NATIONAL SENIOR CERTIFICATE GRADE 12 · 3.2 Calculate the time it takes for ball A to return to its...
Transcript of NATIONAL SENIOR CERTIFICATE GRADE 12 · 3.2 Calculate the time it takes for ball A to return to its...

Copyright reserved Please turn over
MARKS: 150 TIME: 3 hours
This question paper consists of 18 pages, 3 data sheets and 1 graph sheet.
GRADE 12
NATIONAL SENIOR CERTIFICATE
PHYSICAL SCIENCES: PHYSICS (P1)
NOVEMBER 2014

Physical Sciences/P1 2 DBE/November 2014 NSC
Copyright reserved Please turn over
INSTRUCTIONS AND INFORMATION 1. Write your centre number and examination number in the appropriate spaces
in the ANSWER BOOK and on the GRAPH PAPER.
2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12.
This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK. Start EACH question on a NEW page in the ANSWER BOOK. Number the answers correctly according to the numbering system used in this question paper. Leave ONE line between two subsections, for example between QUESTION 2.1 and QUESTION 2.2. You may use a non-programmable calculator. You may use appropriate mathematical instruments. You are advised to use the attached DATA SHEETS. Show ALL formulae and substitutions in ALL calculations. Round off your final numerical answers to a minimum of TWO decimal places. Give brief motivations, discussions, et cetera where required. Write neatly and legibly.

Physical Sciences/P1 3 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 1: MULTIPLE-CHOICE QUESTIONS Four options are provided as possible answers to the following questions. Each question has only ONE correct answer. Write only the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 Which ONE of the following physical quantities is a measure of the inertia of
a body?
A
B C D
Mass Energy Velocity Acceleration
(2) 1.2 The magnitude of the gravitational force exerted by one body on another body
is F. When the distance between the centres of the two bodies is doubled, the magnitude of the gravitational force, in terms of F, will now be …
A
B C
D
41 F
21 F
2F 4F
(2) 1.3 An object is thrown vertically upwards. Which ONE of the following regarding
the object's velocity and acceleration at the highest point of its motion is CORRECT? Ignore the effects of friction.
VELOCITY ACCELERATION
A Zero Zero
B Zero Upwards
C Maximum Zero
D Zero Downwards
(2)

Physical Sciences/P1 4 DBE/November 2014 NSC
Copyright reserved Please turn over
BEFORE COLLISION AFTER COLLISION
m m
vf = 0 v v v
2m 2m
1.4 An object of mass m moving at velocity v collides head-on with an object of
mass 2m moving in the opposite direction at velocity v. Immediately after the collision the smaller mass moves at velocity v in the opposite direction and the larger mass is brought to rest. Refer to the diagram below.
Ignore the effects of friction.
Which ONE of the following is CORRECT?
MOMENTUM MECHANICAL ENERGY
A Conserved Conserved
B Not conserved Conserved
C Conserved Not conserved
D Not conserved Not conserved
(2)
1.5 Two balls, P and Q, are dropped simultaneously from the same height.
Ball P has TWICE the mass of ball Q. Ignore the effects of air friction. Just before the balls hit the ground, the kinetic energy of ball P is x. The kinetic energy of ball Q, in terms of x, will be …
A
B C
D
41 x
21 x
x 2x
(2)

Physical Sciences/P1 5 DBE/November 2014 NSC
Copyright reserved Please turn over
+ 2 nC - 2 nC S T × ×
P
R
Q
S
E3
E2
E1
E0
1.6 The diagram below shows the electron transitions P, Q, R and S between
different energy levels in an atom.
Which ONE of the transitions will result in an emission of a radiation with the
longest wavelength?
A
B C D
P Q R S
(2) 1.7 Two charges of + 2 nC and - 2 nC are located on a straight line. S and T are
two points that lie on the same straight line as shown in the diagram below.
Which ONE of the following correctly represents the directions of the
RESULTANT electric fields at S and at T?
DIRECTION OF THE RESULTANT ELECTRIC FIELD
AT POINT S
DIRECTION OF THE RESULTANT ELECTRIC FIELD
AT POINT T
A Right Left
B Left Left
C Right Right
D Left Right
(2)

Physical Sciences/P1 6 DBE/November 2014 NSC
Copyright reserved Please turn over
X Z
A1
Y
A2 A3
A
S
1.8 Three light bulbs, X, Y and Z with resistances R, 2R and R respectively, are
connected in a circuit as shown below. The battery has negligible internal resistance. When switch S is closed, all the bulbs light up. The reading on ammeter A is 2,5 A.
Which ONE of the following correctly describes the readings on the ammeters
(in amperes) when bulb Z burns out?
A1 A2 A3 A
A 1,25 1,25 0 2,5
B 1,6 0,8 0,1 2,5
C 0,75 0,75 0 1,5
D 1 0,5 0 1,5
(2)

Physical Sciences/P1 7 DBE/November 2014 NSC
Copyright reserved Please turn over
em
f (V
)
t (s)
B
1.9 The coils of an AC generator make one complete rotation. The resulting graph
for the output emf is shown below.
The position B on the graph is obtained when the plane of the coil is at an
angle of … to the magnetic field.
A
B C D
0º 60º 90º 120º
(2)
1.10 A learner makes the observations below after conducting an experiment using
a photocell with frequencies of the incident light being above the threshold frequency (cut-off frequency).
(i)
(ii) (iii)
The photocurrent increases as the intensity of the incident light increases. The ammeter in the circuit registers a current immediately after the incident light is radiated on the cathode. The photocurrent increases as the frequency of the incident light increases.
Which of the observation(s) is/are CORRECT? A
B C D
(i) only (ii) only (i) and (ii) only (ii) and (iii) only
(2)
[20]

Physical Sciences/P1 8 DBE/November 2014 NSC
Copyright reserved Please turn over
•
20 kg
250 N
5 kg
T2
T1
Q
P
QUESTION 2 (Start on a new page.) Two blocks of masses 20 kg and 5 kg respectively are connected by a light inextensible string, P. A second light inextensible string, Q, attached to the 5 kg block, runs over a light frictionless pulley. A constant horizontal force of 250 N pulls the second string as shown in the diagram below. The magnitudes of the tensions in P and Q are T1 and T2 respectively. Ignore the effects of air friction.
2.1 State Newton's Second Law of Motion in words. (2) 2.2 Draw a labelled free-body diagram indicating ALL the forces acting on the
5 kg block.
(3) 2.3 Calculate the magnitude of the tension T1 in string P. (6) 2.4 When the 250 N force is replaced by a sharp pull on the string, one of the two
strings break.
Which ONE of the two strings, P or Q, will break? (1)
[12]

Physical Sciences/P1 9 DBE/November 2014 NSC
Copyright reserved Please turn over
h
A
B 15 m∙s-1
Ground
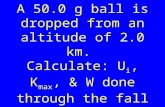
QUESTION 3 (Start on a new page.) A ball, A, is thrown vertically upward from a height, h, with a speed of 15 m∙s-1. AT THE SAME INSTANT, a second identical ball, B, is dropped from the same height as ball A as shown in the diagram below. Both balls undergo free fall and eventually hit the ground.
3.1 Explain the term free fall. (2) 3.2 Calculate the time it takes for ball A to return to its starting point. (4) 3.3 Calculate the distance between ball A and ball B when ball A is at its
maximum height.
(7) 3.4 Sketch a velocity-time graph in the ANSWER BOOK for the motion of ball A
from the time it is projected until it hits the ground. Clearly show the following on your graph: • The initial velocity • The time it takes to reach its maximum height • The time it takes to return to its starting point
(4) [17]

Physical Sciences/P1 10 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 4 (Start on a new page.)
Dancers have to learn many skills, including how to land correctly. A dancer of mass 50 kg leaps into the air and lands feet first on the ground. She lands on the ground with a velocity of 5 m∙s-1. As she lands, she bends her knees and comes to a complete stop in 0,2 seconds.
4.1 Calculate the momentum with which the dancer reaches the ground. (3) 4.2 Define the term impulse of a force. (2) 4.3 Calculate the magnitude of the net force acting on the dancer as she lands. (3) Assume that the dancer performs the same jump as before but lands without bending her knees.
4.4 Will the force now be GREATER THAN, SMALLER THAN or EQUAL TO the
force calculated in QUESTION 4.3?
(1) 4.5 Give a reason for the answer to QUESTION 4.4. (3)
[12]

Physical Sciences/P1 11 DBE/November 2014 NSC
Copyright reserved Please turn over
25o
300 kg
rope motor
QUESTION 5 (Start on a new page.) 5.1 The diagram below shows a track, ABC. The curved section, AB, is
frictionless. The rough horizontal section, BC, is 8 m long.
An object of mass 10 kg is released from point A which is 4 m above the
ground. It slides down the track and comes to rest at point C.
5.1.1 State the principle of conservation of mechanical energy in words. (2) 5.1.2 Is mechanical energy conserved as the object slides from A to C?
Write only YES or NO.
(1) 5.1.3 Using ENERGY PRINCIPLES only, calculate the magnitude of the
frictional force exerted on the object as it moves along BC.
(6) 5.2 A motor pulls a crate of mass 300 kg with a constant force by means of a light
inextensible rope running over a light frictionless pulley as shown below. The coefficient of kinetic friction between the crate and the surface of the inclined plane is 0,19.
5.2.1 Calculate the magnitude of the frictional force acting between the
crate and the surface of the inclined plane.
(3) The crate moves up the incline at a constant speed of 0,5 m∙s-1. 5.2.2 Calculate the average power delivered by the motor while pulling the
crate up the incline.
(6) [18]
4 m
B C
A
8 m

Physical Sciences/P1 12 DBE/November 2014 NSC
Copyright reserved Please turn over
Diagram 1
Diagram 2
Blue Red
Blue Red
QUESTION 6 (Start on a new page.) 6.1 The siren of a stationary ambulance emits a note of frequency 1 130 Hz.
When the ambulance moves at a constant speed, a stationary observer detects a frequency that is 70 Hz higher than that emitted by the siren.
6.1.1 State the Doppler effect in words. (2) 6.1.2 Is the ambulance moving towards or away from the observer?
Give a reason for the answer.
(2) 6.1.3 Calculate the speed at which the ambulance is travelling. Take the
speed of sound in air as 343 m∙s-1.
(5) 6.2 A study of spectral lines obtained from various stars can provide valuable
information about the movement of the stars. The two diagrams below represent different spectral lines of an element. Diagram 1 represents the spectrum of the element in a laboratory on Earth. Diagram 2 represents the spectrum of the same element from a distant star.
Is the star moving towards or away from the Earth? Explain the answer by
referring to the shifts in the spectral lines in the two diagrams above.
(2) [11]

Physical Sciences/P1 13 DBE/November 2014 NSC
Copyright reserved Please turn over
10 cm 20 cm
S R T
R + 8 μC S - 4 μC
QUESTION 7 (Start on a new page.) The diagram below shows two small identical metal spheres, R and S, each placed on a wooden stand. Spheres R and S carry charges of + 8 μC and - 4 μC respectively. Ignore the effects of air.
7.1 Explain why the spheres were placed on wooden stands. (1) Spheres R and S are brought into contact for a while and then separated by a small distance.
7.2 Calculate the net charge on each of the spheres. (2) 7.3 Draw the electric field pattern due to the two spheres R and S. (3) After R and S have been in contact and separated, a third sphere, T, of charge + 1 µC is now placed between them as shown in the diagram below.
7.4 Draw a free-body diagram showing the electrostatic forces experienced by
sphere T due to spheres R and S.
(2) 7.5 Calculate the net electrostatic force experienced by T due to R and S. (6) 7.6 Define the electric field at a point. (2) 7.7 Calculate the magnitude of the net electric field at the location of T due to R
and S. (Treat the spheres as if they were point charges.)
(3) [19]

Physical Sciences/P1 14 DBE/November 2014 NSC
Copyright reserved Please turn over
r
A
V
S
ε
QUESTION 8 (Start on a new page.) NOTE: The graph for QUESTION 8.1.2 must be drawn on the GRAPH SHEET
attached at the end of the QUESTION PAPER.
8.1 A group of learners conduct an experiment to determine the emf (ε ) and
internal resistance (r) of a battery. They connect a battery to a rheostat (variable resistor), a low-resistance ammeter and a high-resistance voltmeter as shown in the diagram below.
The data obtained from the experiment is displayed in the table below.
READING ON VOLTMETER (V)
READING ON AMMETER (A)
2 0,58 3 0,46 4 0,36 5 0,24 6 0,14
8.1.1 State ONE factor which must be kept constant during the experiment. (1) 8.1.2 Using the information in the table above, plot the points and draw the
line of best fit on the attached GRAPH SHEET.
(3) Use the graph drawn in QUESTION 8.1.2 to determine the following: 8.1.3 Emf ( ε ) of the battery (1)
8.1.4 Internal resistance of the battery, WITHOUT USING ANY FORM OF
THE EQUATION ε = I(R + r)
(3)

