free DMEM, 10% DFBS containing 50-100 pCi/ml methionine. This labeling protocol was slightly modified for comparisons of exponential cells with quies- cent cells. After 4 days of starvation in low serum medium at 37 'C, quiescent cells were labeled in DMEM, 0.5% FBS. Exponential cells for comparison were labeled in DMEM, 10% FBS.
For long-term continous labeling experiments, quiescent cells were prepared as described and replated at one-fourth their original density in DMEM, 20% FBS. One flask was placed at 32.5 "C, while the other was returned to 37 "C for 2.5 h to allow cells to attach to the flask. The cells were then labeled for 36 h at their respective temperatures with 10 ml of DMEM, 20% FBS, 20 p~ of methionine containing 100 pCi/ml methionine.
Soluble extracts were prepared by direct addition of extraction buffer (Sherley and Kelly, 1988a) to flasks of labeled cells after briefly rinsing with ice-cold phosphate-buffered saline. Immunoprecipita- tions were performed as previously described (Sherley and Kelly, 1988a) using 200 pl of supernatants from saturated cultures of hy- bridoma cell lines which produce specific anti-p53 monoclonal anti- bodies (Yewdell et al., 1986). In all experiments, comparisons were made based on immunoprecipitations with extract volumes contain- ing equal quantities of trichloroacetic acid-precipitable radioactivity. Autoradiograms were quantitated by scanning densitometry with a Model 620 video densitometer (Bio-Rad).
Nucleotide Biosynthesis Analyses-Analysis of the incorporation rates of radioactive precursors into cytoplasmic RNA was performed in the following manner. Cells cultured in 75-cmZ flasks as for growth analyses were labeled for 2 h at their growth temperature with 5 ml of culture medium containing the appropriate radioactive nucleotide precursor. Total cytoplasmic RNA was prepared from labeled cells by the method of Ricciardi et al. (1979) (except without the addition of vanadyl ribonucleoside), dissolved in distilled water, and quanti- tated by optical density. Each RNA preparation was divided equally into two samples. One sample was precipitated directly with trichlo- roacetic acid, while the other was first subjected to alkaline hydrolysis as described by Spyrou and Reichard (1983). Filtered precipitates were dried and quantitated by scintillation counting. In double label- ing experiments, corrections were made for I4C channel spillage by using determinations from RNA samples labeled with a single radio- isotope. RNA cpm were defined as: prehydrolysis cpm - posthydro- lysis cpm. 72 f 24% (average for all experiments f standard devia- tion) of the total trichloroacetic acid-precipitable cpm were sensitive to alkaline hydrolysis.
To determine the [14C]guanine to ["Cladenine ratio in [14C]for- mate-labeled RNA, the RNA was prepared and hydrolyzed as for purine pathway activity analyses. Hydrolyzed samples were lyophi- lized and redissolved in distilled H20 containing 50 nmol each of AMP and GMP as thin-layer chromatography standards. Samples were chromatographed on polyethyleneimine-cellulose in a single dimension with solvent Sb (74.0 g of (NH4)&04, 0.4 g of (NH,)HSO,, 4.0 g of Na2EDTA, 100 ml of HzO) according to the method of Bochner and Ames (1982). Regions corresponding to UV-detected markers were scraped and the chromatographic resin added to BiofluorTM (Du Pont) for scintillation counting.
For kinetic analysis of the AMP and GMP branches of the purine nucleotide pathway, cells were labeled as above for pathway flux studies with 2 ml of media containing 50 pCi/ml [14C]inosine. Label cells were harvested, and neutralized acid hydrolysates were prepared by the method of Kemp et al. (1986) and stored at -80 "C. Labeled purine nucleotides were separated by thin-layered chromatography according to the procedures of Jackson et al. (1977). Chromatographed samples were combined with 20 nmol each of IMP, sAMP, AMP, XMP, and GMP. Separated "C-labeled nucleotides were identified and quantitated as above.
RESULTS
Construction of p53-inducible Cell Lines-Gene expression elements from bovine papilloma virus (BPV) were used to develop an inducible expression system for p53. The tran- scription of BPV early genes is highly dependent on the expression of the viral transactivator E2 (Yang et al., 1985b). This protein binds to an E2-dependent enhancer located in a region upstream of the viral early genes called the long control region (LCR) (Spalholz et al., 1985; Spalholz et al., 1987; Hawley-Nelson et al., 1988). Recently, DiMaio and Settleman (1988) constructed a temperature-sensitive BPV-E2 allele (hereafter referred to as tsE2) by insertional mutagenesis and showed that the expressed tsE2 protein failed to transactivate an LCR-SV40 hybrid promoter at 37 "C. At 32.5 "C the mu- tant protein functioned like its wild type counterpart to induce gene expression from the hybrid promoter.
The two genetic elements described above were employed to construct a temperature-dependent p53-inducible system. The target plasmid for the system, pLCRcala, contains the wild type murine p53 cDNA inserted downstream of the previously described LCR-SV40 hybrid promoter (Spalholz et al., 1985). Two related plasmids, pJSla and pJSld, which express the temperature-sensitive transactivator, were de- rived from the original plasmid which contained the tsE2 allele under the control of the SV40 early promoter. Both plasmids contain a 3' deletion of the original viral sequences that removes the reading frame for the transforming E5 protein (Horwitz et al., 1988). Plasmid pJSla contains a promoterless neomycin resistance gene inserted in tandem with the tsE2 gene. This plasmid was constructed to minimize the number of plasmids required to stably introduce the p53- inducible system into cells.
The cell line chosen for the introduction of the p53-induc- ible system was C127. A number of considerations led to the selection of this cell line as the prototype parental line. C127 cells were used for the initial characterization of the effect of the tsE2 allele on BPV replication and transformation (DiMaio and Settleman, 1988). Such studies demonstrated that in C127 cells the tsE2 allele functions in a temperature- dependent manner. Since C127 cells are nontransformed mu- rine cells, they provide a homologous species background for analysis of the effects of murine p53 overproduction. The endogenous p53 protein expressed by C127 cells was shown to complex with the SV40 T-antigen and to bind anti-p53 monoclonal antibody Pab246. No complex formation was detected between C127 p53 and the cellular hsc70 protein (see Fig. 1). These three properties distinguish wild type p53 from mutant forms of the protein (Tan et al., 1986; Hinds et al., 1987; Finlay et al., 1988; Gannon et al., 1990). Given these characteristics, C127 cells were ideal for studying effects on the growth of nontransformed cells due to elevating the con- centration of wild type p53.
Neomycin-resistant lines were clonally derived at 37 "C (the nonpermissive temperature for tsE2 function) from C127 cells cotransfected with the complete p53-inducible system and a neomycin resistance gene (see Table I). In addition to cell lines derived with the complete p53-inducible system, control lines were derived with plasmid systems lacking either the tsE2 transactivator plasmid or the p53 target gene. The fre- quency of G418-resistant colonies arising from transfections of either the intact inducible system or control systems lack- ing p53 sequences were remarkably similar (Table I). This result was in marked contrast to previous findings on the effect introducing wild type p53 expression vectors into cells in culture. A number of earlier studies have shown that cotransfection of plasmids expressing the wild type p53 pro-

24818 p53 Concentration Regulates GTP S y n t h e s i s
200K,
97.4K.
68K
43K
29K
C127 lq-P6 M 419'421'246'2481 419'421
c Tag
e p53
FIG. 1. Characterization of the wild type properties of p53 in C127 cells. The p53 protein in exponentially growing C127 cells and a second line, lq-P6, derived by stable transfection of (2127 cells with an expression construct for the SV40 large T antigen, was analysed by immunoprecitation as detailed under "Experimental Pro- cedures." 419, T antigen-specific monoclonal antibody PAR419; 421, panspecific anti-p53 monoclonal antibody PAB421; 246, wild type conformation-specific anti-murine p53 monoclonal antibody PAB246; 248, conformation independent anti-murine p53 monoclonal antibody PAB248; M , "C-labeled molecular weight markers; Tag, SV40 large T antigen.
tein greatly reduces selectable colony formation (Baker et al., 1990; Diller et al., 1990; Mercer et al., 1990a). The failure of the intact p53-inducible system to reduce G418-resistant col- ony formation suggested that the system conferred low expres- sion to the transfected p53 genes. An early indication that the exogenous p53 gene was, in fact, expressed a t a biologically significant level, even a t 37 "C, came when attempts were made to expand selected colonies. Although cells from all colonies were viable, the majority of those derived from trans- fections of the complete inducible system were essentially "dormant." The doubling time of these dormant cultures was estimated to be greater than 100 h (Table I).
