NEONATAL HYPOXIC-ISCHEMIC INJURY: ULTRASOUND · PDF fileNEONATAL HYPOXIC-ISCHEMIC INJURY:...
Transcript of NEONATAL HYPOXIC-ISCHEMIC INJURY: ULTRASOUND · PDF fileNEONATAL HYPOXIC-ISCHEMIC INJURY:...
B C
CONCLUSION
NEONATAL HYPOXIC-ISCHEMIC INJURY: ULTRASOUND AND DYNAMIC COLOR DOPPLER SONOGRAPHY PERFUSION QUANTIFICATION OF THE BRAIN
AND ABDOMEN WITH PATHOLOGIC CORRELATIONG. Cassia1, R. Faingold1 , G. Sant’Anna2 , A. Ben Ely1 , C. Bernard3
1Medical Imaging, 2Department of Neonatology, 3Department of PathologyThe Montreal Children’s Hospital, McGill University Health Centre, Montreal, Qc, Canada
Hypoxic-ischemic encephalopathy (HIE) is a major contributor to neonatal death and morbidity. An estimated 23% of the 4 million neonatal deaths and 8% of all deaths at <5 years of age throughout the world each year are associated with signs of asphyxia at birth (1, 2). Perinatal asphyxia (PA) represents impaired gas exchange that results in hypoxia, hypercarbia and acidosis. If the metabolic abnormalities last long enough, the brain vascular auto regulation will fail. This, combined with low blood pressure will result in decreased cerebral blood flow and potential ischemic brain injury (3, 4). The brain of a term neonate is relatively resistant to hypoxia, but the combination of hypoxia and hypoperfusion as seen in most PA cases results in Hypoxic-ischemic injury (HII).
Brain assessment and monitoring of these infants is usually done by Electroencephalography, Near Infrared Spectroscopy (5-8) and Magnetic Resonance Imaging (MRI) of the brain (9, 10). Cranial and abdominal Ultrasound (US) are performed in neonates, as part of the clinical care, in some centers.Head Ultrasound (HUS) may be helpful in the routine care of neonates with HII (11). Pulsed Doppler Flow velocities and Resistive index (RI) is altered in the cerebral arteries and splancnic arteries such as SMA (12) in asphyxiated neonates. Dynamic color Doppler Sonography (CDS) is a simple non-invasive technique that has been used to assess and quantify tissue perfusion in different organs and systems (13-15).
Multiple-organ failure occurs in 50-60% of neonates with severe HII (12). Blood flow redistribution to the brain, heart and adrenal glands with decrease in flow to the kidneys, bowel and skin plays an important role in the pathophysiology of HII.
Therefore, US is a non-invasive modality, by the bedside, that can provide comprehensive imaging of multiple organs and systems. Our exhibit is a pictorial review with illustration and discussion of US and CDS perfusion intensity findings of the brain and abdomen in neonatal HII with pathologic correlation.
Even at referral centers in developed countries, death or moderate to severe disability occurs in 53% to 61% of infants diagnosed as having moderate to severe HII (16-18).
During the post-asphyxiated period a consistent observation has been a marked increase in cerebral blood flow, which continues for several hours and may or may not decline towards baseline. Importantly, a delayed increase in cerebral blood flow may unfold, with onset generally between 12 to 24 hours and with duration of many hours or days (19). This chain of events is called reperfusion phase and is responsible for secondary brain injury. Indeed, neurological outcome is established to a substantial degree by mechanisms occurring during this period. In HIE infants, high levels of cerebral blood flow measured at 12 – 24 h of life were associated with poorest neurological outcomes (20, 21). It is well known in the literature that involvement of basal ganglia and thalami is associated with poor outcome in HIE (9, 22).
Therapeutic hypothermia has been used more frequently in infants with moderate to severe encephalopathy. It results in improved neurological outcome in survivors (1). Cerebral MRI is the imaging standard in HIE (2), however requires transportation from NICU to MRI suite, which is a complex operation in acute ill infants.