Physical Sciences/P1 15 DBE/November 2014 NSC
Copyright reserved Please turn over
r
A
X
S1 ε =24 V
Y
Z S2
20 V, 100 W
150 W
8.2 Three electrical devices, X, Y and Z, are connected to a 24 V battery with
internal resistance r as shown in the circuit diagram below. The power rating of each of the devices X and Y are indicated in the diagram.
With switch S1 closed and S2 open, the devices function as rated. Calculate the: 8.2.1 Current in X (3) 8.2.2 Resistance of Y (3) 8.2.3 Internal resistance of the battery (5)
Now switch S2 is also closed. 8.2.4 Identify device Z which, when placed in the position shown, can still
enable X and Y to operate as rated. Assume that the resistances of all the devices remain unchanged.
(1)
8.2.5 Explain how you arrived at the answer to QUESTION 8.2.4. (2)
[22]

Physical Sciences/P1 16 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 9 (Start on a new page.) The diagram below represents a simplified version of an electrical machine used to light up a bulb.
9.1 Name the principle on which the machine operates. (1) 9.2 State ONE way in which to make this bulb burn brighter. (1) Some changes have been made to the machine and a new device is obtained as shown below.
9.3 Name part X in the new device. (1)
commutator
X
brushes

Physical Sciences/P1 17 DBE/November 2014 NSC
Copyright reserved Please turn over
em
f (V
)
t (s)
339,45
9.4 The graph of output emf versus time obtained using the device in
QUESTION 9.3 is shown below.
9.4.1 Define the term root mean square value of an AC voltage. (2) 9.4.2 Calculate the rms voltage. (3)
[8]

Physical Sciences/P1 18 DBE/November 2014 NSC
Copyright reserved
potassium cathode incident light
e- e-
DC source
QUESTION 10 (Start on a new page.) Ultraviolet light is incident onto a photocell with a potassium cathode as shown below. The threshold frequency of potassium is 5,548 x 1014 Hz.
10.1 Define the term threshold frequency (cut-off frequency). (2) The maximum speed of an ejected photoelectron is 5,33 x 105 m∙s-1. 10.2 Calculate the wavelength of the ultraviolet light used. (5) The photocell is now replaced by another photocell with a rubidium cathode. The maximum speed of the ejected photoelectron is 6,10 x 105 m∙s-1 when the same ultraviolet light source is used.
10.3 How does the work function of rubidium compare to that of potassium?
Write down only GREATER THAN, SMALLER THAN or EQUAL TO.
(1) 10.4 Explain the answer to QUESTION 10.3. (3)
[11] TOTAL: 150

Physical Sciences/P1 1 DBE/November 2014 NSC
Copyright reserved Please turn over
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 1 (PHYSICS)
GEGEWENS VIR FISIESE WETENSKAPPE GRAAD 12
VRAESTEL 1 (FISIKA) TABLE 1: PHYSICAL CONSTANTS/TABEL 1: FISIESE KONSTANTES
NAME/NAAM SYMBOL/SIMBOOL VALUE/WAARDE
Acceleration due to gravity Swaartekragversnelling
g 9,8 m·s-2
Universal gravitational constant Universele gravitasiekonstant
G 6,67 x 10-11 N·m2·kg-2
Speed of light in a vacuum Spoed van lig in 'n vakuum
c 3,0 x 108 m·s-1
Planck's constant Planck se konstante
h 6,63 x 10-34 J·s
Coulomb's constant Coulomb se konstante
k 9,0 x 109 N·m2·C-2
Charge on electron Lading op elektron
-e -1,6 x 10-19 C
Electron mass Elektronmassa
me 9,11 x 10-31 kg
Mass of Earth Massa van Aarde
M 5,98 x 1024 kg
Radius of Earth Radius van Aarde
RE 6,38 x 106 m

Physical Sciences/P1 2 DBE/November 2014
NSC
Copyright reserved Please turn over
TABLE 2: FORMULAE/TABEL 2: FORMULES MOTION/BEWEGING
tavv if ∆+= 2
21
i taΔtvΔx ∆+= or/of 2
21
i taΔtvΔy ∆+=
xa2vv2
i
2
f ∆+= or/of ya2vv2
i
2
f ∆+= Δt2
vvΔx fi
+= or/of Δt2
vvΔy fi
+=
FORCE/KRAG
maFnet = mvp=
Nμ=f s
max
s Nμ=f kk
if
net
mv- mv=pΔpΔ=tΔF
mgw =
2
21
d
mmG=F or/of
2
21
r
mmG=F
2d
MG=g
2r
MG=g
WORK, ENERGY AND POWER/ARBEID, ENERGIE EN DRYWING
xFW ∆= cosθ mghU= or/of mghEP =
2mv2
1K= or/of 2
k mv2
1E =
KWnet ∆= or/of knet EW ∆=
if KKK −=∆ or/of kikfk EEE −=∆
UKWnc ∆+∆= or/of pknc EEW ∆+∆= t
WP ∆=
Pav = Fvav / Pgemid = Fvgemid
WAVES, SOUND AND LIGHT/GOLWE, KLANK EN LIG
λ= fv f
1T =
s
s
LL f
vv
vvf ±
±=
b
b
LL f
vv
vvf ±
±= hfE= or /of λ= chE
k(max)o E+ W=E or/of maxo K+ W=E where/waar
hf E = and/en 00 hfW = and/en 2
max(max)k mv2
1=E or/of
2
maxmax mv2
1=K
or/of

Physical Sciences/P1 3 DBE/November 2014 NSC
Copyright reserved
ELECTROSTATICS/ELEKTROSTATIKA
2
21
r
QkQF=
2r
kQE =
q
WV =
q
FE =
e
Q=n
eq
Q=n
ELECTRIC CIRCUITS/ELEKTRIESE STROOMBANE
I
VR=
emf ( ε )= I(R + r)
emk ( ε )= I(R + r)
...RRR 21s ++=
...R
1
R
1
R
1
21p
++= I=q ∆ t
W = Vq W = VI∆ t W = I
2R∆ t
W = R
ΔtV2
ΔtW
P=
P = VI
R=P 2I
R
VP
2=
ALTERNATING CURRENT/WISSELSTROOM
2
maxrms
II = /
2
makswgk
II =
2
VV max
rms = / 2
VV maks
wgk =
rmsrmsave VP I= / wgkwgkgemiddeld VP I=
RP 2
rmsave I= / RP 2
wgkgemiddeld I=
R
VP
2
rmsave = /
R
VP
2
wgk
gemiddeld =
or/of

Physical Sciences/P1 DBE/November 2014 NSC
Copyright reserved
CENTRE NUMBER:
EXAMINATION NUMBER:
QUESTION 8.1.2
ATTACH THIS GRAPH SHEET TO YOUR ANSWER BOOK.
Graph of potential difference vs current
I (A)
V (
V)
2,0
4,0
6,0
0 0,2 0,4 0,6 0,8
8,0
1,0
10,0

Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
MARKS/PUNTE: 150
This memorandum consists of 20 pages. Hierdie memorandum bestaan uit 20 bladsye.
PHYSICAL SCIENCES: PHYSICS (P1) FISIESE WETENSKAPPE: FISIKA (V1)
NOVEMBER 2014
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
NASIONALE SENIOR SERTIFIKAAT
GRADE/GRAAD 12

Physical Sciences P1/Fisiese Wetenskappe V1 2 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 1/VRAAG 1 1.1 A (2) 1.2 A (2) 1.3 D (2) 1.4 C (2) 1.5 B (2) 1.6 C (Accept/ Aanvaar R) (2) 1.7 A (2) 1.8 D (2) 1.9 A (2) 1.10 C (2) [20]
QUESTION 2/VRAAG 2
2.1
When a resultant (net) force acts on an object, the object will accelerate in the direction of the force. This acceleration is directly proportional to the force and inversely proportional to the mass of the object. Wanneer 'n resulterende (netto) krag op 'n voorwerp inwerk, sal die voorwerp in die rigting van die krag versnel. Hierdie versnelling is direk eweredig aan die krag en omgekeerd eweredig aan die massa van die voorwerp. OR/OF The net force acting on an object is equal to the rate of change of momentum of the object (in the direction of the force). (2 or 0) Die netto krag wat op 'n voorwerp inwerk is gelyk aan die tempo van verandering in momentum van die voorwerp (in die rigting van die krag).(2 of 0) (2)

Physical Sciences P1/Fisiese Wetenskappe V1 3 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
2.2
(3) 2.3 OPTION 1/OPSIE 1
Fnet = ma For 5 kg block/Vir 5 kg-blok T2 + (-mg) + (-T1 )= ma 250 – (5)(9,8) – T1= 5 a
201 – T1 = 5 a T1 = 201 - 5a........(1) For 20 kg block/Vir 20 kg-blok T1 + (-mg) = ma.......(2) T1 + [-20(9,8)] = 20a
5 = 25 a a = 0,2 m∙s-2 upwards/opwaarts ∴ T1 = 201 - 5(0,2)
= 200 N
OR/OF T1 = 20(9,8) + 20(0,2)
= 200 N (6) OPTION 2 /OPSIE 2
Fnet = ma For 5 kg block/Vir 5 kg-blok T2 + (-mg) + (-T1)= ma 250 - (5)(9,8) - T1= 5 a
201 - T1 = 5a T1 = 201 - 5a........(1) For 20 kg block/Vir 20 kg-blok , T1 + (-mg) = ma.......(2) T1 + [-20(9,8)] = 20a
(1) x 4 : 4T1 = 804 – 20a
∴T1 - 196 = 804 – 4T1
∴5T1 = 1 000
∴T1 = 200 N (6)
T1
w
T2

Physical Sciences P1/Fisiese Wetenskappe V1 4 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 3/OPSIE 3
Fnet = ma For 5 kg block/Vir 5 kg-blok T2 + (-mg) + (-T1 )= ma 250 - (5)(9,8) - T1= 5 a
201 - T1 = 5 a T1 = 201 - 5a........(1) ∴ a =
5T201 1−
For 20 kg block/Vir 20 kg-blok , T1 + (-mg) = ma.......(2) T1+ [-(20)(9,8)] = 20a ∴T1 - 196 = 20(
5T201 1−
) ∴T1 = 200 N (6)
2.4 Q (1) [12]
QUESTION 3/VRAAG 3
3.1 An object moving / Motion under the influence of gravity / weight / gravitational force only (and there are no other forces such as friction). (2 or/of 0) ('n Voorwerp wat / Beweging slegs onder die invloed van swaartekrag / gewig / gravitasiekrag (en daar is geen ander kragte soos wrywing nie). (2)
3.2 OPTION 1/OPSIE 1
Upwards positive/Opwaarts positief: ∆y = vi∆t + ½ a∆t2 0 = 15 ∆t + ½ (-9,8) ∆t2 ∆t = 3,06 s It takes/Dit neem 3,06 s
Downwards positive/Afwaarts positief: ∆y = vi∆t + ½ a∆t2 0 = -15 ∆t + ½ (9,8) ∆t2 ∆t = 3,06 s It takes/Dit neem 3,06 s
(4) OPTION 2/OPSIE 2
Upwards positive/Opwaarts positief : vf = vi + a∆t 0 = 15 +(-9,8)∆t ∆t = 1,53 s It takes (2)(1,53) = 3,06 s
Downwards positive/Afwaarts positief: vf = vi + a∆t 0 = -15 +(9,8)∆t ∆t = 1,53 s It takes/Dit neem 3,06 s (4)
OPTION 3 / OPSIE 3 Upwards positive/Opwaarts positief: vf = vi + a∆t -15 = 15 + (-9,8)∆t ∆t = 3,06 s
Downwards positive/Afwaarts positief: vf = vi + a∆t 15 = -15 + (9,8)∆t ∆t = 3,06 s (4)

Physical Sciences P1/Fisiese Wetenskappe V1 5 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 4/OPSIE 4
Upwards positive/Opwaarts positief: Fnet ∆t = ∆p mg ∆t = m (vf -vi )
∆t = 8,9-)15-0(
∆t = 1,53 s It takes/Dit neem (2)(1,53s) = 3,06 s
Downwards positive /Afwaarts positief : Fnet ∆t = ∆p mg ∆t = m (vf -vi )
∆t = 8,9
)15(0 −−
∆t = 1,53 s It takes/Dit neem (2)(1,53s) = 3,06 s (4)
OPTION 5/OPSIE 5
Upwards positive/Opwaarts positief: Fnet ∆t = ∆p mg ∆t = m (vf -vi )
∆t = 8,9
)15(15−−−
= 3,06 s
Downwards positive/Afwaarts positief: Fnet ∆t = ∆p mg ∆t = m (vf -vi )
∆t = 8,9
)15(15 −−
∆t = 3,06 s (4) OPTION 5/OPSIE 6
Upwards positive/Opwaarts positief:
yΔa2+v=v 2i
2f
For ball A/Vir bal A 0 = (15)2 + 2 (-9,8)∆y ∆yA = 11,48 m
Δy = t2
vv if ∆
+
11,48 = t2
015 ∆
+
Δt = 1,53 s It takes/Dit neem (2)(1,53s) = 3,06 s
Downwards positive/Afwaarts positief :
yΔa2+v=v 2i
2f
For ball A/Vir bal A 0 = (-15)2 + 2 (9,8)∆y ∆yA = -11,48 m
Δy = t2
vv if ∆
+
-11,48 = t2
015 ∆
+−
Δt = 1,53 s It takes/Dit neem (2)(1,53s) = 3,06 s