Characterization of p53 Expression in Inducible Cell Lines- Cell lines derived from transfections of the complete p53- inducible system were analyzed by primer extension for expression of the transfected p53 gene. The endogenous C127 p53 mRNA is readily distinguishable from the exogenous transcript because of 5' end differences due to the presence of sequences from the hybrid LCR-SV40 promoter. Since there are two transcriptional start sites in the SV40 promoter separated by 5 base pairs (Spalholz et al., 1987), two similarly spaced extension products are predicted from the transfected gene. The single primer extension product obtained with '2127 poly-A RNA corresponded to the previously defined major transcriptional start site of the endogenous p53 gene (Bienz et al., 1984; Lamb and Crawford et al., 1986; Tuck and Craw- ford et al., 1989). In addition to the endogenous transcript product, lines transfected with the complete inducible system also exhibited two new extension products with lengths as predicted for the transfected p53 gene (data not shown). By this measurement, the level of expression of the transfected p53 gene was similar to or less than that of the endogenous gene a t 37 "C.
The inducibility of p53 protein expression in lines derived
with the intact inducible system was assessed by comparison of the accumulation rate of the protein at 37 and 32.5 "C. The accumulation rate of p53 in these lines, as measured by the incorporation of ["S]methionine into p53 polypeptide, was about 70% higher at 32.5 "C than at 37 "C (Fig. 2R). Labeling continuously for times in great excess of the half-life of wild type p53 proteins (Finlay et al., 1988) gave similar results, indicating that the increase in p53 accumulation rate at the induction temperature of 32.5 "C resulted in about a %fold elevation in the steady-state level of p53 protein (Fig. 2 A ) . Neither the parental C127 line nor control lines derived with incomplete plasmid systems showed induction of p53 at 32.5 "C (Fig. 2 and data not shown). Since the complete lines had an increased basal level of p53 even a t 37 "C (see Fig. 2 A ) , the 2-fold induction resulted in an actual 3-6-fold in- creased level of p53 protein as compared to C127 cells a t 32.5 "C.
Together, the primer extension analysis and the ["S]me- thionine incorporation analyses characterize the expression parameters of the stably introduced BPV-tsE2 inducible expression system. The uninduced LCR-SV40 hybrid pro- moter exhibits a low level of basal expression that is evidenced both by the exogenous p53 transcripts and by the small increase in p53 protein concentration detected a t 37 "C. The leakiness of the system is likely to be responsible for the occurrence of dormant cell clones primarily in transfections with the complete p53-inducible system (see Table I). This observation was a first indication that C127 cells are acutely sensitive to small elevations of p53 protein levels within the physiologic range.
Characterization of thep53 Induction Phenotype-Cell lines characterized for p53 expression were subsequently assessed for the effect of p53 induction on cellular growth by the generation of growth curves for each line a t 37 and 32.5 "C (Fig. 3 and data not shown). Consistent with the leakiness of the p53-inducible expression system noted above, a t 37 "C cell lines derived with the complete system had greater doubling times than control lines (Table IIA). Even so, a p53 induction phenotype was readily apparent upon examination of the growth properties of inducible and control lines at 32.5 "C. In general, all cell types had a somewhat decreased growth rate at the lower temperature. However, while parental C127 cells and control lines derived with incomplete plasmid systems showed on average a 1.6-fold increase in doubling time at the induction temperature, the p53-inducible lines showed on average a 2.6-fold increase (Table IIA). The p53-related growth defect was greater a t early times after the shift of cultures from 37 to 32.5 "C. During the first 24 h after shift to the induction temperature, the doubling time of p53-induc- ible cell lines increased as much as 4-fold. The greater dou- bling time seen a t early times after the shift to the induction temperature reflected unusual growth kinetics that were spe- cific to p53-inducible lines a t 32.5 "C. As shown in Fig. 3, cell lines derived with the intact p53-inducible system exhibited biphasic growth kinetics specifically at 32.5 "C. The first phase of growth reproducibly lasted about 4 days and was followed by a second phase characterized by on average a 5- fold increase in growth rate. This kinetic behavior can be demonstrated by comparison of the doubling times for each 24-h interval of growth. For instance, in Fig. 3 the interval doubling time of p53-inducible line ln-3 a t 37 "C is initially about 30 h and remains constant as predicted for exponential growth kinetics. However, a t 32.5 "C the interval-doubling time of this cell line varies from 125 h in the first 24-h interval to 25 h in the fifth interval. C127 cells and incomplete control

p53 Concentration Regulates GTP Synthesis
A B
24819
200K-
97.4K"
- I -
29K. ."- "" . - " - - FIG. 2. Immunoprecipitation analyses of p53 expression in inducible cell lines. The expression of p.58
protein in p53-inducible cell lines lh-3 and ln-3 was compared to that of parental C127 cells at either 37 "C ( H 7 3 or the induction temperature of 32.5 "C (L7'). A , for analyses of steady state levels of p53, extracts were prepared from cells labeled at the indicated temperature for 36 h, and immunoprecipitations performed with monoclonal antibody PAB421 (unless otherwise indicated) as detailed under "Experimental Procedures." The p53 pol.vpeptide was further identified by failure to be recognized by the unrelated monoclonal antihodv PAR419 (NO and competition by 5.0 p g of synthetic peptide corresponding to the epitope for PAB421 ( P ) . R , for comparisons of the accumulation rate of p53 protein, cells growing in either DMEM, 10% DFRS or DMEM, 10% DFRS supplemented with 400 p~ hypoxanthine (+H) were labeled for 2 h and analyzed as described under "Experimental Procedures." M, '.IC-labeled molecular weight markers.
lines exhibited typical exponential growth kinetics a t both temperatures.
The p53-related induction phenotype of decreased growth was further characterized by determination of the plating efficiencies of p53-inducible lines and control lines a t high and low temperatures. The frequency at which cells form colonies after plating at low density reflects the growth poten- tial of the culture from which those cells were derived. Con- sistent with this relationship, results from plating efficiency assays mirrored the results from growth curve analyses in all respects. At the noninducing temperature of 37 "C, cell lines derived with the complete p53 inducible system plated less efficiently than the parental C127 line or control lines derived with incomplete plasmid systems (see Fig. 4A). In accord with results from growth curve analyses, incubation at the induc- tion temperature reduced the plating efficiency of p53-induc- ible cells to a greater extent than that of control cells (Table IIB; Fig. 4.4). This p53-related plating defect was greatly enhanced by plating cells in culture medium supplemented with dialyzed serum (Tables IIB and IIIB). Under these conditions, the plating efficiency of p53-inducible cells a t 32.5 "C was reduced to less than one-third of the efficiency a t 37 "C. By the measure of plating efficiency reduction, the complete lines were 1.5-%fold more sensitive to plating at the p53 induction temperature than control cells.
In addition to serving as a second measure of cellular growth potential, the plating efficiency assay provided an approach to assessing the reversibility of the p53 induction phenotype. Cells were first plated a t 32.5 "C for different lengths of time, shifted to 37 "C, and then scored for subsequent colony for- mation (Fig. 423). This procedure permits the detection of p53-induced "quiescent cells" by their subsequent formation of colonies at the nonrestrictive temperature. This approach demonstrated that p53-induced quiescent cells were com-
pletely viable at 32.5 "C and maintained their growth potential for periods as long as 2 weeks. The induction and subsequent accumulation of these quiescent cells in populations of p53- inducible cells growing at the induction temperature give rise to the observed first growth phase a t 32.5 "C.'
The plating behavior of p53-inducible cells was shown to result from a problem with cell growth and not cell attach- ment. To make this distinction, p53-inducible cells were plated and allowed to incubate a t 37 "C long enough to insure complete cell attachment. The attached cells were then shifted to 32.5 "C for subsequent growth and analysis. Such preplat- ings at the nonrestrictive temperature for growth had essen- tially no effect on the final plating efficiency of p53-inducible cells at the restrictive temperature (Fig. 4C). Altogether, the results from the plating analyses demonstrate that the salient feature of the p53 induction phenotype is a decrease in growth potential.
It was initially surprising that such a moderate elevation of p53 protein levels resulted in the observed alterations in the growth behavior of cells. However, the comparison of pulse- labeling results from exponentially growing cells and quies- cent cells prepared by serum deprivation demonstrated that quiescent cells exhibit a 2-fold greater p53 protein accumu- lation rate (Fig. 5). This finding indicates that if p53 levels are an important determinant of the growth status of cells, then it is not surprising that even a modest increase in the cellular concentration of p53 is of great consequence for the proliferative capacity of cells.