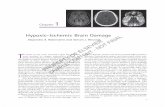
A recently described CDS technique has been used to assess and quantify tissue perfusion or perfusion intensity in different organs and systems (13-15). This technique can also be used to assess and quantify Cerebral Perfusion Intensity (CPI), more specifically the CPI of the basal ganglia and thalamic areas (Fig.1).
These areas are of particular interest since a substantial proportion of post asphyxiated infants (35% to 85%) exhibit predominantly cerebral deep nuclear neuronal involvement (23, 24 ) and injury to these areas have been associated with unfavorable neurological outcome (9, 22, 25, 26). (Fig.2).
Our unpublished data regarding Basal Ganglial Color Doppler perfusion measurements performed in 30 neonates with HII, during hypothermia, provided important information.
Head Ultrasound (HUS) was performed routinely in all HIE neonates submitted to hypothermia, as part of their routine care. CDS was obtained with an 11LW4 MHz linear transducer to obtain DICOM color Doppler videos of the blood flow in the basal ganglia. Three videos of 3 seconds each are obtained for the ROI, in the coronal plane, and used to calculate the CPI. Measurements of CPI were evaluated retrospectively by 2 radiologists using a dedicated software. It allows automatic quantification of color Doppler data from a region of interest (ROI) by dynamically assessing color pixels and flow velocity during the heart cycle. CPI is expressed in cm/sec and is calculated by multiplying the mean velocity of all pixels by the area of the pixels, divided by the area of the ROI. Clinical and radiological data were evaluated retrospectively.
Unpublished data showed that increased cerebral perfusion intensity to the basal ganglia in neonates with HIE, treated with hypothermia, was associated with poor outcome. CPI values were significantly higher in the 7 neonates that died (0.226 ± 0.221 cm/s vs. 0.111± 0.082 cm/sec; p= 0.02). CPI quantification with dynamic CDS opens a window to better understand reperfusion injury in HII.
A
C D
C
C
Mild HII. A. HUS showing brain swelling with hyperechogenic subcortical white-matter and increased grey-white matter differentiation B. HUS with color Doppler and quantification of CPI with ROI in basal ganglia. C. ADC map with restrictive diffusion in watershed areas bilaterally
Adrenal gland involvement. A. Adrenal swelling with ill defined central echogenic stripe B. Adrenal hemorrhage C. Section of adrenal gland with swelling and focal bleed.
Adrenal swelling and thickening has been described in infants with PA and also other causes of perinatal stress (30). Sonographically they may be enlarged or may loose their central echogenic stripe (Fig.7a). Diffuse congestion of sinusoids has been described in histological specimens.
Adrenal hemorrhage is relatively uncommon in neonates (Fig.7b). It has been described in association with PA, birth trauma, septicemia and bleeding diathesis. The incidence ranges from approximately 1.7 per 1000 of autopsied neonates to approximately 3% in abdominal ultrasound (31).
A B C
PA has devastating consequences in neonates. In a study by Martin-Ancel (27) et al, over a period of 2 years, 29% of neonates with PA showed GI disfunction. Multiple-organ failure occurs in 50-60% of neonates with severe HII (12). Blood flow redistribution to the brain, heart and adre-nal glands with decrease in flow to the kidneys, bowel and skin plays an important role in the pathophysiology of HII. In severe HII, significant decrease in the mean blood flow velocities and increase in RI are detected in the superior mesenteric artery (SMA) (12). Accurate assessment of the mesenteric perfusion changes in asphyxia may help staging this devastating disease and understanding the mechanism of intestinal auto-regulation in neonates.
Our unpublished data regarding intestinal Color Doppler perfusion measurements performed in 28 neonates with HII, during hypothermia, provided important information.