Physical Sciences P1/Fisiese Wetenskappe V1 6 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
3.3 OPTION 1/OPSIE 1
Upwards positive/Opwaarts po sitief:
yΔa2+v=v 2i
2f
For ball A/Vir bal A 0 = (15)2 + 2 (-9,8)∆y ∆yA = 11,48 m When A is at highest point Wanneer A op hoogste punt is ∆yB = vi∆t + ½ a∆t2 = 0 + ½ (-9,8) (1,53)2 ∆yB = -11,47 m ∆yB = 11,47 m downward/afwaarts Distance/Afstand = yA + yB = 11,47 + 11,48 = 22,95 m
Downwards positive/Afwaarts positief :
yΔa2+v=v 2i
2f
For ball A/Vir bal A 0 = (-15)2 + 2 (9,8)∆y ∆yA = -11,48 m When A is at highest point Wanneer A op hoogste punt is ∆yB = vi∆t + ½ a∆t2 = 0 + ½ (9,8) (1,53)2 ∆yB = 11,47 m ∆yB = 11,47 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,47 = 22,95 m
OPTION 2/OPSIE 2 Upwards positive/Opwaar ts positief: At maximum height vf = 0: By maksimum hoogte vf = 0: Ball/Bal A ∆yA = vi∆t + ½ a∆t2 = 15 (1,53) + ½ (-9,8) (1,53)2 = 11,48 m When A is at highest/point Wanneer A op hoogste punt is ∆yB = vi∆t + ½ a∆t2 = 0 + ½ (-9,8) (1,53)2 ∆yB = -11,47 m ∆yB = 11,47 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,47 = 22,95 m
Downwards positive/Afwaarts positief : At maximum height vf = 0: By maksimum hoogte vf = 0: Ball/Bal A ∆yA = vi∆t + ½ a∆t2 = (-15) (1,53) + ½ (9,8) (1,53)2 = -11,48 m When A is at highest point Wanneer A by hoogste punt is ∆yB = vi∆t + ½ a∆t2 = 0 + ½ (-9,8) (1,53)2 ∆yB = -11,47 m ∆yB = 11,47 m downward/afwaarts Distance/Afstand = (yA + yB) = 11,48 + 11,47 = 22,95 m (7)

Physical Sciences P1/Fisiese Wetenskappe V1 7 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 3/OPSIE 3
Upwards positive/Opwaarts positief: Ball A/Bal A ∆yA = vi∆t + ½ a∆t2 ∆yA = 15(1,53) + ½ (-9,8) (1,53)2 = 11,48 m For ball B/Vir bal B vf = vi + a∆t vf = 0 + (-9,8)(1,53) vf = 14,99 m∙s-1
xa2vv 2i
2f ∆+=
14,992 = 0 + 2(-9,8) ∆yB ∆yB = -11,47 (m) = 11,47 m downward/afwaarts Distance/Afstand = (yA + yB) = 11,48 + 11,47 = 22,95 m
Downwards positive/Afwaarts positief : Ball A/Bal A yA = vi∆t + ½ a∆t2 ∆yA = -15 (1,53) + ½ (9,8) (1,53)2 = -11,48 (m) = 11,48 m upward/opwaarts For ball B/Vir bal B vf = vi + a∆t vf = 0 + (9,8)(1,53) vf = 14,99 m∙s-1
xa2vv 2i
2f ∆+=
14,992 = 0 + 2(9,8) ∆yB ∆yB = 11,47 (m) Distance/Afstand = (yA + yB) = 11,48 + 11,47 = 22,95 m (7)
OPTION 4/OPSIE 4 Upwards positive/Opwaarts positief: Ball A/Bal A
∆yA = tΔ2
v+v fi = )53,1(2
)015( +
= 11,48 m For ball B/Vir bal B vf = vi + a∆t = 0 +( -9,8) (1,53) = -15 m∙s-1
∆y = tΔ2
v+v fi = 2
53,1)15-0( ×
= -11,47 m = 11,47 m downward/afwaarts Distance/Afstand = (yA + yB) = 11,48 + 11,47 = 22,95 m
Downwards positive/Afwaarts positief : Ball A/Bal A
∆yA = tΔ2
v+v fi = )53,1(2
)015-( +
= -11,48 (m) = 11,48 m upwards/opwaarts vf = vi∆t + a∆t = 0 +( 9,8) (1,53) = 15 m∙s-1
∆y = tΔ2
v+v fi = 2
53,1)150( ×+
= 11,47m Distance/Afstand = yA + yB = 11,48 + 11,47 = 22,95 m (7)

Physical Sciences P1/Fisiese Wetenskappe V1 8 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 5/OPSIE 5 Upwards positive/Opwaarts positief : Ball A/Bal A Wnet = ∆K
OR/OF ½ m( v2
f – v2i) = mg(hf – h i)cosθ
½ m(0 -152) = m(9,8)hfcos180o h = 11,48 m
OR/OF For Ball B when A is at highest point./ Vir Bal B wanneer A by sy hoogste punt is. vf = vi + a∆t = 0 +( -9,8) (1,53) = -15 m∙s-1
∆y = tΔ2
v+v fi = 2
53,1)15-0( ×
=-11,48 m = 11,48 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,48 = 22,96 m
Downwards positive/Afwaarts positief : Ball A/Bal A Wnet = ∆K
OR/OF ½ m( v2
f – v2i) = mg(hf – h i)cosθ
½ m(0 -152) = m(9,8)hfcos180o h = 11,48 m
OR/OF For Ball B when A is at highest point./ Vir Bal B wanneer A by sy hoogste punt is. vf = vi + a∆t = 0 +(9,8) (1,53) = 15 m∙s-1
∆y = tΔ2
v+v fi = 2
)53,1)(150( +
= 11,48 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,48 = 22,96 m (7)
OPTION 7/OPSIE 7
Upwards positive/Opwaarts positief : Ball A ½ m v2
i + mgh i = ½ m v2f + mghf
½ m(152) +0 = ½ m(0) + m(9,8)h h = 11,48 m
OR/OF For Ball B when A is at highest point. Vir Bal B wanneer A by sy hoogste punt is. vf = vi + a∆t = 0 +( -9,8) (1,53) = -15 m∙s-1
∆y = tΔ2
v+v fi
= 2
)53,1)(15-0(
=-11,48 m = 11,48 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,48 = 22,96 m
Downwards positive/Afwaarts positief : Ball A ½ m v2
i + mgh i = ½ m v2f + mghf
½ m(152) +0 = ½ m(0) + m(9,8)h h = 11,48 m
OR/OF For Ball B when A is at highest point. Vir Bal B wanneer A by sy hoogste punt is. vf = vi + a∆t = 0 +(9,8) (1,53) = 15 m∙s-1
∆y = tΔ2
v+v fi
= 2
)53,1)(150( +
= 11,48 m downward/afwaarts Distance/Afstand = yA + yB = 11,48 + 11,48 = 22,96 m (7)

Physical Sciences P1/Fisiese Wetenskappe V1 9 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
15
-15
1,53 3,06 t (s) v
(m·s
-1)
0
3.4
(4) [17]
CONSIDER MOTION DOWNWARD AS POSITIVE/BESKOU BEWEGING AFWAARTS AS POSITIEF
Criteria/Kriteria Marks/Punte Graph starts at correct Initial velocity shown./Grafiek begin by korrekte beginsnelheid aangetoon.
Time for maximum height shown (1,53 s)./Tyd vir maksimum hoogte aangetoon.(1,53 s)
Time for return shown (3,06 s) /Tyd om terug te keer (3,06) aangetoon.
Shape/Vorm: Straight line extending beyond 3,06 s/ Reguitlyn wat verby 3,06 s strek.
(4) [17]
15
-15
1,53 3,06
v (m
·s-1
)
0

Physical Sciences P1/Fisiese Wetenskappe V1 10 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 4/VRAAG 4 4.1 p = mv
= 50(5) = 250 kg∙m∙s-1 (downward/afwaarts) OR/OF p = mv = 50(-5) = - 250 kg∙m∙s-1 = 250 kg∙m∙s-1 (downward/afwaarts) (3)
4.2
The product of the (net) force and the time interval (during which the force acts) (2 or 0) Die produk van die (netto) krag en die tydinterval (waartydens die krag inwerk) (2 of 0).
(2) 4.3 OPTION 1/OPSIE 1
∆p = Fnet∆t 0 - 250 = Fnet(0,2) Fnet = -1 250 N = 1 250 N
∆p = Fnet∆t 250 - 0 = Fnet(0,2) Fnet = 1 250 N
∆p = Fnet∆t 50(0 – (-5))= Fnet(0,2) Fnet = 1 250 N (3)
OPTION 2/OPSIE 2
m(vf – vi) = Fnet∆t 50 (0 - 5) = Fnet (0,2) Fnet = -1 250 N = 1 250 N
m(vf – vi) = Fnet∆t 50 (5 - 0) = Fnet (0,2) Fnet = 1 250 N
(3) OPTION 3 /OPSIE 3
vf = vi + a∆t 0 = 5 + a(0,2) a = -25 m∙s-2 Fnet = ma = 50 (-25) = - 1 250 N = 1 250 N
vf = vi + a∆t 5 = 0 + a(0,2) a = 25 m∙s-2 Fnet = ma = 50 (25) = 1 250 N
(3)

Physical Sciences P1/Fisiese Wetenskappe V1 11 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
4.4 Greater than/Groter as (1) 4.5 For the same momentum change,
the stopping time (contact time) will be smaller (less) ∴the (upward) force exerted (on her) is greater. Vir dieselfde verandering in momentum, sal die stilhoutyd (kontaktyd) kleiner wees ∴die (opwaartse)krag wat (op haar) uitgeoefen word, sal groter wees.
(3) [12]
QUESTION 5/VRAAG 5 5.1.1 In an isolated/closed system, the total mechanical energy is conserved /
remains constant In 'n geïsoleerde/geslote sisteem bly die totale meganiese energie behoue / bly konstant. OR/OF The total mechanical energy of a system is conserved/ remains constant in the absence of friction. Die totale meganiese energie van 'n sisteem bly behoue/bly konstant in die afwesigheid van wrywing. OR/OF The total mechanical energy of a system remains constant provided the net work done by external non conservative forces is zero. Die totale meganiese energie van 'n sisteem bly konstant, mits die arbeid verrig deur eksterne nie-konserwatiewe kragte, nul is. OR/OF In the absence of a non-conservative force, the total mechanical energy is conserved/remains constant In die afwesigheid van ‘n nie-konserwatiewe krag, bly die totale meganiese energie behoue / konstant OR/OF In an isolated/closed system, the sum of kinetic and gravitational potential energy is conserved / remains constant In 'n geïsoleerde/geslote sisteem bly som van kinetiese en gravitasionele potensiële energie behoue / bly konstant.
5.1.2 No/Nee (1) 5.1.3 OPTION 1/OPSIE 1
(6)
Along AB/Langs AB Emechanical at A = Emechanical at B
(Ep + Ek)A = (Ep + Ek)B (mgh + ½ mv2)A= (mgh + ½ mv2)B
(10)(9,8)(4) + 0 = 0 + ½ (10) v f2
vf = 8,85 m∙s-1
Along AB/Langs AB Wnet = ΔEk FgΔhcosθ = ½ m(vf
2 – vi2)
(10)(9,8)(4)cos0o = ½ (10)(v f2 – 0)
vf = 8,85 m∙s-1

Physical Sciences P1/Fisiese Wetenskappe V1 12 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
Along AB/Langs AB
Wnc = ΔK + ΔU 0 = ½ (10)(v f
2 – 0) + 10(9,8)(4 – 0) vf = 8,85 m∙s-1
Substitute 8,85 m∙s-1 in one of the following options Vervang 8,85 m∙s-1 in een van die volgende opsies
Along BC/Langs BC
Wnet = ∆K f∆x cosθ = ∆K f(8)cos 180o = ½ (10)(0 – 8,852) f = 48,95 N
Along BC/Langs BC Wnc = ∆K + ∆U f ∆xcosθ = ∆K + ∆U f(8)cos 180 = ½ (10)(0 - 8,852) + 0 f = 48,95 N (Accept/ Aanvaar 49 N)
OPTION 2/OPSIE 2
Along AC/Langs AC Wnc = ∆K + ∆U f∆xcosθ = ∆K + ∆U (f)(8)(cos180o) = (0 - 0) + 10 (9,8)(0 - 4) f = 49 N (6)
5.2.1 fk = µkN
= µkmgcosθ = (0,19)(300)(9,8) cos 25o = 506,26 N (3)
5.2.2 OPTION 1/OPSIE 1
Fnet = 0 Fapp + (-Fgsinθ) + (-f) = 0 Fapp - (300)(9,8)sin 25o - 506,26 = 0 Fapp = 1 748,76 N Pave = Fvave = 1748,76 x 0,5 = 874,38 W (6)
FN
f
Fg
Fapp
θ