The final approach employed to characterize the p53 in- duction phenotype was cell cycle analysis by flow cytometry. For the first 4 days after shift to the induction temperature, p53-inducible cell lines showed only limited growth (see Fig. 3). The possibility existed that this growth lag was not due to
' J. Sherley and K. Voss, unpublished data.

24820 p53 Concentration Regulates GTP Synthesis
'*]l------ ea
111-3 I
"1 A / 40
20
0
80
40 - C127 - lrn-5 - lf-4
20
- . 0 4 8 9 6 144
Hour
1 9 2 2 4 0
FIG. 3. Growth kinetics of p53-inducible lines. Growth curves for p53-inducible lines, lh-3 and ln-3, (top two panels) and control lines (bottom panel) were derived as detailed under "Experi- mental Procedures." Data points represent the average of duplicates. Error bars indicate the range of the data. Open symbols, 37 "C; closed symbols, 32.5 "C, the p53 induction temperature.
a general slowing of growth, but instead to a specific block in the cell cycle. Therefore, DNA content histograms generated by flow cytometry were compared for p53-inducible cells during the period of the growth lag at 32.5 "C and for cells in parallel at 37 "C. The data presented in Fig. 6 show that such histograms are quite similar and demonstrate that the growth lag is not due to a gross block in cell cycle progression. However, there are notable differences between the cell cycle pattern of p53-inducible cells at 32.5 "C versus 37 "C that do not exist for parental C127 cells. During the period of the growth lag at 32.5 "C, populations of p53-inducible cells show a relative decrease in cells with a G1 phase DNA content and a reciprocal increase in cells with an S phase DNA content. The newly appearing cell fraction with an S phase DNA content is disproportionately distributed in early S phase as delineated by flow cytometry (Fig. 6D) . In kinetic terms, the relative integrated area of each region of a flow cytometry
TABLE I1 Growth analvses of ~53-inducible cell lines
A. Growth curve analysis" ~~
Cell line DT at 37 "C DT at 32.5 "C (32,5,37 oc) DT ratio
Parental C127
p53 Only lf-3 lf-4
tsE2 Only lg-1 lm-5
p53-inducible lh-3 111-3
h
23 37 1.6
17 31 1.8 19 30 1.6
18 21 1.5 18 21 1.5
27 64 2.4 25 67 2.7
B. Plating assayb Average plating ratio (32.5/37 'C)
FBS (no. of exu) DFBS (no. of exu) Cell line
Parental C127
p53 Only lf-3 lf-4
tsE2 Only lg-1 lm-1 lm-5
p53-inducible lh-3 ln -3
1.0 (2)
ND' ND
ND ND ND
0.86 (1) 0.64 (2)
0.71 (3)
0.63 (1) 0.55 (1)
0.77 (1) 0.44 (1) 0.45 (1)
0.24 (3) 0.29 (4)
"Doubling times (DT) were determined as described under "Ex- perimental Procedures."
Plating efficiencies were determined as described under "Experi- mental Procedures." The average plating ratio is defined as the average number of colonies a t 32.5 "C divided by the average number of colonies a t 37 "C.
e ND, not done.
DNA histogram reflects the relative amount of time cells spend in the phase of the cell cycle represented by that region (Muirhead et al., 1985). Thus, the changes in the cell cycle profile of p53-inducible cells at 32.5 "C suggested a delay in the progession of cells through early S phase. This particular interpretation of the data implicated p53 in interfering with cellular DNA replication and, subsequently, led to the first important clue to the role of the protein in cellular growth.
Phenotypic Reversal of the p53-related Growth Defect by Hypoxanthine-The initial phenotypic observations de- scribed above were treated as clues to the underlying action of p53. The experimental plan developed to take advantage of these new clues was based on the concept of phenotypic reversion. Specifically, agents with reasonably well-defined cellular effects were tested for the ability to reverse the p53 induction phenotype. The identification of such agents would increase the information base for p53 function by linking the known properties of such agents to the action of p53. Such functional links would represent new and finer clues that were, perhaps, closer to the actual molecular function of the protein.
The initial agents tested for the ability to reverse the p53 induction phenotype were the nucleosides hypoxanthine and uridine. These nucleotide precursors have been well charac- terized for their ability to complement defects in the de novo synthesis of purine and pyrimidine nucleotides, respectively, via cellular nucleotide salvage pathways (Lehninger, 1975; Kornberg, 1980). Preliminary growth curve analyses demon-

p53 Concentration Regulates GTP Synthesis 24821
TABLE I11 Hypoxanthine metabolism and the p53 induction phenotype
A. Hypoxanthine reverses the plating defect of p53-inducible line 111-3"
250
-r
0.44
C127 I Q - 1 1 h - 3 1 n - 3
Cell Line
100 B 1
80 -
60 -
40 7 I T 1-
o m CT 7d 14d
1 n-3
Csll Line
100 IC 1 80 -
60 -
40 -
Direct Post 3d @ HT
90 1 1 "
0 1 h - 3 1 n-3
Csll Llnn
FIG. 4. Plating efficiency analysis of p53-inducible lines. The plating efficiency of p53-inducible lines lh-3 and 111-3 was compared to that of control lines at 37 "C ( H T ) and the induction temperature, 32.5 "C (LT) as detailed under "Experimental Proce- dures." Analyses were initiated with exponentially growing cells and performed in DMEM, 10% DFBS. A, control line lg-1 and parental line C127 uersus p53-inducible lines. Ear heights represent the average of four replicates. The numbers above the error bars represent average number of colonies a t 32.5 "C per average number of colonies a t 37 "C. B , the reversibility of the p53-induction phenotype of decreased plating efficiency was assessed by shifting dishes to 37 "C for 2 weeks of growth after they first spent either 7 days (7d) or 14 days (14d) a t 32.5 "C. Ear heights represent the average of five replicates. C, plated p53-inducible cells were either placed directly at 32.5 "C (Direct) or after placement first at 37 "C for 3 days (Post 3d @ HT). Bar heights represent the average of five replicates. All error bars indicate the standard deviation of the data.
Media addition (100 pM) Colony number f S.D.b
37 "C 32.5 "(1 .. - None 103 f 13 56 f 20 Deoxynucleosides 94 i 4.1 47 f 4.9 Hypoxanthine + uri- 101 f 7.3 71 f 14
Hypoxanthine 113 f 10 77 f 10 Uridine 101 f 13 46 f 5.3
dine
(32.5 T / 3 7 "C) Ratio
0.54 0.50 0.70
0.68 0.46
B. Serum dialysis increases the p53-induced plating defect'
Cell line Media type Colony number f S.D. Ratio
37 mc 32.5 -c (32.5 "C/37 "C)
C127 FBS 47 i 6.8 49 f 7.8 1.0 DFBS 64 f 5.7 49 f 4.2 0.77 400 p M Hx 56 f 12 44 t 4.7 0.79
111-3 FBS 50 rt 7.9 37 f 6.4 0.74 DFBS 60 f 8.2 23 f 2.6 0.38 400 IIM Hx 45 i 9.7 38 & 4.1 0.84
sensitivity to p5sd C. Cell lines deficient for hypoxanthine utilization exhibit increased
Colony number ? S.D. Ratio
Cell line (at 37 "C) Neo Neo + system (+System/neo) C127 105 f 11 42 f 7.4 0.40
HUD-2 40 f 5.0 4.4 f 2.6 0.11 HUD-1 19 f 3.6 2.4 f 0.89 0.13
Cell line (+system) 37 "C 32.5 "C (32.5 "C/37 "C) C127 42 f 7.4 25 f 3.8 0.60
2.4 f 0.89 0.0 f 0.0 <0.08 HUD-1 HUD-2 4.4 f 2.6 0.60 f 0.55 0.14
Quiescent p53-inducible ln-3 cells, derived by postconfluent maintenance a t 37 "C for 4 days in DMEM, 0.5% FBS, were replated a t either 37 "C or at 32.5 "C as detailed under "Experimental Proce- dures" in DMEM, 10% FBS without supplementation (None) or supplemented with one of the following a t 100 p~ concentration: all four of the deoxynucleosides, deoxyadenosine, deoxycytosine, deoxy- guanosine, and thymidine; hoth hypoxanthine and uridine; hypoxan- thine alone; uridine alone.
Average of five replicates f S.D. Quiescent p53-inducible 111-3 cells and quiescent parental (2127
cells, derived by postconfluent maintenance a t 37 "C for 2 days in DMEM, 0.5% FBS followed by 2 days in DMEM, 0.5% DFBS, were replated at either 37 "C or a t 32.5 'C in DMEM with the following supplements: 10% FBS; 10% DFBS; 10% dialyzed FBS + 400 ~ L M hypoxanthine (400 p~ Hx).