Gray-scale abdominal US images of the bowel and DICOM color Doppler videos of the mural blood flow were acquired with an 11LW4 MHz linear transducer to assess the Intestinal Perfusion Intensity (IPI), with similar technique and analyzed with same software as described previously. The IPI, bowel wall thickness and echotexture were evaluated in all neonates. Evidence of sloughed mucosa was defined as the presence of a halo or echogenic material with echo-texture similar to the mucosa, within the intestinal lumen. (Fig.3)
Seven neonates died, and 6 of them had sloughed mucosa on US. The presence of sloughed mucosa was highly predictable of mortality with a sensitivity = 86, specificity = 100, PPV = 100 and NPV = 95. Bowel wall was relatively thicker in the neonates that died (1.600 ± 0.39 mm vs. 1.439 ± 0.31 mm; p< 0.01). There was a trend to decreased mural perfusion (0.040 ± 0.015 cm/s vs. 0.052 ± 0.029 cm/s) in non-survivors. (Fig.4)
Severe HII. A. HUS showing hyperechogenic basal ganglia B. HUS with color Doppler and quantification of CPI, with ROI in basal ganglia, demonstrating increased perfusion C. ADC map with restrictive diffusion in basal ganglia and thalami. D. Histology: presence of necrotic acidophil neurons (black arrows), gliosis and reactive astrocytes (red arrows) H&E 60X
A
Gray-scale renal appearances. A. Normal echotexture and cortico-medullary differentiation B. hyperechogenic kidney with loss of cortico-medullary differentiation C. Kidney section with areas of medullary hemorrhage and cortical necrosis.
Renal CDS findings. A. Normal CDS. B. Very decreased CDS within renal parenchyma with loss of cortico-medullary differentiation.
Shah et al (28) examined 130 term neonates and found that all neonates with HIE developed either renal injury or involvement of other organs. In another study conducted by Martin-Ancel et al (27), 2-year follow up of 72 term neonates with PA revealed end organ dysfunction incidence as follows: 42% renal, 26% pulmonary, 29% cardiac and 29% gastrointestinal complications. In both these studies, clinical as well as laboratory findings were used as a measure of organ dysfunction. Imaging is not routinely used, however a decrease in blood flow velocity has been described in the renal artery with Doppler interrogation in severe HII (12). (Figs. 5,6)
PA is one of the most common causes of acute renal injury in neonates. The presence of multi-organ dysfunction appear to predict worse outcome. The prevalence of acute renal failure ranges from 30 to 56% according to the literature (29). Due to limitations in the diagnostic criteria, these numbers are likely an underestimate. It may course with or without oliguria or increase in serum creatinine.
A B C
A B
Hepatic injury. A. The liver parenchyma is heterogenous with geographic areas of increased echogenicity B. Section of pathology specimen with areas of ischemic necrosis and hemorrhage.
The liver is commonly affected in PA despite generally being excluded from the classification of multi-organ involvement. One study found hepatic injury (Fig.8) in up to 39% of neonates with PA (32). Monitoring of hepatic dysfunction involves quantification of liver enzymes, especially AST, released due to hepatocyte injury. Imaging does not play an important role in this population.
A B
INTRODUCTION
A B
A B
B
US and dynamic CDS of the brain and abdomen provides reliable and comprehensive bedside multi-organ imaging information in HII. This pictorial review with pathologic correlation provides an overview of the systemic impact of HII. It also helps understanding the complex pathophysiology of the disease.
E
C
D
Brain
Adrenal
Liver
Bowel
Kidneys
NEONATAL HYPOXIC-ISCHEMIC INJURY: ULTRASOUND AND DYNAMIC COLOR DOPPLER SONOGRAPHY PERFUSION QUANTIFICATION OF THE BRAIN
AND ABDOMEN WITH PATHOLOGIC CORRELATION
FIGURE 1
FIGURE 5
FIGURE 6
FIGURE 7
FIGURE 8
FIGURE 2
FIGURE 3
Gray-scale intestinal appearances. A. Normal B. Echogenic bowel wall and ascites C. Sloughed mucosa (white arrows) D. Sloughed mucosa (white arrow) E. Pathology specimen with areas of ischemic necrosis (white arrow) and sloughed mucosa (black arrow).
Intestinal CDS and IPI measurements. A. Preserved flow with CDS and IPI calculated with 1 cm ROI (B). C. Decreased CDS with corresponding IPI quantification.
FIGURE 4