Physical Sciences P1/Fisiese Wetenskappe V1 13 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 2/OPSIE 2
W f + Wapp + WN + Wg = 0 F∆xcosθ + Fapp∆x cosθ + 0 + Fg∆x cosθ = 0 (506,26∆x cos180o) + (Fapp∆x cos0) + 300(9,8)Δxcos115o= 0 Fapp = 1748,76 N Pave = Fvave = (1748,76 ) (0,5) = 874,38 W (6)
OPTION 3/OPSIE 3
W f + Wapp + WN + Wg = 0 F∆xcosθ + Fapp∆x cosθ +0 + Fg sinθ∆x cosθ = 0 (506,26∆x cos 0) + (Fap∆x cos0) + 300 (9,8)sin 25o Δxcos180) = 0 Fapp = 1 748,76 N Pave = Fvave = (1 748,76 )(0,5) = 874,38 W
(6) [18]
QUESTION 6/VRAAG 6 6.1.1 An (apparent) change in observed/detected frequency (pitch), (wavelength) as
a result of the relative motion between a source and an observer (listener). 'n Skynbare verandering in waargenome frekwensie (toonhoogte),(golflengte) as gevolg van die relatiewe beweging tussen die bron en 'n waarnemer/luisteraar. (2)
6.1.2 Towards/Na
Observed/detected frequency is greater than the actual frequency. Waargenome frekwensie is groter as die werklike frekwensie. (2)
6.1.3
ss
LL f
vvvv
f ±±= OR/OF s
sL f
vvv
f −=
1130 v-343
343 =(1200)
s
vs = 20,01 m∙s-1 Accept/Aanvaar: (19,42 – 20,01 m∙s-1) (5)
6.2 The star is approaching the earth. Die ster nader die aarde. OR/OF The earth and the star are approaching (moving towards) each other. Die aarde en die ster nader mekaar. The spectral lines in diagram 2 are shifted towards the blue end/blue shifted. Die spektrumlyne in diagram 2 het verskuif na die blou ent/blou verskuiwing
(2)
[11]

Physical Sciences P1/Fisiese Wetenskappe V1 14 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 7/VRAAG 7
7.1 To ensure that charge does not leak to the ground/insulated. Om te verseker dat die lading nie na die grond toe lek nie/isoleer. (1)
Notes/Aantekeninge Accept/Aanvaar In order retain original charge/To insulate the charges./ Om oorspronklike lading te behou/ Om lading te isoleer.
7.2 Net charge/Netto lading =
2Q+Q SR =
2-4)(+8+
= 2 µC (2)
7.3 (3)
7.4 (2)
7.5
OPTION 1/OPSIE 1
F = 221
r
QQk
FST = 2
-6-69
)2,0()10 )(210 (1
)109(××× = 0,45 N / 4,5 x 10-1 N left/links
OR/OF
FTS = 41
FRT = 41
(1,8) = 0,45 N
FRT = 2
-6-69
)1,0()10 × )(110 × (2
×10×9 = 1,8 N right/regs
OR/OF FRT = 4FST = 4(0,45) = 1,8 N right /regs Fnet = FST + FRT = 1,8 + (-0,45) = 1,35 N or towards sphere S / na sfeer or/of right/regs S (6)
Criteria for sketch:/Kriteria vir skets : Marks/ Punte
Correct direction of field lines Korrekte rigting van veldlyne
Shape of the electric field Vorm van elektrieseveld
No field line crossing each other / No field lines inside the spheres/ Geen veldlyne wat maekaar kruis nie / Geen veldlyne binne sfeer nie
T
FS on T F R on T

Physical Sciences P1/Fisiese Wetenskappe V1 15 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 2/OPSIE 2
2Rr
kQE = =
2
69
)1,0(
)102)(109( −×× = 1,8 x 106 N.C-1 right/regs
2sr
kQE = =
2
69
)2,0(
)102)(109( −×× = 4,5 x 105 N.C-1 left/links
Enet = 1,8 x 106 - 4,5 x 105 = 1,35 x 106 N.C-1right/regs F = EQ = (1,35 x 106)(1 x 10-6) = 1,35 N towards sphere S / na sfeer S right/regs (6)
7.6 Force experienced per unit positive charge placed at that point.
Krag ondervind per eenheid positiewe lading by daardie punt. (2) 7.7 OPTION 1/OPSIE 1
E = qF = 6-10×1
35,1 = 1,35 x 106 N∙C-1
(3) OPTION 2/OPSIE 2
2Rr
kQE = =
2
69
)1,0(
)102)(109( −×× = 1,8 x 106 N∙C-1 right/regs
2sr
kQE = =
2
69
)2,0(
)102)(109( −××= 4,5 x 105 N∙C-1 left/links
Enet = 1,8 x 106 - 4,5 x 105 = 1,35 x 106 N∙C-1 (3) OPTION 3/OPSIE 3
E = qF =
6-1018,1
× = 1,8 x 106 N∙C-1
E = qF
= 6-101
45,0× = 4,5 x 105 N∙C-1
Enet = 1,8 x 106 - 4,5 x 105 = 1,35 x 106 N∙C-1 (3) [19]

Physical Sciences P1/Fisiese Wetenskappe V1 16 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
Grafiek van potensiaalverskil teenoor stroom
QUESTION 8/VRAAG 8 8.1.1
Keep the temperature (of battery) constant. Hou die temperatuur (van battery) konstant
(1)
8.1.2
(3) 8.1.3 7,2 V
(Accept any readings between 7,0 V and 7,4 V or the value of the y-intercept /Aanvaar enige lesing tussen 7,0 V en 7,4 V of die waarde van die y-afsnit
(1)
8.1.4
Slope/Helling =IΔVΔ
= 0-0,8
7,2-0 = - 9
r = 9 Ω (3)
Criteria for drawing line of best fit:/Kriteria vir teken van lyn van beste pas : Marks/ Punte
ALL points correctly plotted (at least 4 points) ALLE punte korrek gestip (ten minste 4 punte)
Correct line of best fit if all plotted points are used ( at least 3 point) Korrekte lyn van beste pas indien alle punte gebruik word (ten minste 3 punte)

Physical Sciences P1/Fisiese Wetenskappe V1 17 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
8.2.1 OPTION 1/OPSIE 1
P = VI 100 = 20(I) I = 5 A (3)
OPTION 2/OPSIE 2
P = RV 2
100 = R
)20( 2
R = 4 Ω V = IR 20 = I(4) I = 5 A (3)
OPTION 3/OPSIE 3
P = RV 2
100 = R
)20( 2
R = 4 Ω P=I2R 100 = I2(4) I = 5 A
8.2.2 OPTION 1/OPSIE 1
P = RV2
R = 150
)20( 2
= 2,67 Ω (3) OPTION 2/OPSIE 2
P = VI 150 = (20)I I = 7,5 A V = IR 20 = (7,5)R R = 2,67 Ω OR/OF P = I2R 150 = (7,5)2R R = 2,67 Ω (3)

Physical Sciences P1/Fisiese Wetenskappe V1 18 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 3/OPSIE 3
IX : IY 5 : 7,5 1 : 1,5 RX : RY 1,5 : 1 4 : 2,67 Ω (3)
8.2.3 OPTION 1/OPSIE 1
P = VI OR/OF P = I2R
I150W = 20
150 = 7,5 A I150W = 67,2
150 = 7,5 A
Itot = (5 + 7,5) ε = I(R + r) 24 = 12,5(R + r) 24 = Vext + V ir 24 = 20 + 12,5(r ) r = 0,32 Ω
(5)
OPTION 2/OPSIE 2
V = Ir Itot = (5 + 7,5) (24 - 20) = 12,5 r
∴r = 5,12
4
r = 0,32 Ω (5) OPTION 3/OPSIE 3
21 R1
R1
R1 +=
//R1
=67,21
41 + OR/OF R// =
67,24)67,2)(4(
+
∴R|| =1,6 Ω
Itot = 6,1
20 = 12,5 A
ε = I(R + r)
24 = 12,5(R + r) 24 = Vext + V ir 24 = 20 + 12,5(r ) r = 0,32 Ω
(5)

Physical Sciences P1/Fisiese Wetenskappe V1 19 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 4/OPSIE 4
P = VI 250 = (20)I I = 12,5 A V = Ir 4 = (12,5)r r = 0,32 Ω (5)
8.2.4 Device Z is a voltmeter .
Toestel Z is 'n voltmeter (1) 8.2.5 Device Z should be a voltmeter (or a device with very high resistance) because
it has a very high resistance and will draw very little current. The current through X and Y will remain the same hence the device can operate as rated. Toestel Z moet 'n voltmeter wees (of 'n toestel met 'n baie hoë weerstand) omdat dit 'n baie hoë weerstand het en baie min sal stroom trek Die stroom deur X en Y sal dieselfde bly, gevolglik kan die toestel werk soos ontwerp. (2)
[22] QUESTION 9/VRAAG 9
9.1 Electromagnetic induction / Elektromagnetiese induksie (1)
9.2 Rotate the coil faster/Increase the number of coils/ Increase the strength of the magnetic field. Roteer die spoel vinniger/Verhoog die aantal spoele / Verhoog die sterkte van die magneetveld.
(1)
9.3 Slip rings/Sleepringe (1) 9.4.1 It is the value of the voltage in a DC circuit that will have the same heating
effect as an AC circuit. Dit is die waarde van die potensiaalverskil in 'n GS-stroombaan wat dieselfde verhittingseffek het as 'n WS-stroombaan (2)
9.4.2 Vrms =
2
Vmax
= 2
45,339
Vrms = 240,03 V
(3) [8]

Copyright reserved/Kopiereg voorbehou
QUESTION 10/VRAAG 10 10.1 The minimum frequency (of a photon/light) needed to emit electrons from
(the surface of) a metal. (substance) Die minimum frekwensie (van 'n foton/lig) benodig om elektrone vanaf die (oppervlakte van)'n metaal (stof) vry te stel.
(2) 10.2 OPTION 1/OPSIE 1
k(max)o E+ W=E
2maxo mv
21
+ W=E
2max0 mv
21
+hf =λc
h
λ×× )103)(1063,6( 8-34 = )10548,5)(1063,6( 14-34 ×× 2531- )1033,5)(1011,9(
21 ××+
λ = 4 x 10-7 m (5) OPTION 2/OPSIE 2
k(max)o E+ W=E
2maxo mv
21
+ W=E
2max0 mv
21
hf hf +=
(6,63 x 10-34)f = )10548,5)(1063,6( 14-34 ×× 2531- )1033,5)(1011,9(21 ××+
f = 7,5 x 1014 Hz c = fλ 3 x 108 = (7,5 x 1014)λ λ = 4 x 10-7 m (5)
10.3 Smaller (less) than
Kleiner (minder) as (1) 10.4 The wavelength/frequency/energy of the incident light (photon/hf) is constant.
Die golflengte/frekwensie/energie van die invallende lig (foton/hf) is konstant Since the speed is larger, the kinetic energy is larger the work function/W0/threshold frequency smaller. Aangesien die spoed vergroot, is die kinetiese energie groter, is die arbeidsfunksie / W0 / drumpel frekwensie kleiner
(3)
[11]
GRAND TOTAL/GROOTTOTAAL : 150
Any one / Enige een
Any one / Enige een

Copyright reserved Please turn over
MARKS: 150 TIME: 3 hours
This question paper consists of 16 pages and 4 data sheets.
PHYSICAL SCIENCES: CHEMISTRY (P2)
NOVEMBER 2014
NATIONAL SENIOR CERTIFICATE
GRADE 12

Physical Sciences/P2 2 DBE/November 2014 NSC
Copyright reserved Please turn over
INSTRUCTIONS AND INFORMATION 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12.
Write your examination number and centre number in the appropriate spaces on the ANSWER BOOK. This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK. Start EACH question on a NEW page in the ANSWER BOOK. Number the answers correctly according to the numbering system used in this question paper. Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2. You may use a non-programmable calculator. You may use appropriate mathematical instruments. You are advised to use the attached DATA SHEETS. Show ALL formulae and substitutions in ALL calculations. Round off your final numerical answers to a minimum of TWO decimal places. Give brief motivations, discussions, et cetera where required. Write neatly and legibly.