Wild type C127 cells and derivative mutant cells deficient for hypoxanthine utilization (HUD-1 and HUD-2) were transfected as detailed under "Experimental Procedures" with either plasmids pJSla (expressing tandem tsE2 and neomycin resistance genes; see text for details of plasmids) and pLCR-0 (a derivative of the LCRp53 target plasmid deleted of all p53 sequences) (Neo) or plasmids pJSla and pLCRcala (the LCRp53 target plasmid) (Neo + system). The tsEZLCRp53 ratio for C127 cell transfections was 1:lO. The ratio for mutant cell transfections was 1:5. Both transfections and selection of G418 sulfate resistant colonies were performed at 37 "C. Transfec- tions with the intact p53-inducible system (ie. plasmids pJSla and pLCRcala) were tested for the appearance of resistant colonies a t 37 uersus 32.5 "C, the induction temperature for p53.
strated that the addition of hypoxanthine and uridine to the medium of p53-inducible cells rescued them from the growth defect at 32.5 "C. This nucleoside rescue was characterized by both a decrease in doubling time and a return to exponential growth kinetics (data not shown).
Because the plating efficiency assay is more amenable than growth curve analysis to the testing of many parameters in parallel, it was chosen to further characterize the phenotypic

24822 p53 Concentration Regulates GTP Synthesis G I 27 l h - 3 ln -3
M N E O N E O N E O "~
"
Z O O K -
97.4K-
68K-
P63") 43K-
29K-
FIG. 5. Comparison of p53 express ion in qu iescent and ex- ponentially growing cells. The accumulation rate of p.53 protein in quiescent (Q) and exponentially growing cells ( E ) was assessed by immunoprecitiation analysis of extracts from parental C127 and p53- inducible cells labeled for 2 h at 37 "C. Unless otherwise indicated, immunoprecipitations were performed with monoclonal antibody PAR421 as detailed under "Experimental Procedures." NI, control immunoprecipitation of labeled extracts from growing cells with the unrelated monoclonal antibody PAB419. M , '.'C-labeled molecular weight markers.
reversal of the p53-related growth defect by hypoxanthine and uridine. Similar to preliminary growth curve analyses, the addition of the two nucleotide precursors to platings of p53-inducible cells resulted in a 50% rescue of colonies a t 32.5 "C (Table IIIA). By adding each of the two nucleosides separately, it could be shown that only hypoxanthine was required for phenotypic reversal. These results specifically implicated the purine nucleotide biosynthetic pathway as a cellular target for p53 action. The findings also explained the aforementioned observation that the p53 induction phenotype is greatly enhanced by plating cells in culture medium supple- mented with dialyzed serum (Tables IIB and IIIB). Dialysis removes exogenous purine nucleotide precursors like hypo- xanthine and guanine which exist at biologically significant levels in serum (Harkness et al., 1980; Hayashi et al., 1990a; Hayashi et al., 1990b). In plating experiments performed with media supplemented with dialyzed serum, complete pheno- typic reversal was achieved, as compared to parental C127 cells under the same conditions, by the addition of hypoxan- thine at 400 WM (see Table IIIB). This concentration of hypoxanthine also conferred complete phenotypic reversal in growth curve analyses (data not shown).
A final experiment to demonstrate the importance of purine nucleotide biosynthesis in the p53 inc'action phenotype re- quired the derivation of mutant C127 cells that were defective for hypoxanthine utilization. Such mutant C127 lines were derived by first selecting for spontaneously occurring C127 clones which were resistant to 8-azaguanine. These mutant clones occurred a t a frequency of in C127 populations. Selected 8-azaguanine-resistant clones were expanded and, subsequently, screened for sensitivity to hypoxanthinelami- nopterinlthymidine medium. Clones which met this second criteria by definition were defective for hypoxanthine utili- zation. Typically, cells selected by this procedure are deficient in the enzyme hypoxanthinelguanine phosphoribosyltrans- ferase which is required for the salvage of purine nucleosides (Fenwick, 1985). In plating assays for G418-resistant colonies immediately after transfection with the p53 inducible system,
, A
LT
c127
1. .L, . , . , , .
B
C
D
- C127 - lh .3 - ln .3
I
0 5 0 100 150 200 2 5 0 Retmlive Fluorescence
FIG. 6. Cell cycle analysis of p53-inducible l ines during the per iod of the f i rs t growth phase at the induction temperature. DNA histograms, derived by flow cytometry as detailed under "Ex- perimental Procedures," for p53-inducible cell lines l h - 3 and In-3 ( R and C, respectively) were compared to those of the parental (2127 line ( A ) for cell cultures growing a t either 37 "C (H77 or 32.5 "C (L71 during the period of the first growth phase at the induction temper- ature (see Fig. 3 and "Results"). A relative fluorescence of 85 corre- sponds to G1 cells; a relative fluorescence of 170 corresponds G2 cells; and S phase cells are located between these two extreme values. I) is the calculated difference after subtraction of each 37 "C histogram from its corresponding 32.5 "C histogram. A relat,ive fluorescence of 100 corresponds to cells in early S phase.

p53 Concentration Regulates GTP Synthesis 24823
the mutant cell lines were found to be more sensitive to the effects the p53 (Table IIIC). As compared to the parental (2127 line, cell lines defective for hypoxanthine utilization plated &fold less efficiently at the induction temperature after transfection with the intact p53-inducible system (Table IIIC). Because these transfection-plating assays allowed the evaluation of numerous cell clones, the results obtained estab- lished the relationship of purine nucleotide biosynthesis to the p53-related growth defect as a general property of C127 cells.
A surprising result which proved to be of great significance was the fact that although addition of hypoxanthine rescued p53-inducible cells from the 32.5 "C growth defect, addition of all four deoxynucleosides under similar conditons did not (see Table IIIA). Deoxynucleosides are immediate salvage precursors for DNA synthesis (Kornberg, 1980). If the main defect in p53-induced cells was, in fact, insufficient precursors for DNA synthesis, then the cells should have also been rescued by the addition of these exogenous precursors. The failure of deoxyribonucleosides to reverse the p53 induction phenotype indicated that the p53-related growth defect was not simply due to a problem with DNA synthesis. This con- clusion represented an important refinement of the initial working hypothesis for the primary effect of p53 induction in cells.
The p53 Induction Phenotype Is Characterized by a Defect in Purine Nucleotide Biosynthesis-The ability of hypoxan- thine to reverse the p53 induction phenotype suggested that the purine nucleotide biosynthetic pathway was involved in the mechanism of p53 action. The integrity of the purine nucleotide pathway was assessed in p53-inducible cell lines by measurement of the flux of the radioactive tracer ["C] formate through the pathway and into RNA (Smellie et al., 1958). The single carbon moiety of formate is utilized for the construction of the purine ring at steps 4 and 10 of the purine pathway. Formate is also utilized in the pyrimidine pathway in the de novo synthesis of thymidine 5'-monophosphate (Lehninger, 1975). To avoid the simultaneous detection of the pyrimidine pathway's activity, only alkali-sensitive cyto- plasmic nucleic acid (i.e. RNA only) was analyzed for the incorporation of Il4C]forrnate. Cell lines derived with the complete p53-inducible system exhibited a 56-91% decrease in their ability to incorporate [14C]formate into RNA at the induction temperature. This negative effect of low tempera- ture on formate incorporation in cell lines derived with the complete system was at least 4-fold greater than for control lines derived with incomplete plasmid systems (Table IVA).