Physical Sciences/P2 3 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 1: MULTIPLE-CHOICE QUESTIONS Four options are provided as possible answers to the following questions. Each question has only ONE correct answer. Write only the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11. D.
1.1 Which ONE of the following is a primary nutrient for plants? A
B C D
Oxygen Carbon Potassium Magnesium
(2)
1.2 Which ONE of the following statements is CORRECT?
Alkenes ...
A
B C D
have the general formula CnH2n+2. are unsaturated hydrocarbons. readily undergo substitution reactions. have one triple bond between two carbon atoms.
(2)
1.3 Consider the reaction represented by the balanced equation below:
Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
In the above reaction, Cu(s) is the ...
A
B C D
oxidising agent and is reduced. oxidising agent and is oxidised. reducing agent and is reduced. reducing agent and is oxidised.
(2)

Physical Sciences/P2 4 DBE/November 2014 NSC
Copyright reserved Please turn over
1.4 Which ONE of the following describes the effect of a positive catalyst on the
net activation energy and the heat of reaction (∆H) of a specific reaction?
NET ACTIVATION ENERGY
∆H
(2)
A Increases No effect
B Decreases Increases
C No effect Decreases
D Decreases No effect
1.5 The following equation represents the cracking of a hydrocarbon at high
temperature and pressure:
C11H24 → 2C2H4 + Y + C4H10
Which ONE of the following is the IUPAC name of product Y?
A
B C D
Prop-1-ene Propane Ethene Ethane
(2)
1.6 When 2-chlorobutane is strongly heated in the presence of concentrated
sodium hydroxide, the major product formed is …
A
B C D
but-1-ene. but-2-ene. butan-1-ol. butan-2-ol.
(2)

Physical Sciences/P2 5 DBE/November 2014 NSC
Copyright reserved Please turn over
1.7 A hypothetical reaction reaches equilibrium at 10 °C in a closed container
according to the following balanced equation:
A(g) + B(g) AB(g) ∆H < 0 The temperature is now increased to 25 °C. Which ONE of the following is correct as the reaction approaches a new equilibrium?
REACTION RATE YIELD OF
PRODUCTS
(2)
A Increases Remains the same
B Increases Decreases
C Increases Increases
D Decreases Decreases
1.8 Which ONE of the following represents the products formed during the
hydrolysis of ammonium chloride?
A
B
C
D
NH3(aq) and +OH3 (aq)
+4NH (aq) and Cℓ− (aq)
HCℓ(aq) and OH− (aq)
Cℓ− (aq) and +OH3 (aq)
(2) 1.9 An electrochemical cell is used to electroplate an iron spoon with nickel.
Which ONE of the following half-reactions takes place at the positive electrode of this cell?
A
B C
D
Fe2+(aq) + 2e− → Fe(s)
Fe(s) → Fe2+(aq) + 2e−
Ni2+(aq) + 2e− → Ni(s)
Ni(s) → Ni2+(aq) + 2e−
(2)

Physical Sciences/P2 6 DBE/November 2014 NSC
Copyright reserved Please turn over
1.10 The following reaction reaches equilibrium in a closed container at a certain
temperature:
2O3(g) 3O2(g) The pressure is now decreased by increasing the volume of the container at constant temperature. Which ONE of the following is correct as the reaction approaches a new equilibrium?
NUMBER OF MOLES OF O3(g)
NUMBER OF MOLES OF O2(g)
CONCENTRATION OF O2(g)
A Increases Decreases Decreases
(2)
B Decreases Increases Increases
C Decreases Increases Decreases
D Increases Decreases Increases
[20]

Physical Sciences/P2 7 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 2 (Start on a new page.)
Consider the organic compounds represented by the letters A to F in the table below.
A
2,2,4-trimethylhexane
B
CH3CH2CH2CH2CHO
C
D
E
F Pentan-2-one
2.1 Write down the LETTER that represents the following:
2.1.1 An aldehyde (1)
2.1.2 A condensation polymer (1)
2.1.3 A compound which has a carbonyl group bonded to two carbon atoms as its functional group
(1)
2.2 Write down the IUPAC name of: 2.2.1 Compound C (3) 2.2.2 The monomer of compound D (1) 2.3 Write down the structural formula of: 2.3.1 Compound A (2) 2.3.2 Compound F (2) 2.4 The table contains compounds which are functional isomers. 2.4.1 Define the term functional isomer. (2) 2.4.2 Write down the LETTERS that represent two compounds that are
functional isomers.
(1) [14]
C C
H
H
H
C
C
H
H
C
Cℓ
H
C
Br
H
H
H
H
H
H
C C
H
H H
H
n
C C
H
H
O
n
C
O
O C
H
H
C
H
H
O

Physical Sciences/P2 8 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 3 (Start on a new page.) 3.1 Give a reason why alkanes are saturated hydrocarbons. (1) 3.2 Write down the structural formula of: 3.2.1 The functional group of alcohols (1) 3.2.2 A tertiary alcohol that is a structural isomer of butan-1-ol (2) 3.3 Learners investigate factors that influence the boiling points of alkanes and
alcohols. In one of the investigations they determine the boiling points of the first three alkanes.
3.3.1 Write down an investigative question for this investigation. (2) 3.3.2 Fully explain why the boiling point increases from methane to
propane.
(3) 3.4 The learners find that the boiling point of propan-1-ol is higher than that of
propane. Explain this observation by referring to the TYPE of INTERMOLECULAR FORCES present in each of these compounds.
(3) [12]

Physical Sciences/P2 9 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 4 (Start on a new page.) The flow diagram below shows the preparation of an ester using prop-1-ene as a starting reagent. P, Q, R and S represent different organic reactions.
4.1 Write down the type of reaction represented by: 4.1.1 Q (1) 4.1.2 R (1) 4.2 For reaction P write down the: 4.2.1 Type of addition reaction (1) 4.2.2 Balanced equation using structural formulae (3) 4.3 Write down the structural formula of the haloalkane formed in reaction Q. (2) 4.4 In reaction S propan-1-ol reacts with ethanoic acid to form the ester.
For this reaction write down the:
4.4.1 Name of the reaction that takes place (1) 4.4.2 FORMULA or NAME of the catalyst needed (1) 4.4.3 Structural formula of the ester formed (2) 4.4.4 IUPAC name of the ester formed (2) 4.5 The propan-1-ol formed in reaction R can be converted to prop-1-ene. Write
down the FORMULA or NAME of the inorganic reagent needed.
(1) [15]
Ester
Propan-1-ol
Propane Haloalkane Q
R
S
Prop-1-ene P
Cℓ2

Physical Sciences/P2 10 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 5 (Start on a new page.)
5.1 Define the term reaction rate in words. (2)
Learners use the reaction between IMPURE POWDERED calcium carbonate and excess hydrochloric acid to investigate reaction rate. The balanced equation for the reaction is:
CaCO3(s) + 2HCℓ(aq) → CaCℓ2(aq) + H2O(ℓ) + CO2(g)
They perform four experiments under different conditions of concentration, mass and temperature as shown in the table below. They use identical apparatus in the four experiments and measure the volume of gas released in each experiment.
EXPERIMENT
1 2 3 4
Concentration of acid (mol·dm-3) 1 0,5 1 1
Mass of impure calcium carbonate (g) 15 15 15 25
Initial temperature of acid (°C) 30 30 40 40
5.2 The results of experiments 1 and 3 are compared in the investigation.
Write down the:
5.2.1 Independent variable (1)
5.2.2 Dependent variable (1)
5.3 Use the collision theory to explain why the reaction rate in experiment 4 will be higher than that in experiment 3.
(3)
The learners obtain graphs A, B, C and D below from their results.
5.4 Which ONE of the graphs (A, B, C or D) represents experiment 1? Fully explain the answer by comparing experiment 1 with experiments 2, 3 and 4.
(6)
5.5 When the reaction in experiment 4 reaches completion, the volume of gas formed is 4,5 dm3. Assume that the molar gas volume at 40 °C is equal to 25,7 dm3.
Calculate the mass of the impurities present in the calcium carbonate.
(5) [18]
Time (s)
A
Vo
lum
e C
O2 (
cm
3)
D C
B

Physical Sciences/P2 11 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 6 (Start on a new page.) A certain amount of nitrogen dioxide gas (NO2) is sealed in a gas syringe at 25 °C. When equilibrium is reached, the volume occupied by the reaction mixture in the gas syringe is 80 cm3. The balanced chemical equation for the reaction taking place is:
2NO2(g) N2O4(g) ∆H < 0 dark brown colourless
6.1 Define the term chemical equilibrium. (2)
6.2 At equilibrium the concentration of the NO2(g) is 0,2 mol·dm-3. The equilibrium constant for the reaction is 171 at 25 °C.
Calculate the initial number of moles of NO2(g) placed in the gas syringe.
(8)
6.3 The diagram below shows the reaction mixture in the gas syringe after equilibrium is established.
The pressure is now increased by decreasing the volume of the gas syringe
at constant temperature as illustrated in the diagram below.
6.3.1 IMMEDIATELY after increasing the pressure, the colour of the
reaction mixture in the gas syringe appears darker than before. Give a reason for this observation.
(1)
After a while a new equilibrium is established as illustrated below. The colour of the reaction mixture in the gas syringe now appears lighter than the initial colour.
6.3.2 Use Le Chatelier's principle to explain the colour change observed
in the gas syringe.
(3)
6.4 The temperature of the reaction mixture in the gas syringe is now increased and a new equilibrium is established. How will each of the following be affected?
6.4.1 Colour of the reaction mixture Write down only DARKER, LIGHTER or REMAINS THE SAME.
(1)
6.4.2 Value of the equilibrium constant (Kc) Write down only INCREASES, DECREASES or REMAINS THE SAME.
(1) [16]
80 cm3

Physical Sciences/P2 12 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 7 (Start on a new page.) 7.1 Nitric acid (HNO3), an important acid used in industry, is a strong acid. 7.1.1 Give a reason why nitric acid is classified as a strong acid. (1) 7.1.2 Write down the NAME or FORMULA of the conjugate base of
nitric acid.
(1) 7.1.3 Calculate the pH of a 0,3 mol∙dm-3 nitric acid solution. (3) 7.2 A laboratory technician wants to determine the percentage purity of
magnesium oxide. He dissolves a 4,5 g sample of the magnesium oxide in 100 cm3 hydrochloric acid of concentration 2 mol∙dm-3.
7.2.1 Calculate the number of moles of hydrochloric acid added to the
magnesium oxide.
(3) He then uses the apparatus below to titrate the EXCESS hydrochloric acid in
the above solution against a sodium hydroxide solution.
7.2.2 Write down the name of apparatus Q in the above diagram. (1) 7.2.3 The following indicators are available for the titration:
INDICATOR pH RANGE
A 3,1 – 4,4
B 6,0 – 7,6
C 8,3 – 10,0
Which ONE of the above indicators (A, B or C) is most suitable
to indicate the exact endpoint in this titration? Give a reason for the answer.
(3)
Q
Erlenmeyer flask
Hydrochloric acid
Sodium hydroxide solution
Retort stand

Physical Sciences/P2 13 DBE/November 2014 NSC
Copyright reserved Please turn over
7.2.4 During the titration, the technician uses distilled water to wash any
sodium hydroxide spilled against the sides of the Erlenmeyer flask into the solution. Give a reason why the addition of distilled water to the Erlenmeyer flask will not influence the results.
(1) 7.2.5 At the endpoint of the titration he finds that 21 cm3 of a
0,2 mol dm-3 sodium hydroxide solution has neutralised the EXCESS hydrochloric acid. Calculate the number of moles of hydrochloric acid in excess.
(3) 7.2.6 The balanced equation for the reaction between hydrochloric acid
and magnesium oxide is:
MgO(s) + 2HCℓ(aq) → MgCℓ2(aq) + 2H2O(ℓ) Calculate the percentage purity of the magnesium oxide. Assume that only the magnesium oxide in the 4,5 g sample reacted with the acid.
(5) [21]

Physical Sciences/P2 14 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 8 (Start on a new page.) A standard electrochemical cell is set up using a standard hydrogen half-cell and a standard X|X2+ half-cell as shown below. A voltmeter connected across the cell, initially registers 0,31 V.
8.1 Besides concentration write down TWO conditions needed for the hydrogen
half-cell to function under standard conditions.
(2) 8.2 Give TWO reasons, besides being a solid, why platinum is suitable to be used
as electrode in the above cell.
(2) 8.3 Write down the: 8.3.1 NAME of component Q (1) 8.3.2 Standard reduction potential of the X|X2+ half-cell (1) 8.3.3 Half-reaction that takes place at the cathode of this cell (2) 8.4 The hydrogen half-cell is now replaced by a M|M2+ half-cell. The cell notation
of this cell is: M(s) | M2+(aq) || X2+(aq) | X(s)
The initial reading on the voltmeter is now 2,05 V.
8.4.1 Identify metal M. Show how you arrived at the answer. (5) 8.4.2 Is the cell reaction EXOTHERMIC or ENDOTHERMIC? (1) 8.5 The reading on the voltmeter becomes zero after using this cell for several
hours. Give a reason for this reading by referring to the cell reaction.
(1) [15]
Q Hydrogen gas
Platinum
1 mol·dm-3 H+(aq)
V
X
)aq(X2+
+
_

Physical Sciences/P2 15 DBE/November 2014 NSC
Copyright reserved Please turn over
QUESTION 9 (Start on a new page.) The simplified diagrams below represent two electrochemical cells, A and B. A concentrated copper(II) chloride solution is used as electrolyte in both cells.
ELECTROCHEMICAL CELL A ELECTROCHEMICAL CELL B
9.1 Are A and B ELECTROLYTIC or GALVANIC cells? (1) 9.2 Which of the electrodes (P, Q, R or T) will show a mass increase? Write down
a half-reaction to motivate the answer.
(4) 9.3 Write down the NAME or FORMULA of the product formed at: 9.3.1 Electrode P (1) 9.3.2 Electrode R (1) 9.4 Fully explain the answer to QUESTION 9.3.2 by referring to the relative
strengths of the reducing agents involved.
(3) [10]
Copper Copper
Carbon Carbon
P Q R T
Concentrated CuCℓ2(aq) Concentrated CuCℓ2(aq)

Physical Sciences/P2 16 DBE/November 2014 NSC
Copyright reserved
QUESTION 10 (Start on a new page.) 10.1 The flow diagram below shows the processes involved in the industrial
preparation of fertiliser Q.
Write down the: 10.1.1 NAMES or FORMULAE of the reactants used in the Haber process (2) 10.1.2 Balanced equation for the formation of fertiliser Q (3) 10.2 The diagram below shows a bag of NPK fertiliser. Calculate the mass of nitrogen in the bag. (4)
[9] TOTAL: 150
3 – 1 – 5 (36)
20 kg
Haber process
Ostwald process
Product A Reactants Main product B
Fertiliser Q

Physical Sciences/P2 1 DBE/November 2014 NSC
Copyright reserved Please turn over
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
GEGEWENS VIR FISIESE WETENSKAPPE GRAAD 12
VRAESTEL 2 (CHEMIE) TABLE 1: PHYSICAL CONSTANTS/TABEL 1: FISIESE KONSTANTES
NAME/NAAM SYMBOL/SIMBOOL VALUE/WAARDE
Standard pressure Standaarddruk
θp 1,013 x 105 Pa
Molar gas volume at STP Molêre gasvolume by STD
Vm 22,4 dm3∙mol-1
Standard temperature Standaardtemperatuur
θT 273 K
Charge on electron Lading op elektron
e -1,6 x 10-19 C
Avogadro's constant Avogadro-konstante
NA 6,02 x 1023 mol-1
TABLE 2: FORMULAE/TABEL 2: FORMULES
M
mn=
AN
Nn=
V
nc = or/of
MV
mc =
mV
Vn=
b
a
bb
aa
n
n
vc
vc = pH = -log[H3O+]
Kw = [H3O+][OH-] = 1 x 10-14 at/by 298 K
θanode
θcathode
θcell EEE −= / θ
anode
θkatode
θsel EEE −=
or/of
θoxidation
θreduction
θcell EEE −= / θ
oksidasie
θreduksie
θsel EEE −=
or/of
θagent reducing
θagent oxidising
θcell EEE −= / θ
ddelreduseermi
θddeloksideermi
θsel EEE −=

Physical Sciences/P2 2 DBE/November 2014 NSC
Copyright reserved Please turn over
TABLE 3: THE PERIODIC TABLE OF ELEMENTS TABEL 3: DIE PERIODIEKE TABEL VAN ELEMENTE
1 (I)
2 (II)
3
4
5
6
7
8
9
10
11
12 13 (III)
14 (IV)
15 (V)
16 (VI)
17 (VII)
18 (VIII)
2,1
1
H 1
2
He 4
1,0
3
Li 7
1,5
4
Be 9
2,0
5
B 11
2,5
6
C 12
3,0
7
N 14
3,5
8
O 16
4,0
9
F 19
10
Ne 20
0,9
11
Na 23
1,2
12
Mg 24
1,5
13
Aℓ 27
1,8
14
Si 28
2,1
15
P 31
2,5
16
S 32
3,0
17
Cℓ 35,5
18
Ar 40
0,8
19
K 39
1,0
20
Ca 40
1,3
21
Sc 45
1,5
22
Ti 48
1,6
23
V 51
1,6
24
Cr 52
1,5
25
Mn 55
1,8
26
Fe 56
1,8
27
Co 59
1,8
28
Ni 59
1,9
29
Cu 63,5
1,6
30
Zn 65
1,6
31
Ga 70
1,8
32
Ge 73
2,0
33
As 75
2,4
34
Se 79
2,8
35
Br 80
36
Kr 84
0,8
37
Rb 86
1,0
38
Sr 88
1,2
39
Y 89
1,4
40
Zr 91
41
Nb 92
1,8
42
Mo 96
1,9
43
Tc
2,2
44
Ru 101
2,2
45
Rh 103
2,2
46
Pd 106
1,9
47
Ag 108
1,7
48
Cd 112
1,7
49
In 115
1,8
50
Sn 119
1,9
51
Sb 122
2,1
52
Te 128
2,5
53
I 127
54
Xe 131
0,7
55
Cs 133
0,9
56
Ba 137
57
La 139
1,6
72
Hf 179
73
Ta 181
74
W 184
75
Re 186
76
Os 190
77
Ir 192
78
Pt 195
79
Au 197
80
Hg 201
1,8
81
Tℓ 204
1,8
82
Pb 207
1,9
83
Bi 209
2,0
84
Po
2,5
85
At
86
Rn
0,7
87
Fr
0,9
88
Ra 226
89
Ac
58
Ce 140
59
Pr 141
60
Nd 144
61
Pm
62
Sm 150
63
Eu 152
64
Gd 157
65
Tb 159
66
Dy 163
67
Ho 165
68
Er 167
69
Tm 169
70
Yb 173
71
Lu 175
90
Th 232
91
Pa
92
U 238
93
Np
94
Pu
95
Am
96
Cm
97
Bk
98
Cf
99
Es
100
Fm
101
Md
102
No
103
Lr
Electronegativity Elektronegatiwiteit
Approximate relative atomic mass Benaderde relatiewe atoommassa
Atomic number Atoomgetal
29
Cu 63,5
1,9
Symbol Simbool
KEY/SLEUTEL

Physical Sciences/P2 3 DBE/November 2014 NSC
Copyright reserved Please turn over
TABLE 4A: STANDARD REDUCTION POTENTIALS TABEL 4A: STANDAARDREDUKSIEPOTENSIALE
Half-reactions/Halfreaksies θ
E (V)
F2(g) + 2e− 2F− + 2,87
Co3+
+ e− Co2+
+ 1,81
H2O2 + 2H+ +2e− 2H2O +1,77
MnO−4 + 8H
+ + 5e− Mn
2+ + 4H2O + 1,51
Cℓ2(g) + 2e− 2Cℓ− + 1,36
Cr2O−2
7 + 14H+ + 6e− 2Cr
3+ + 7H2O + 1,33
O2(g) + 4H+ + 4e− 2H2O + 1,23
MnO2 + 4H
+ + 2e− Mn
2+ + 2H2O + 1,23
Pt2+
+ 2e− Pt + 1,20
Br2(ℓ) + 2e− 2Br− + 1,07
NO−3 + 4H
+ + 3e− NO(g) + 2H2O + 0,96
Hg2+
+ 2e− Hg(ℓ) + 0,85
Ag+ + e− Ag + 0,80
NO−3 + 2H
+ + e− NO2(g) + H2O + 0,80
Fe3+
+ e− Fe2+
+ 0,77
O2(g) + 2H+ + 2e− H2O2 + 0,68
I2 + 2e− 2I− + 0,54
Cu+ + e− Cu + 0,52
SO2 + 4H+ + 4e− S + 2H2O + 0,45
2H2O + O2 + 4e− 4OH− + 0,40
Cu2+
+ 2e− Cu + 0,34
SO−2
4 + 4H+ + 2e− SO2(g) + 2H2O + 0,17
Cu2+
+ e− Cu+ + 0,16
Sn4+
+ 2e− Sn2+
+ 0,15
S + 2H+ + 2e−
H2S(g) + 0,14
2H+ + 2e− H2(g) 0,00
Fe3+
+ 3e− Fe − 0,06
Pb2+
+ 2e− Pb − 0,13
Sn2+
+ 2e− Sn − 0,14
Ni2+
+ 2e− Ni − 0,27
Co2+
+ 2e− Co − 0,28
Cd2+
+ 2e− Cd − 0,40
Cr3+
+ e− Cr2+
− 0,41
Fe2+
+ 2e− Fe − 0,44
Cr3+
+ 3e− Cr − 0,74
Zn2+
+ 2e− Zn − 0,76
2H2O + 2e− H2(g) + 2OH− − 0,83
Cr2+
+ 2e− Cr − 0,91
Mn2+
+ 2e− Mn − 1,18
Aℓ3+ + 3e− Aℓ − 1,66
Mg2+
+ 2e− Mg − 2,36
Na+ + e− Na − 2,71
Ca2+
+ 2e− Ca − 2,87
Sr2+
+ 2e− Sr − 2,89
Ba2+
+ 2e− Ba − 2,90
Cs+ + e
- Cs - 2,92
K+ + e− K − 2,93
Li+ + e− Li − 3,05
Inc
reas
ing
ox
idis
ing
ab
ilit
y/T
oe
ne
me
nd
e o
ks
ide
ren
de
ve
rmo
ë
Inc
reas
ing
re
du
cin
g a
bil
ity
/To
en
em
en
de r
ed
us
ere
nd
e v
erm
oë

Physical Sciences/P2 4 DBE/November 2014 NSC
Copyright reserved
TABLE 4B: STANDARD REDUCTION POTENTIALS TABEL 4B: STANDAARDREDUKSIEPOTENSIALE
Half-reactions/Halfreaksies θ
E (V)
Li+ + e− Li − 3,05
K+ + e− K − 2,93
Cs+ + e− Cs − 2,92
Ba2+
+ 2e− Ba − 2,90
Sr2+
+ 2e− Sr − 2,89
Ca2+
+ 2e− Ca − 2,87
Na+ + e− Na − 2,71
Mg2+
+ 2e− Mg − 2,36
Aℓ3+ + 3e− Aℓ − 1,66
Mn2+
+ 2e− Mn − 1,18
Cr2+
+ 2e− Cr − 0,91
2H2O + 2e− H2(g) + 2OH− − 0,83
Zn2+
+ 2e− Zn − 0,76
Cr3+
+ 3e− Cr − 0,74
Fe2+
+ 2e− Fe − 0,44
Cr3+
+ e− Cr2+
− 0,41
Cd2+
+ 2e− Cd − 0,40
Co2+
+ 2e− Co − 0,28
Ni2+
+ 2e− Ni − 0,27
Sn2+
+ 2e− Sn − 0,14
Pb2+
+ 2e− Pb − 0,13
Fe3+
+ 3e− Fe − 0,06
2H+ + 2e− H2(g) 0,00
S + 2H+ + 2e−
H2S(g) + 0,14
Sn4+
+ 2e− Sn2+
+ 0,15
Cu2+
+ e− Cu+ + 0,16
SO−2
4 + 4H+ + 2e− SO2(g) + 2H2O + 0,17
Cu2+
+ 2e− Cu + 0,34
2H2O + O2 + 4e− 4OH− + 0,40
SO2 + 4H+ + 4e− S + 2H2O + 0,45
Cu+ + e− Cu + 0,52
I2 + 2e− 2I− + 0,54
O2(g) + 2H+ + 2e− H2O2 + 0,68
Fe3+
+ e− Fe2+
+ 0,77
NO−3 + 2H
+ + e− NO2(g) + H2O + 0,80
Ag+ + e− Ag + 0,80
Hg2+
+ 2e− Hg(ℓ) + 0,85
NO−3 + 4H
+ + 3e− NO(g) + 2H2O + 0,96
Br2(ℓ) + 2e− 2Br− + 1,07
Pt2+
+ 2 e− Pt + 1,20
MnO2 + 4H
+ + 2e− Mn
2+ + 2H2O + 1,23
O2(g) + 4H+ + 4e−
2H2O + 1,23
Cr2O−2
7 + 14H+ + 6e− 2Cr
3+ + 7H2O + 1,33
Cℓ2(g) + 2e− 2Cℓ− + 1,36
MnO−4 + 8H
+ + 5e− Mn
2+ + 4H2O + 1,51
H2O2 + 2H+ +2 e− 2H2O +1,77
Co3+
+ e− Co2+
+ 1,81
F2(g) + 2e− 2F− + 2,87
Inc
reas
ing
ox
idis
ing
ab
ilit
y/T
oe
ne
me
nd
e o
ks
ide
ren
de
ve
rmo
ë
Inc
reas
ing
re
du
cin
g a
bil
ity
/To
en
em
en
de r
ed
us
ere
nd
e v
erm
oë

Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
MARKS/PUNTE: 150
This memorandum consists of 20 pages.
Hierdie memorandum bestaan uit 20 bladsye .
PHYSICAL SCIENCES: CHEMISTRY (P2) FISIESE WETENSKAPPE: CHEMIE (V2)
NOVEMBER 2014
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
NASIONALE SENIOR SERTIFIKAAT
GRADE/GRAAD 12