The ['4C]formate flux results were consistent with the refined hypothesis that p53 induction caused a defect in purine nucleotide biosynthesis. However, it was also possible that the apparent decrease in purine pathway activity resulted indirectly from the fact that p53-induced cells grow more slowly. Because of the nature of the analysis, if slow growth resulted in decreased RNA synthesis, this would be reflected as a decrease in formate incorporation. Support for the former hypothesis was obtained by simultaneous analysis of tracer flux through the pyrimidine pathway. If the apparent purine pathway defect was a general effect of decreased RNA syn- thesis, then the pyrimidine pathway should show a similar defect. To rule out this possibility, pathway activity analyses were performed with [3H]orotate. Orotate is a pyrimidine- specific intermediate whose formation is completed at step 3 of the pathway (Lehninger, 1975). In double-labeling experi- ments with ['4C]formate and [3H]orotate, the negative effect of low temperature on the incorporation of [ 14C]formate into the RNA of p53-inducible cells was %fold greater than for
TABLE IV Nucleotide biosynthetic pathway anal.yses
A. Purine pathway activity versus p53 induction"
Cell line 37 "C cpmlea 32.5 "C cpmlrm Decrease ~~
tsE2 only lg-1 lm-5
p53-inducible lh-3 111-3
%
88 68 23 28 34 -21
68 30 56 49 4.6 91 61 27 56
B. Comparison of purine and pyrimidine pathway activities in p53- inducible line 111-3~
Precursor 37 "C cpm/ue 32.5 "C cwm/rg Decrease %
89 47 47 8.6 7.3 15
C. Serum dialysis effect on the p53-induced purine pathway defect'
Cell line 37 "C "C/W 32.5 "C "C/SH Decrease %
D-FBS lm-5 (tsE2 only) 8.3 7.6 8 111-3 (p53-inducible) 10 6.4 36
lm-5 1.7 1.9 -12 111-3 2.3 1.5 35
FBS
D. RNA G:A ratio distortion in p53-inducible cellsd
Cell line 31 "C "C-G/"C-A 32.5 "C ''C-GYC-A
tsE2 only lg-1 ND' 0.89 lm-5 ND 0.92
p53-inducible lh-3 0.71 0.68 111-3 0.90 0.67
[I4C]Formate incorporation into cytoplasmic RNA was deter- mined (as detailed under "Experimental Procedures") as a measure of the synthetic activity of the purine nucleotide pathway in cells growing at the indicated temperature. Line lg-1 is the control com- parison for the first listed ln-3 experiment. Line lm-5 is the control comparison for both the lh-3 experiment and the second listed 111-3 experiment.
* ["CIFormate and [3H]orotate incorporation in cytoplasmic RNA were determined after double labeling as a measure of the synthetic activity of the purine and pyrimidine nucleotide pathways, respec- tively, in p53-inducible ln-3 cells growing at the indicated tempera- ture.
Cells were double-labeled with ["Clformate and ['Hlorotate, and the ratio of 14C to 'H incorporated into cytoplasmic RNA was calcu- lated to assess temperature-induced changes in nucleotide biosyn- thetic activity specific to the purine pathway.
Cytoplasmic RNA purified from cells labeled with [14C]formate as in A was hydrolyzed as described under "Experimental Proce- dures," and the products analyzed by quantitative thin-layered chro- matography to determine the ratio of 14C incorporated into the adenine and guanine bases.
e ND, not done.
[3H]orotate incorporation (Table IVB). This result demon- strated that the p53-related nucleotide pathway flux defect was specific for the purine biosynthetic pathway.
In subsequent analyses of the effect of p53 induction on purine nucleotide biosynthesis, [3H]orotate incorporation served as the ideal standard to normalize for general changes in the activity of the nucleotide pathways due to culture and growth conditions. Justification for the use of [3H]orotate incorporation as a normalization factor comes from the ob-

24824 ~53 Concentration Regulates GTP Synthesis
servation that uridine, a pyrimidine nucleotide precursor, does not reverse the p53-related growth defect (see Table IRA). Therefore, in subsequent studies, changes in the activity of the purine pathway were assessed by measure of the ratio of [%]formate incorporation to [3H]orotate incorporation. By this measure, p53-inducible cells showed at least a 4.5-fold greater purine-specific defect at 32.5 “C, as compared to con- trol cells. Unlike the original induction phenotype of de- creased growth, the p53-related purine pathway defect was independent of culture conditions (Table IVC). This newly established p53-related biochemical phenotype represented a significant refinement over the original cellular phenotype of decreased growth.
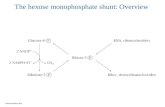
Conversion of IMP to XMP Is Regulated by the Cellular p53 Concentration-The preceding experiments established that elevation of the cellular ~53 concentration resulted in a de- crease in the activity of the purine nucleotide biosynthetic pathway. The aim of subsequent studies was to determine whether this p53-related biochemical effect was executed at a specific step in the pathway. The purine nucleotide biosyn- thetic pathway is comprised of 15 enzymatic steps. The path- way branches after step 11, the production of IMP, to ulti- mately yield AMP and GMP (Lehninger, 1975; see Fig. 8). Hypoxanthine, which enters the pathway at the branchpoint, might rescue p53-inducible cells in one of two ways. The nucleoside might serve to either bypass an upstream block in the pathway or to overcome a downstream block by increasing the amount of available substrate.
The first indication that a specific step in the purine pathway was affected upon ~53 induction came from exami- nation of RNA from [%]formate incorporation studies. The ‘%-1abeIed guanine and adenine bases in such RNA result primarily from label incorporated via the purine pathway. Therefore, the ratio of [‘%]guanine to [14C]adenine bases in the RNA is a measure of the relative activities of the two branches of the purine pathway, While RNA isolated from control lines growing at the induction temperature of 32.5 “C had a [‘4C]guanine/[‘4C]adenine ratio of 0.9, that of p53- inducible lines had a lower value of 0.7. More importantly, this ratio distortion could be relieved by growing p53-inducible cells at the noninducing temperature of 37 “C (Table IVD). One p53-inducible line (lh-3) showed little increase in the [14C]guanine/[‘4C]adenine ratio when grown at 37 “C. How- ever, the same line also exhibited poorer growth than other lines at 37 “C in all assays. Since it was known from the [‘“Cl formate incorporation studies that overall purine pathway activity decreased upon p53-induction, the coincident de- crease in the [‘4C]guanine/[‘4C]adenine ratio suggested that the p53-related defect localized to the GMP branch of the pathway.
The GMP synthesis branch of the purine pathway is com- prised of two enzymatic steps. In the first step IMP is con- verted to XMP by the enzyme IMP dehydrogenase. This enzyme catalyzes the rate-limiting step for GTP synthesis in mammalian cells (Jackson et ai., 1977; Weber, 1983). In the second step XMP is converted to GMP by GMP synthetase (Lehninger, 1975). In order to map more precisely the p53- related defect in GMP synthesis, changes in the kinetics of labeling purine nucleotides with [‘4C]inosine were assessed in p53-inducible cells upon shift to the induction temperature. [ “‘C]Inosine is converted directly into IMP via cellular nucle- oside kinases (Kornberg, 1980). The appearance of label in the downstream nucleotides, sAMP, AMP, XMP, and GMP is due to the action of specific enzymes in the branched pathway. Effects that perturb the activities of the these en- zymes result in changes in the labeling kinetics of the pathway
nucleotides. In [ “C]inosine labeling analyses normalized to total labeled cellular nucleotides, p53-inducible lines showed increased accumulation of label in IMP when cultured at the induction temperature, as compared to control lines (Fig. 7A). With respect to the distribution of label specifically within the branched purine pathway, the increased [14C]IMP accu- mulation represented a shift of 21% of the label in the system from GMP and XMP to IMP. No compensatory shift was seen with control lines. Altogether, these data indicated that a specific defect in the conversion of IMP to XMP was induced upon elevation of the cellular ~53 concentration. Consistent with this conclusion, extracts derived from p53- inducible cells growing at the induction temperature were
A I
G/XMP IMP AMP
H(i0) H(i00) X(50) X(400)
Addltlon (uhf)
FIG. 7. Localization of the p53-induced purine nucleotide biosynthetic pathway defect. A, changes in the l%linosine-label- ing kinetics of purine pathway nucleotides upon ~53 induction. p53- inducible cells and control cells were labeled with 1”Clinosine for 2 h at either 37 or 32.5 “C!, and acid hydrolysates containing total cellular nucleotides were prepared. An equivalent amount of hydrol- ysate %-cpm was spotted on thin-layered chromatography plates, and the indicated nucleotides were isolated and quantitated for in- corporated 14C as detailed under “Experimental Procedures.” y-axis, for each nucleotide the difference in accumulated “C-cpm between 37 (HZ’) and 32.5 “C! (LT), the induction temperature for ~53. B, comparison of the ability of purine nucleotide salvage precursors to reverse the p53-induction phenotype of decreased growth. p53-induc- ible line ln-3 cells growing exponentially at 37 ‘C in media supple- mented with FBS were replated at 32.5 “C in media supplemented with dialyzed FBS and the indicated concentrations of salvage pre- cursors (H, hypoxanthine; X, xanthosine). The doubling time was determined for the interval from 1 to 4 days of growth from the analysis of growth curves derived as detailed undei “Experimental Procedures.” Bar heights represent the average of duplicates, except for X(400) which represents data from a single growth curve. Error bars indicate the range of the data.

p53 Concentration Regulates GTP Synthesis 24825
diminished in IMP dehydrogenase enzymatic activity (Table V).
A final prediction could be made given the kinetic mapping of the p53-related purine pathway defect to the step of IMP conversion to XMP. If this were, indeed, the target enzymatic step, then exogenously added purine precursors utilized down- stream of this step should reverse the p53-induction pheno- type of decreased growth. Xanthosine is such a downstream salvage precursor which is converted directly into XMP by cellular nucleoside kinases (Kornberg, 1980). Not only did xanthosine reverse the p53-induction phenotype of decreased growth, but it was also more effective than hypoxanthine (Fig. 7B).
DISCUSSION
As an approach to defining the role of the cellular protein p53 in cellular proliferation, cell lines were derived which contain a p53-inducible expression system. The system based on the gene expression elements of bovine papilloma virus affords a 3-6-fold physiologic elevation of the cellular p53 concentration by simply growing the cells at 32.5 "C. This modest induction of p53 in cells results in a profound change in their growth behavior, characterized by a 2-4-fold increase in doubling time and biphasic growth kinetics. The first slow phase of growth can be explained by the induction and accu- mulation of quiescent cells in p53-induced cell populations. Colony formation assays demonstrate that p53-induced quies- cent cells can remain dormant for as long as 2 weeks while still retaining their capacity for growth. The explanation for the second phase of growth is presently unclear. The 5-fold increase in the growth rate of p53-inducible cells after 4 days at the induction temperature is manifest in growth curve analyses, but not in colony formation studies. Since growth curve analyses are performed at cell densities at least 100- fold greater than that of cell colony studies, the second growth phase may be due to the accumulation of cellular products in the media that can reverse the p53-induced growth arrest. Based on the results reported here, purine metabolites are likely candidates.
Systematic analysis of the p53 induction phenotype of altered growth led to the identification of the cellular target of p53 action. A number of lines of experimental evidence support the conclusion that p53 is a cellular regulator of GTP synthesis and performs this function by ultimately controlling
TABLE V Analysis of IMP dehydrogenase actiuity inp53-inducible cell extracts
Cells were grown for the indicated amount of time a t either 37 or 32.5 "C, harvested by trypsinization, and stored frozen at -80 'C. Cell extracts were prepared from thawed cell pellets by the method of Sherley and Kelly (1988a). IMP dehydrogenase activity was assayed by measurement of the conversion of [8-14C]IMP to [8-14C]XMP (Hodaes et al.. 1989).
IMPDH activitv Cell line
(days of growth) (XMP,cpm/pg k) Change"
37 'C 32.5 "C
Control lines C127 (1) 1g-1 (1) k-1 (2) lm-4 (1)
p53-inducible
ln-3 (1) 111-3 (2)
lh-3 (1)
%
33 37 12 18 27 50 25 22 -12 11 12 9
244 27
4.6 7.3
-98
33 16 -73 -52
a [(32.5 "C - 37 "C)/37 "C] x 100%.
the conversion of IMP to XMP, the rate-limiting step for the production of GTP. To begin with, culturing p53-inducible cells in dialyzed fetal bovine serum enhances the induction phenotype. This effect can be attributed to the removal of serum purine nucleotide precursors (Harkness et al., 1980; Hayashi et al., 1990a; Hayashi et al., 1990b), as supplemen- tation of dialyzed serum with hypoxanthine leads to the complete reversal of the p53-induction phenotype. Consistent with the ability of hypoxanthine to rescue cells from the effects of p53 induction, mutant cells which are defective for the utilization of hypoxanthine have increased sensitivity to p53 induction. Biochemical analyses demonstrate a specific decrease in the activity of the purine nucleotide biosynthetic pathway upon p53 induction. By examining the labeling ki- netics of purine pathway intermediates with [14C]inosine, this defect was mapped specifically to the step of IMP conversion to XMP (Fig. 8); and extracts from p53-inducible cells growing at the induction temperature are diminished in the activity of the enzyme responsible for this conversion, IMP dehydrogen- ase. Consistent with the conclusion that this enzymatic step is the ultimate biochemical target of p53 action, xanthosine, which is a direct salvage precursor for XMP, not only reverses the p53-induction phenotype, but also does so more effectively that hypoxanthine.
It is clearly the case that the described biochemical sequelae of p53 induction are not peculiar properties of particular cell clones that bear no relation to the stably transfected gene sequences. Regarding this issue, the two independently de- rived p53-inducible cell lines which serve as the basis for this study share very similar, though not identical, properties. The lack of identity is likely to reflect, in part, the prescribed compositional differences in the transfected plasmid systems (see Table I). The transfection plating assay employed to assess cell lines defective for hypoxanthine utilization pro- vides the best indication that the observed behaviors of p53- inducible lines are not irrelevant peculiarities of rare cell clones. With this assay the p53-induction phenotype is evi- dent in numerous independent transfection events, indicating that sensitivity to p53 elevation is a general property of C127 cells. More importantly, the same assay clearly demonstrates that involvement of the purine nucleotide biosynthetic path- way is a general feature of the antiproliferative action of p53 in c127 cells.
(De novo Purine Pathway)
Hyporanthine ___* HGPRT r I sAMP XMP +XXanthoslne
NSK
I I + c AMP GMP
FIG. 8. The proposed biochemical target of p53. Depicted is the branched segment of the purine nucleotide biosynthetic pathway. Elevation of the cellular concentration of p53 ultimately results in the inhibiton of IMP dehydrogenase (IMP-DH). This enzyme cata- lyzes the conversion of IMP to XMP, the rate-limiting step for GTP synthesis in mammalian cells. Hypoxanthine overcomes the cellular effects of this inhibition by increasing the concentration of the substrate for the reaction, IMP, while xanthosine bypasses the block by supplying the product of the reaction, XMP. HGPRT, hypoxan- thine/guanine phosphoribosyltransferase; NsK, nucleoside kinase; IMP, inosine 5'-monophosphate.

24826 p53 Concentration Regulates GTP Synthesis
Although the considerations above rule out the rare cell clone hypothesis, they do not address the possibility that the described effects of the elevation of the cellular p53 concen- tration is peculiar to C127 cells. To address this issue, similar analyses have been performed in another cell system whose growth properties are correlated, in part, with changes in the concentration of wild type p53. Recently, transformed rat embryo fibroblast (REF) cell lines have been described which were derived by cotransfection of an activated ras oncogene with a temperature sensitive allele of the murine p53 gene (Michaolvitz et al., 1990; Martinez et al., 1991). In such lines about 27% of the expressed murine p53 protein exists in a wild type conformation at 39.5 "C (as judged by the status of wild type and mutant specific monoclonal antibody epitopes), while at 32.5 "C the wild type fraction increases to 82%. Correlated with this effective increase in the cellular concen- tration of wild type p53 protein, these cell lines exhibit com- plete growth arrest a 32.5 "C (Martinez et at., 1991). Prelimi- nary analyses show that addition of xanthosine, a direct purine salvage precursor for XMP, results in partial rescue of such lines from the p53-related growth arrest. More impor- tantly, at the time of the growth arrest the conditional p53 lines exhibit a specific 50% decrease in the rate of cellular GTP ~ynthesis .~ The reproduction of these two key aspects of the C127 p53-inducible cell system in the conditional REF system strongly suggests that the regulation of cellular GTP synthesis is the central role of p53 in cellular proliferation.
The mechanism by which p53 concentration regulates IMP conversion to XMP is presently under investigation. This enzymatic step catalyzed by IMP dehydrogenase is ideal for such a regulatory mechanism, as it is the rate-limiting step for GTP synthesis (Jackson et al., 1977; Weber, 1983). As is common for metabolic pathway enzymes, IMP dehydrogenase is known to be feedback inhibited by the pathway product GMP (Lehninger, 1975). With regards to cell proliferation, the enzyme shares ideal reciprocity with p53. While over- expression of wild type p53 is incompatible with cell growth and transformation, IMP dehydrogenase activity is elevated both in actively growing cells and in malignant tissues (Jack- son et al., 1975; Jackson et al., 1977; Proffitt et al., 1983; Shimura et al., 1983; Weber, 1983). Inhibitors of the enzyme have been used to demonstrate its importance in cell growth (Cohen et al., 1981; Cohen and Sadee, 1983). It is of note that just as p53-inducible cells have an apparently greater sensi- tivity to the effects of elevated p53 concentration in S phase, it has been observed that cells also have a greater sensitivity to inhibitors of IMP dehydrogenase when in S phase (Cohen et al., 1981; Cohen and Sadee, 1983). Recently both gene probes and immunological reagents have been described for human and hamster IMP dehydrogenase (Collart and Hub- erman, 1987; Collart and Huberman, 1988 Natsumeda et al., 1990). The availability of such important experimental tools should greatly aid the elucidation of the p53 regulatory mech- anism.
Given that p53 concentration regulates GTP synthesis, an important question is what is the primary cellular response to this regulation that leads to the observed alterations in cell growth. GTP is an important cellular nucleotide as a substrate for nucleic acid synthesis, an energy source for metabolic reactions, and a regulatory molecule for cellular processes as diverse as microtubule assembly and protein translation (Lehninger, 1975). Considering the staggering list of potential GTP-related perturbations upon p53 induction, a simple ex- planation is that there is no critical function which is primar- ily responsible for the observed changes in growth behavior.