Physical Sciences P2/Fisiese Wetenskappe V2 2 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 1 / VRAAG 1 1.1 C (2) 1.2 B (2) 1.3 D (2) 1.4 D (2) 1.5 A (2) 1.6 B (2) 1.7 B (2) 1.8 A (2) 1.9 D (2) 1.10 C (2)
[20] QUESTION 2 / VRAAG 2 2.1 2.1.1
B
(1)
2.1.2 E (1) 2.1.3 F (1) 2.2 2.2.1
2-bromo-3-chloro-4-methylpentane 2-bromo-3-chloro-4-metielpentaan / 2-broom-3-chloor-4-metielpentaan
Marking criteria / Nasienriglyne: • Correct stem i.e. pentane. / Korrekte stam d.i. pentaan. • All substituents correctly identified. / Alle substituente korrek geïdentifiseer. • Substituents correctly numbered, in alphabetical order, hyphens and commas correctly used. Substituente korrek genommer, in alfabetiese volgorde, koppeltekens en kommas korrek gebruik.
(3) 2.2.2 Ethene / Eteen (1)

Physical Sciences P2/Fisiese Wetenskappe V2 3 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
2.3 2.3.1
Marking criteria / Nasienriglyne: • Six saturated C atoms in longest chain i.e. hexane. Ses versadigde C-atome in langste ketting d.i. heksaan. • Three methyl substituents on second C and fourth C. Drie metielsubstituente op tweede C en vierde C.
Notes / Aantekeninge: • If correct structure, but H atoms omitted / Indien korrekte struktuur, maar H-atome
weggelaat: Max / Maks. 21
• Condensed or semi-structural formula:
Gekondenseerde of semistruktuurformule: Max./Maks. 21
• Molecular formula / Molekulêre formule: 20
(2) 2.3.2
Marking criteria / Nasienriglyne: • Whole structure correct / Hele struktuur
korrek: 2
2
• Only functional group correct / Slegs
funksionele groep korrek: 2
1
Notes / Aantekeninge:
• If two or more functional groups/Indien twee of meer funksionele groepe: 20
• Condensed or semi-structural formula: Gekondenseerde of semistruktuurformule: Max / Maks 2
1
• Molecular formula / Molekulêre formule: 20
(2)
2.4 2.4.1
(Compounds with) the same molecular formula but different functional goups / different homologous series. (Verbindings met) dieselfde molekulêre formule, maar verskillende funksionele groepe / verskillende homoloë reekse.
(2) 2.4.2 B & F (1)
[14]
C C C C C
O H
H
H H
H
H
H
H
H
H
C C C C C C H
H
H H
H
C
H
H
C
C
H
H
H
H
H
H
H
H
H
H
H
H
H

Physical Sciences P2/Fisiese Wetenskappe V2 4 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 3 / VRAAG 3 3.1 ANY ONE / ENIGE EEN: • Alkanes have ONLY single bonds.
Alkane het SLEGS enkelbindings. • Alkanes have single bonds between C atoms. Alkane het enkelbindings tussen C-atome.
• Alkanes have no double OR triple bonds OR multiple bonds. • Alkane het geen dubbel- OF trippelbindings OF meervoudige bindings nie. • Alkanes contain the maximum number of H atoms bonded to C atoms. Alkane bevat die maksimum getal H-atome gebind aan C-atome.
(1) 3.2 3.2.1
ANY ONE / ENIGE EEN:
– OH – O – H
R OH R O H
(1) 3.2.2
Marking criteria / Nasienriglyne: • - OH group on second C atom of longest chain. - OH-groep op tweede C-atoom van langste ketting. • Tertiary group consisting of four C atoms with methyl group on 2nd C atom. Tersiêre groep bestaande uit vier C-atome met metielgroep op 2de C-atoom. • If two or more functional groups / Indien
twee of meer funksionele groepe: 20
Notes / Aantekeninge: • Accept / Aanvaar – OH • If correct structure and number of bonds, but H atoms omitted / Indien korrekte
struktuur en getal bindings, maar H-atome weggelaat: Max / Maks. 21
• Condensed or semi-structural formula / Gekondenseerde of
semistruktuurformule: Max / Maks. 21
• Molecular formula / Molekulêre formule: 20
(2)
H
C C
H
H
H O
C
C
H
H
H
H
H
H
C O H C OH

Physical Sciences P2/Fisiese Wetenskappe V2 5 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
3.3 3.3.1
Criteria for investigative question / Riglyne vir ondersoekende vraag:
The dependent and independent variables are stated. Die afhanklike en onafhanklike veranderlikes is genoem.
Ask a question about the relationship between the independent and dependent variables. Vra 'n vraag oor die verwantskap tussen die onafhanklike en afhanklike veranderlikes.
Examples / Voorbeelde : • How does an increase in chain length / molecular size / molecular structure / molecular mass / surface area influence boiling point? Hoe beïnvloed 'n toename in kettinglengte / molekulêre grootte / molekulêre struktuur / molekulêre massa / reaksieoppervlak die kookpunt?
• What is the relationship between chain length / molecular size / molecular structure / molecular mass / surface area and boiling point? Wat is die verwantskap tussen kettinglengte / molekulêre grootte / molekulêre struktuur / molekulêre massa / oppervlakte en kookpunt?
(2)
3.3.2 • Structure / Struktuur : The chain length / molecular size / molecular structure / molecular mass / surface area increases. Die kettinglengte / molekulêre grootte / molekulêre struktuur / molekulêre massa / oppervlakte neem toe. • Intermolecular forces / Intermolekulêre kragte : Increase in strength of intermolecular forces / induced dipole / London / dispersion / Van der Waals forces. Toename in sterkte van intermolekulêre kragte / geïnduseerde dipoolkragte / London-kragte / dispersiekragte / Van der Waalskragte. • Energy / Energie :
More energy needed to overcome / break intermolecular forces. Meer energie benodig om intermolekulêre kragte te oorkom / breek. OR / OF • Structure / Struktuur :
From propane to methane the chain length / molecular size / molecular structure / molecular mass / surface area decreases. Van propaan na metaan neem die kettinglengte / molekulêre grootte / molekulêre struktuur / molekulêre massa / oppervlakte af. • Intermolecular forces / Intermolekulêre kragte : Decrease in strength of intermolecular forces / induced dipole forces / London forces / dispersion forces. Afname in sterkte van intermolekulêre kragte / geïnduseerde dipoolkragte / London-kragte / dispersiekragte. • Energy / Energie :
Less energy needed to overcome / break intermolecular forces. Minder energie benodig om intermolekulêre kragte te oorkom / breek.
(3)
-

Physical Sciences P2/Fisiese Wetenskappe V2 6 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
3.4 • Between propane molecules are London forces / dispersion forces / induced dipole forces. Tussen propaanmolekule is Londonkragte / dispersiekragte / geïnduseerde dipoolkragte. • Between propan-1-ol molecules are London forces / dispersion forces / induced dipole forces and hydrogen bonds. Tussen propan-1-ol molekule is Londonkragte / dispersiekragte / geïnduseerde dipoolkragte en waterstofbindings. • Hydrogen bonds / Forces between alcohol molecules are stronger or need more energy than London forces / dispersion forces / induced dipole forces. Waterstofbindings / Kragte tussen alkoholmolekule is sterker of benodig meer energie om oorkom te word as Londonkragte / dispersiekragte / geïnduseerde dipoolkragte.
OR/OF Between propane molecules are weak London forces / dispersion forces / induced dipole forces and between propan-1-ol molecules are strong hydrogen bonds. Tussen propaanmolekule is swak Londonkragte / dispersiekragte / geïnduseerde dipoolkragte en tussen propan-1-olmolekule is sterk waterstofbindings.
(3)
[12]
QUESTION 4 / VRAAG 4 4.1 4.1.1
Substitution / chlorination / halogenation Substitusie / chlorering / halogenering / halogenasie
(1)
4.1.2 Substitution / hydrolysis Substitusie / hidrolise
(1)
4.2 4.2.1
Hydrogenation / Hidrogenasie / Hidrogenering
(1)
4.2.2 Notes / Aantekeninge : • Ignore/Ignoreer • Accept H2 if condensed. / Aanvaar H2 as gekondenseerd. • Any additional reactants and/or products
Enige addisionele reaktanse en / of produkte: Max./Maks. 3
2
• Accept coefficients that are multiples. Aanvaar koëffisiënte wat veelvoude is. • Molecular / condensed formulae
Molekulêre-/ gekondenseerde formule: Max./Maks. 3
2
(3)
+ C C C
H
H
H H
H
H
H
H
C C C
H
H
H H H
H
H H

Physical Sciences P2/Fisiese Wetenskappe V2 7 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
4.3
Marking criteria / Nasienriglyne:
• Whole structure correct:/ Hele struktuur korrek: 2
2
• Only ONE Cℓ atom as functional group. / Slegs
EEN Cℓ-atoom as funksionele groep. 2
1
Notes / Aantekeninge: . • Condensed or semi-structural formula
Gekondenseerde of semistruktuurformule: Max./Maks. 2
1
• Molecular formula. / Molekulêre formule: 20
• If functional group is incorrect. / Indien funksionele groep verkeerd is: 20
(2) 4.4 4.4.1
Esterification / Condensation Verestering / Esterifikasie / Kondensasie
(1) 4.4.2 (Concentrated) H2SO4 / (Concentrated) sulphuric acid
(Gekonsentreerde) H2SO4 / (Gekonsentreerde) swawelsuur of swaelsuur
(1) 4.4.3
Marking criteria / Nasienriglyne: • Whole structure correct / Hele struktuur
korrek: 2
2
• Only functional group correct / Slegs
funksionele groep korrek: 2
1
Notes / Aantekeninge :
• If two or more functional groups/Indien twee of meer funksionele groepe: 20
• Condensed or semi-structural formula: Gekondenseerde of semistruktuurformule: Max./Maks. 2
1
• Molecular formula / Molekulêre formule: 20
• If functional group is incorrect/Indien funksionele groep verkeerd is: 20
(2) 4.4.4 Propyl ethanoate
Propieletanoaat
(2) 4.5 Sulphuric acid / H2SO4 / Phosphoric acid / H3PO4
Swawelsuur / Swaelsuur / H2SO4 / Fosforsuur / H3PO4
(1) [15]
C
O
O C C C C
H
H H
H H
H
H
H
H
H
C
H
C
Cℓ
H
C
H
H
H
H
H

Physical Sciences P2/Fisiese Wetenskappe V2 8 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 5 / VRAAG 5 5.1 ONLY ANY ONE OF/ SLEGS ENIGE EEN VAN: • Change in concentration of products / reactants per (unit) time.
Verandering in konsentrasie van produkte / reaktanse per (eenheids)tyd. • Rate of change in concentration.
Tempo van verandering in konsentrasie. • Change in amount / number of moles / volume / mass of products or reactants per (unit) time. Verandering in hoeveelheid / getal mol/volume / massa van produkte of reaktanse per (eenheids)tyd. • Amount / number of moles / volume / mass of products formed or reactants used per (unit) time. Hoeveelheid / getal mol / volume / massa van produkte gevorm of reaktanse gebruik per (eenheids)tyd.
(2)
5.2 5.2.1
Temperature / Temperatuur
(1)
5.2.2 Rate of reaction / Volume of gas (formed) per (unit) time
Reaksietempo / Volume gas (gevorm) per (eenheids)tyd
(1) 5.3 • Larger mass / amount / surface area.
Groter massa / hoeveelheid / reaksieoppervlak. • More effective collisions per (unit) time. / Frequency of effective collisions
increase./ More particles collide with sufficient kinetic energy & correct orientation per (unit) time. Meer effektiewe botsings per (eenheids)tyd. / Frekwensie van effektiewe botsings verhoog./ Meer deeltjies bots met genoeg kinetiese energie & korrekte oriëntasie per tyd(seenheid).
IF / INDIEN: • Larger mass / amount / surface area.
Groter massa / hoeveelheid / reaksieoppervlak. • More particles collide. / More collisions.
Meer deeltjies bots. / Meer botsings. Max./Maks. 3
2
Notes / Aantekeninge :
IF/INDIEN: No reference to mass / amount / surface area in answer:
Geen verwysing na massa / hoeveelheid / reaksieoppervlak in antwoord: 3
0
(3)