'' J. Sherley, unpublished data.
Instead, the altered growth pattern is a manifestation of numerous, unrelated cellular disturbances. Given the nature of the p53-induction phenotype, this simple explanation seems unlikely. I t is difficult to imagine how a deranged cell state might give rise to such a unique mode of growth which is completely reversible. There is experimental data to suggest that the nucleic acid synthesis is an unlikely candidate as the primary transducer of the induction phenotype. First, meas- urements using labeled pyrimidine nucleotide precursors in- dicate that the rate of RNA synthesis in p53-inducible lines growing under conditions which elevate the p53 concentration is similar to that of control lines (data not shown). Second, the observation that deoxynucleosides cannot reverse the p53 induction phenotype indicates that the lack of precursors for DNA synthesis is also not the primary problem in these cells. In retrospect, the original finding that elevation of the cellular p53 concentration resulted in a delay in progression through early S should be reinterpreted as reflecting the point in the cell cycle at which cells are most sensitive to the effect of p53 induction as opposed to an inhibition of DNA synthesis per se.
A priori, if, in fact, the induction phenotype is due to a critical cellular function which responds to a regulatory action of p53, then that function must be sensitive to small changes in the rate of GTP synthesis. Such a candidate cellular function is signal transduction. A number of growth factor signal transduction pathways have as important regulatory components proteins called G-proteins which require the binding of GTP to function. Replacement of GTP by GDP results in the inactivation of G-proteins. Since G-proteins have very similar affinities for GTP and GDP, an important parameter which regulates their activity is the concentration ratio of the two guanine nucleotides (Gilman, 1987; Gibbs and Marshall, 1989; Bourne et al., 1990; Reed, 1990; Bourne et al., 1991). This dependency on the ratio of GTP to GDP affords G-proteins a greater sensitivity to changes in GTP synthesis as compared to cellular factors which are regulated simply by GTP concentration. Recently, it has been shown that stimu- lation of quiescent cells to grow with specific growth factors results in a 2-fold increase in the cellular concentration of ras.GTP complexes (Gibbs et al., 1990). The mammalian ras protein is a GTP-binding protein that is well established as an important cellular effector of growth. After consideration of these observations, the argument can be put forth that small decreases in the GTP/GDP ratio may greatly compro- mise the ability of cells to maintain continued growth.
Given the above discussion, a very attractive hypothesis for the role of p53 is that of a global damper of cellular growth signals via regulation of GTP production. Such a model can account for both the oncogenic and antioncogenic properties of the protein. The wild type protein is envisioned to limit the growth capacity of cells by regulating the availability of GTP for growth factor signal transduction. Events which interfere with this regulatory function of p53 such as mutation or complex formation with viral tumor antigens would serve to increase the availability of GTP for the transduction of signals essential for increased cell growth and transformation. Future experiments will be directed at testing the validity of this model.
These studies demonstrate that a modest increase in the cellular concentration of p53 has profound consequences for the future growth of a cell. Induction of p53 expression in exponentially growing cells to a level similar to that seen in cells rendered quiescent by serum deprivation is shown to induce a new quiescent state. This p53-induced state is unique in that it represents a type of "physiologic quiescence" in

p53 Concentration Regulates GTP Synthesis 24827
culture, i.e. quiescence in the presence of serum growth fac- S o h , R. L., Douglass, E. C., and Housman, D. E. (1990) Cell 61, tors. This novel culture state promises to be more similar to 1257-1269 the state of nondividing cells in vivo. A detailed investigation ~ ~ ~ ~ ~ ; ~ $ ' ~ a , n ~ i " ~ ~ , E: F; ~ ~ ~ ~ ~ $ ! , ~ i ~ t ~ , l ~ K., and of the nature of this novel p53-induced quiescent state is in squire, J. A. (1980) Biochem, sot, T ~ ~ ~ , 8, 139 progress. Hawley-Nelson, P., Androphy, E. J., Lowy, D. R., and Schiller, J. T.
Acknowledgments-1 thank the members of the Levine lab for Hayashi, y., Hirai, s., Harawma, H., Saito, T.7 and IChikawa, A. critical reading of the manuscript. Special thanks are given to D. (1990a) JPn J. H~QrmQcol. 6391-9 Olson and Drs. E. Montalvo, J. Momand, I. Georgoff, J. Martinez, Hayashi, y., Hirai, s., and Ichikawa, A. (1990b) In Vitro Cell. Deu. and T. Chittenden for many helpful discussions. Many thanks to M. B i d 26,843-845 0. Cunningham and the WaWa Club for their continued encourage- Hinds, P. W., Finlay, C. A., Frey, A. B., and Levine, A. J. (1987) Mol. ment and support. I am also indebted to J. Zawadzki for many Cell. Biol. 7,2863-2869 insightful conversations and his rigorous approach to flow cytometry. Hedges, S. D.9 Fung, E., McKay, D. J., Renaux, B. s.9 and Snyder, F.
REFERENCES Holt, J. T., Gopal, T. V., Moulton, A. D., and Nienhuis, A. W. (1986)
Baker, s. J., Fearon, E. R., Nigra, J. M-, Hamilton, s. R., Preisinger, Horwitz, B. H., Burkhardt, A. L., Schlegel, R., and DiMaio, D. (1988)
(1988) EMBO J. 7, 525-531
F. (1989) J. Biol. Chem. 264, 18137-18141
Proc. Natl. Acad. Sci. U. S. A. 83, 4794-4798
A. C., Jessup, J. M., vanTuinen, P., Ledbetter, D. H., Barker, D. ~ ~ 1 . Cell. ~ i ~ l . 8,4071-4078 F., Nakamura, Y., White, R., and Vogelstein, B. (1989) Science Hunter, T. (1991) cell 64, 249-270 244,217-221
Baker, S. J., Markowitz, S., Fearon, E. R., Willson, J. K. V., Vogel- 331-333
Bienz, B., Zakut-Houri, R., Givol, D., and Oren, M. (1984) EMBO J. 1-10
Bishop, J. M. (1991) Cell 64, 235-248
Jackson, R. C., Weber, G., and Morris, H. P. (1975) Nature 256,
Jackson, R. C., Morris, H. P., and Weber, G. (1977) Biochem. J. 166,
Johansen, H., van der Straten, A., Sweet, R., Otto, E., Maroni, G.,
B. R . v and B. N. (1982) J. Bioi. Chem. 257* 9759- Kemp, A. J., Lyons, S. D., and Christopherson, R. I. (1986) J. Biol.
stein, B. (1990) Science 249, 912-915
3,2179-2183 and Rosenberg, M. (1989) Genes & Deu. 3, 882-889
Chem. 261, 14891-14895
H. R.r D. A . ~ and McCormick~ F. (lggl) Nature J. G., Smith, J . A,, and Struhl, K., eds) pp. 9.1.1-9.1.4, John Wiley (Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman,
& Sons, New York
(1987) Proc. Natl. Acad. Sci. U. S. A. 84, 2150-2154
9769 Bourne, H. R., Sanders, D. A., and McCormick, F. (1990) Nature Kingston, R. E, (1987) in Current Protocols in Molecular Biology 348,125-131
349,117-127 Buchberg, A. M.9 Cleveland, L. s., N. A., and Copeland, N. Korman, A, J., Frantz, J, D., Strominger, J. L., and Mulligan, R, C,
G. (1990) Nature 347, 291-294
D. A,, Rose, E. A., Kral, A., Yeger, H., Lewis, W. H., Jones, C., and Freeman and Company, Sari Francisco Housman, D. E. (1990) Cell 60, 509-520
A., Kapeller, R., and Soltoff, S. (1991) Cell 64, 281-302
Call, K. M., Glaser, T., Ita, C. Y., Buckler, A. J., Pelletier, J., Haber, Kornberg, A, (1980) DNA Replication, znd Ed., pp, 39-85, W. H.
Cantley, L. C., Auger, K. R., Carpenter, C., Duckworth, B., Graziani, Lane, D. p., and Benchimol, S, (1990) Genes & Deu, 4, 1-8 Chen, P . - L Chen, Y., Bookstein, R., and Lee, W.-H. (1990) Science Levine, A. J., and Momand, J. (1990) Biochim. Biophys, Acta 1032, Chiba, I., Takahashi, T., Nau, M. M., D'Amico, D., Curiel, D. T., Lewin, B. (1991) Cell 64, 303-312
Lamb, P., and Crawford, L. (1986) Mol. Cell. Biol. 6, 1379-1385
Lehninger, A. L. (1975) Biochemistry, 2nd Ed., Worth, New York
250, 1576-1580 119-136
Mitsudomi? T'? Buchhagen, D' L'? D'? Piantadosip s., Lupton, S,, and Levine, A. J. (1985) Mol. Cell. Bioi, 5, 2533-2542 Koga, H., Reissman, P. T., Slamon, D. J., Holmes, E. C., and Minna, J. D. (1990) Oncogene 5, 1603-1610 Malkin, D., Li, F. P., Strong, L. C., Fraumeni, J. F., Jr., Nelson, C.
E., Kim, D. H., Kassel, J., Gryka, M. A., Bischoff, F. Z., Tainsky, Cohen, M. B., and Sadee, W. (1983) Cancer Res. 43, 1587-1591 Cohen, M. B., Maybaum, J., and Sadee, W. (1981) J. Biol. Chem. M' A', and s' H' Science 2509 1233-1238
Collart, F, R., and Huberman, E. (1987) Mol, Cell, Bioi, 7, 3328-3331 J., Georgoff, I., Martinez, J.7 and Levine, A. J. (1991) Genes Collart' F' R'' and Huberman7 E. (1988) J' 2639 15769- McClinton, D., Stafford, J., Brents, L., Bender, T. P., and Kuehl, W. Cross, M., and Dexter, T. M. (1991) Cell 64, 271-280 M. (1990) Mol. Cell. Biol. 10, 705-710 Diller, L., Kassel, J., Nelson, C. E., Gryka, M. A., Litwak, G., Mercer, w. E., Amin, M., Sauve, G . J., Appella, E., Ullrich, s. J.7 and
256,8713-8717 Marshall, C. J. (1991) Cell 64,313-326
& Deu. 5,151-159 15772
Gebhardt, M., Bressac, B., Ozturk, M., Baker, S. J., Vogelstein, B., Romano, J. w. (lggoa) Oncogene 59 973-980 and Friend, S. H. (1990) Mol. Cell. Biol. 10, 5772-5781 Mercer, W. E., Sheilds, M. T., Amin, M., Suave, G. J., Appella, E.,
DiMaio, D., and Settleman, J . (1988) EMBO J. 7, 1197-1204 Romano, J. W., and Ullrich, S. J . (1990b) Proc. Natl. Acad. Sci. U. Eilers, M., Picard, D., Yamamoto, K. R., and Bishop, J. M. (1989) s. A . 879 6166-6170
Nature 340,66-68 Mercer, W. E., Shields, M. T., Lin, D., Appella, E., and Ullrich, S. J. Eliyahu, D., Michalovitz, D., Eliyahu, S., Pinhasi-Kimhi, O., and (lggl) NQtl. Acad. u. 5'. A. 88, 1958-1962
Oren, M. (1989) Proc. Natl. Acad. Sci. U. S. A. 86,8763-8767 Michalovitz, D., Halevy, O., and Oren, M. (1990) Cell 62, 671-680 Fenwick, R. G. (1985) in Molecular Cell Genetics (Gottesman, M., ed.) Muirhead, K. A., Horan, p. K.3 and poster G. (19851 BiolTechnology
pp. 333-373, John Wiley & Sons, New York 3,337-356 Fields, S., and Jang, S. K. (1990) Science 249, 1046-1049 Natsumeda, Y., Ohno, S., Kawasaki, H., Konno, Y., Weber, G., and Finlay, C. A., Hinds, P. W., Tan, T.-H., Eliyahu, D., Oren, M., and Suzuki* K. (1990) J. CJW~. 2659 5292-5295
Levine, A. J. (1988) Mol. Cell. Biol. 8, 531-539 Nigro, J. M., Baker, S. J., Preisinger, A. C., Jessup, J. M., Hostetter, Finlay, C. A., Hinds, P. W., and Levine, A. J. (1989) Cell 57, 1083- R., Cleary, K., Bigner, S. H., Davidson, N., Baylin, S., Devilee, P., 1093 Glover, T., Collins, F. S., Weston, A., Modall, R., Harris, C. C., and
Gannon, J. V., Greaves, R., Iggo, R., and Lane, D. P. (1990) EMBO Vogelstein, B. (1989) Nature 342, 705-708
Gessler, M., Poustka, A., Cavenee, W., Neve, R. L., Orkin, S. H., and 649 Bruns, G. A. P. (1990) Nature 343, 774-778 O'Rourke, R. W., Miller, C. W., Kato, G. J., Simon, K. J., Chen, D.-
Gibbs, J . B., and Marshall, M. S. (1989) Microbiol. Reu. 53, 171-185 L., Dang, C. V., and Koeffler, H. P. (1990) Oncogene 5, 1829-1832 Gibbs, J. B., Marshall, M. S., Scolnick, E. M., Dixon, R. F., and Proffitt, R. T., Pathak, V. K., Villacorte, D. G., and Presant, C. A.
Gilman, A. G., (1987) Annu. Reu. Biochem. 56, 615-649 Raycroft, L., Wu, H., and Lozano, G. (1990) Science 249, 1049-1051 Green, M. R. (1989) Cell 56, 1-3 Reed, R. R. (1990) The New Biologist 2,957-960 Haber, D. A., and Housman, D. E. (1991) Cell 64,5-8 Ricciardi, R. P., Miller, J. S., and Roberts, B. E. (1979) Proc. Natl. Haber, D. A., Buckler, A. J., Glaser, T., Call, K. M., Pelletier, J., Acad. Sci. U. S. A. 76, 4927-4931
J. 9, 1595-1602 Nishikura, K., and Murray, J. M. (1987) Mol. & Cell. Biol. 7, 639-
Vogel, U. S. (1990) J. Biol. Chem. 265, 20437-20442 (1983) Cancer Res. 43, 1620-1623

24828 p53 Concentration Regulates GTP Synthesis Robbins, P. D., Horowitz, J. M., and Mulligan, R. C. (1990) Nature
Sager, R. (1989) Science 246, 1406-1412 Sawyers, C. L., Denny, C. T., and Witte, 0. N. (1991) Cell 64, 337-
Scheinfest, C. W., Fugiwara, S., Lau, L. F., and Papas, T. S. (1988)
Sherley, J. L., and Kelly, T. J. (1988a) J. Bid . Chem. 263, 375-382 Sherley, J. L., and Kelly, T. J. (1988b) J. Biol. Chem. 263, 8350-
Shimura, K., Okada, M., Shiraki, H., and Nakagawa, H. (1983) J.
Smellie, R. M. S., Thomson, R. Y., and Davidson, J. N. (1958)
Spalholz, B. A,, Yang, Y.-C., and Howley, P. M. (1985) Cell 42,183-
Spalholz, B. A., Lambert, P. F., Yee, C. L., and Howley, P. M. (1987)
Spyrou, G., and Reichard, P. (1983) Biochem. Biophys. Res. Commun.
346,668-671
350
Mol. Cell. Biol. 8, 3080-3087
8358
Biochem. (Tokyo) 94, 1595-1603
Biochim. Biophys. Acta 29, 59-74
191
J. Virol. 61, 2128-2137
115, 1022-1026
Srivastava, S., Zou, Z., Pirollo, K., Blattner, W., and Chang, E. H. (1990) Nature 348, 747-749
Takahashi, T., Nau, M. M., Chiba, I., Birrer, M. J., Rosenberg, R. K., Vinocour, M., Levitt, M., Pass, H., Gazdar, A. F., and Minna, J. D. (1989) Science 246, 491-494
Tan, T.-H., Wallis, J., and Levine, A. J. (1986) J. Virol. 69, 574-583 Tuck, S. P., and Crawford, L. (1989) Mol. Cell. Biol. 9, 2163-2172 Weber, G. (1983) Cancer Res. 43, 3466-3492 Xu, G., O’Connell, P., Viskochil, D., Cawthon, R., Robertson, M.,
Culver, M., Dunn, D., Stevens, J., Gesteland, R., White, R., and Weiss, R. (1990a) Cell 62.599-608
Xu, G., Lin, B., Tanaka, K., Dunn, D., Wood, D., Gesteland, R., White, R., Weiss, R., and Tamanoi, F. (1990b) Cell 63,835-841
Yang, Y.-C., Okayama, H., and Howley, P. M. (1985a) Proc. Natl. Acad. Sci. U. S. A . 8 2 , 1030-1034
Yang, Y.-C., Spalholz, B. A., Rabson, M. S., and Howley, P. M. (1985b) Nature 3 18, 575-577
Yewdell, J. W., Gannon, J. V., and Lane, D. P. (1986) J. Virol. 69, 444-452