Physical Sciences P2/Fisiese Wetenskappe V2 9 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
5.4 Marking criteria / Nasienriglyne: Compare
Exp.1 with Exp. 2: Vergelyk Eksp. 1 met Eksp. 2:
The reaction in exp. 1 is faster in exp. 1 than in exp. 2 due to the higher acid concentration. Die reaksie in eksp. 1 is vinniger as dié in eksp. 2 as gevolg van die hoër suurkonsentrasie.
Therefore the gradient of the graph representing exp. 1 is greater / steeper than that of exp. 2. / Graph of Exp. 1 reaches constant volume in shorter time than exp. 2. Dus is die gradiënt van die grafiek wat eksp. 1 voorstel, groter/steiler as dié vir eksp. 2. / Grafiek van exp. 1 bereik konstante volume in korter tyd as dié vir eksp. 2.
Compare Exp. 1 with Exp 3 & 4: Vergelyk Eksp. 1 met Eksp. 3 & 4:
The reaction in exp. 3 is faster than that in exp. 1 due to the higher temperature. Die reaksie in eks. 3 is vinniger as dié in eksp. 1 as gevolg van die hoër temperatuur.
The reaction in exp. 4 is faster than that in exp. 1 due to the higher temperature / larger surface area. Die reaksie in eks. 4 is vinniger as dié in eksp. 1 as gevolg van die hoër temperatuur / groter reaksieoppervlak. OR/OF Graph A represents exp. 4 due to the greater mass of CaCO3 - greater yield of CO2 at a faster rate. Grafiek A stel eksp. 4 voor as gevolg van die groter massa CaCO3 - groter opbrengs CO2 teen vinniger tempo.
Therefore the gradient of the graphs of exp. 3 & 4 are greater/steeper than that of exp. 1. / Graphs of Exp. 3 & 4 reaches constant volume in shorter time than exp. 1. Dus is die gradiënte van die grafieke vir eksp. 3 & 4 is groter/steiler as dié in eksp. 1./ Grafieke van exp. 3 & 4 bereik konstante volume in korter tyd as dié vir eksp. 1.
Final answer Finale antwoord
C
(6) Notes/Aantekeninge • Compare exp. 1 with exp. 2 / Vergelyk eksp. 1 met eksp. 2:
o Factor & rate / Faktor & tempo. o Gradient / volume CO2 per time / gradient / volume CO2 per tyd. • Compare exp. 1 with exp. 3 / Vergelyk eksp. 1 met eksp. 3: o Factor & rate / Faktor & tempo. • Compare exp. 1 with exp. 4/ Vergelyk eksp. 1 met eksp. 4: o Factor & rate / Faktor & tempo. • Compare gradient / volume CO2 per time of exp 1 with that of exp. 3 & 4 Vergelyk gradient/volume CO2 per tyd van eksp 1 met die van eksp. 3 & 4 • Final answer / finale antwoord: C

Physical Sciences P2/Fisiese Wetenskappe V2 10 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
5.5 Marking criteria / Nasienriglyne: • Divide volume by / Deel volume deur: 25,7
• Use ratio / Gebruik verhouding: n(CO2) = n(CaCO3) = 1:1
• Substitute / Vervang 100 in Mm
n = . • Subtraction / Aftrekking. • Final answer / Finale antwoord: 7,00 g to/tot 7,5 g
OPTION 1 / OPSIE 1
)mol175,0(mol18,07,255,4
VV
)CO(nm
2
===
n(CaCO3) = n(CO2) = 0,18 mol
100m
18,0
Mm
)CaCO(n 3
==
∴ m = 18 g (17,5 g) m(CaCO3) not reacted/nie gereageer nie): 25 – 18 = 7,00 g (7,49 g)
(Accept range: 7,00 g – 7,5 g) (Aanvaar gebied: 7.00 g – 7,5 g)
OPTION 2 / OPSIE 2 Calculate mass of CO2: Bereken massa CO2:
)mol175,0(mol18,07,255,4
VV
)CO(nm
2
===
44m
18,0
Mm
)CO(n 2
==
∴m(CO2) = 7,92 g (7,7043 g)
)g5,17(g18
1004492,7
)/neededCaCO(m 3
=×=benodig
m(CaCO3 not reacted/nie gereageer nie): 25 – 18,00 = 7,00 g (7,49 g)
(Accept range: 7,00 g – 7,5 g) (Aanvaar gebied: 7.00 g – 7,5 g)
(5) OPTION 3 / OPSIE 3
25,7 dm3 : 1 mol 4,5 dm3 : 0,18 mol
100 g : 1 mol x : 0,18 mol x = 18 g
m(CaCO3 not reacted/nie gereageer nie): 25 – 18 = 7,00 g
(Accept range: 7,00 g – 7,5 g) (Aanvaar gebied: 7,00 g – 7,5 g)
OPTION 4 / OPSIE 4 100 g CaCO3 → 25,7 dm3 CO2 x g → 4,5 dm3 CO2 ∴x = 17,51 g Mass not reacted/Massa nie gereageer nie = 25 – 17,51 = 7,49 g
(Accept range: 7,00 g – 7,5 g) (Aanvaar gebied: 7,00 g – 7,5 g)
(5)
Ratio/ verhouding

Physical Sciences P2/Fisiese Wetenskappe V2 11 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 6 / VRAAG 6 6.1 The stage in a chemical reaction when the rate of forward reaction equals the
rate of reverse reaction. Die stadium in 'n chemiese reaksie wanneer die tempo van die voorwaartse reaksie gelyk is aan die tempo van die terugwaartse reaksie. OR / OF The stage in a chemical reaction when the concentrations of reactants and products remain constant. Die stadium in 'n chemiese reaksie wanneer die konsentrasies van reaktanse en produkte konstant bly.
(2) 6.2 CALCULATIONS USING NUMBER OF MOLES
BEREKENINGE WAT GETAL MOL GEBRUIK
Mark allocation / Puntetoekenning: • Correct Kc expression (formulae in square brackets). Korrekte Kc uitdrukking (formules in vierkanthakies). • Substitution of concentrations into KC expression. Vervanging van konsentrasies in KC-uitdrukking. • Substitution of KC value / Vervanging van KC-waarde.
• Equilibrium concentration of both NO2 & N2O4 multiplied by 0,08 dm3. Ewewigskonsentrasie van beide NO2 & N2O4 vermenigvuldig met 0,08 dm3
• Change in n(N2O4) = equilibrium n(N2O4) – initial n(N2O4)
Verandering in n(N2O4) = ewewig n(N2O4) – aanvanklike n(N2O4) • USING ratio / GEBRUIK verhouding: NO2 : N2O4 = 2 : 1 • Initial n(NO2)= equilibrium n(NO2) + change n(NO2). Aanvanklike n(NO2)= ewewig n(NO2) + verandering n(NO2). • Final answer / Finale antwoord: 1,11 (mol) Accept range/Aanvaar gebied: 1,11 – 1,12 (mol)

Physical Sciences P2/Fisiese Wetenskappe V2 12 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 1 / OPSIE 1
22
42c ]NO[
]ON[K =
171 = 242
)2,0(]ON[
∴ [N2O4] = 171 x (0,2)2 = 6,84 mol∙dm-3
(8)
NO2 N2O4 Initial quantity (mol)
Aanvangshoeveelheid (mol) 1,11
0
Change (mol)
Verandering (mol) 1,094 0,55
Quantity at equilibrium (mol)/ Hoeveelheid by ewewig (mol) 0,016 0,55
Equilibrium concentration (mol∙dm-3) Ewewigskonsentrasie (mol∙dm-3) 0,2 6,84
OPTION 2 / OPSIE 2
22
42c ]NO[
]ON[K =
171 = 242
)2,0(]ON[
∴ [N2O4] = 171 x (0,2)2 = 6,84 mol∙dm-3
Equilibrium moles / Ewewigsmol: n(N2O4) = (6,84)(0,080) = 0,55 mol n(NO2) = (0,2)(0,080) = 0,016 mol n(N2O4 formed/gevorm) = 0,55 – 0 = 0,55 mol Ratio / Verhouding: n(NO2 reacted / gereageer) = 2n(N2O4 formed/gevorm) = 2(0,55) = 1,094 mol Initial / Aanvanklike n(NO2) = 0,016 + 1,094 = 1,11 (mol)
(8)
Wrong KC expression /Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution /Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7
x 0,08 dm3
ratio verhouding
Wrong KC expression /Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution / Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7
x 0,08

Physical Sciences P2/Fisiese Wetenskappe V2 13 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 3 / OPSIE 3
NO2 N2O4 Initial quantity (mol) Aanvangshoeveelheid (mol) 2x + 0,016
0
Change (mol)
Verandering (mol)
2x
x Quantity at equilibrium (mol)/ Hoeveelheid by ewewig (mol) 0,016 x
Equilibrium concentration (mol∙dm-3) Ewewigskonsentrasie (mol∙dm-3)
0,2 08,0
x
2
2
42c ]NO[
]ON[K =
171 = 2)2,0(
08,0x
∴ x = 0,5472 ∴ n(initial/aanvanklik) = 2(0,5472) + 0,016 = 1,11 mol
(8) OPTION 4 / OPSIE 4
NO2 N2O4 Initial quantity (mol) Aanvangshoeveelheid (mol) x 0
Change (mol)
Verandering (mol) x – 0,016 2016,0x −
Quantity at equilibrium (mol)/ Hoeveelheid by ewewig (mol) 0,016
2016,0x −
Equilibrium concentration (mol∙dm-3) Ewewigskonsentrasie (mol∙dm-3)
0,2 16,0
016,0x −
2
2
42c ]NO[
]ON[K =
171 = 2)2,0(
16,0016,0x −
∴ x = 1,11 mol
(8)
ratio verhouding
Wrong KC expression/Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution/Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7
x 0,08 & ÷0,08
ratio verhouding
Wrong KC expression/Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution/Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7
x 0,08 & ÷0,08

Physical Sciences P2/Fisiese Wetenskappe V2 14 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
CALCULATIONS USING CONCENTRATION
BEREKENINGE WAT KONSENTRASIE GEBRUIK
Mark allocation / Puntetoekenning: • Correct Kc expression (formulae in square brackets). Korrekte Kc uitdrukking (formules in vierkanthakies). • Substitution of concentrations into KC expression. Vervanging van konsentrasies in KC-uitdrukking. • Substitution of KC value. / Vervanging van KC-waarde. • Change in [N2O4] = equilibrium [N2O4] – initial [N2O4]. Verandering in [N2O4] = ewewig [N2O4] – aanvanklike [N2O4]. • USING ratio/GEBRUIK verhouding: NO2 : N2O4 = 2 : 1 • Initial [NO2] = equilibrium [NO2] + change in [NO2]. Aanvanklike [NO2] = ewewigs [NO2] + verandering in [NO2]. • Equilibrium concentration of [NO2] multiplied by 0,08 dm3. Ewewigskonsentrasie van [NO2] vermenigvuldig met 0,08 dm3. • Final answer/Finale antwoord: 1,11 (mol) Accept range/Aanvaar gebied: 1,11 – 1,12 (mol)
OPTION 5 / OPSIE 5
22
42c ]NO[
]ON[K =
171 = 242
)2,0(]ON[
∴ [N2O4] = 171 x (0,2)2 = 6,84 mol∙dm-3
NO2 N2O4 Initial concentration (mol∙dm-3) Aanvangskonsentrasie (mol∙dm-3) 13,88
0
Change (mol∙dm-3)
Verandering (mol∙dm-3)
13,68 6,84
Equilibrium concentration (mol∙dm-3) Ewewigskonsentrasie (mol∙dm-3) 0,2 6,84
n(NO2) = cV = (13,88)(0,08) = 1,11 mol (8)
ratio verhouding
Wrong KC expression/Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution/Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7

Physical Sciences P2/Fisiese Wetenskappe V2 15 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
OPTION 6 / OPSIE 6
NO2 N2O4 Initial concentration (mol∙dm-3) Aanvangskonsentrasie (mol∙dm-3) x 0
Change (mol∙dm-3)
Verandering (mol∙dm-3) x – 0,2 2
2,0x −
Equilibrium concentration (mol∙dm-3) Ewewigskonsentrasie (mol∙dm-3)
0,2 2
2,0x −
2
2
42c ]NO[
]ON[K =
171 = 2)2,0(
22,0x −
∴ x = 13,88 mol⋅dm-3 n(NO2) = cV = (13,88)(0,08) = 1,11 mol
(8) 6.3 6.3.1
Concentration (of the gases) increases. / Molecules become more condensed or move closer to each other. Konsentrasie (van die gasse) verhoog. / Molekule word meer saamgepers of beweeg nader aan mekaar.
(1)
6.3.2 • Increase in pressure favours the reaction that leads to smaller number of moles / volume of gas. Toename in druk bevoordeel die reaksie wat tot die kleiner getal mol / volume gas lei. • Forward reaction is favoured. / Voorwaartse reaksie word bevoordeel. • Number of moles/amount of N2O4 / colourless gas increases. Aantal mol/hoeveelheid N2O4 / kleurlose gas neem toe. OR / OF Number of moles/amount of NO2 / brown gas decreases. Aantal mol/hoeveelheid NO2 / bruin gas neem af.
(3)
6.4 6.4.1
Darker / Donkerder
(1)
6.4.2 Decreases / Verlaag (1) [16]
ratio verhouding
Wrong KC expression/Verkeerde Kc-uitdrukking:
Max./Maks. 8
5
No KC expression, correct substitution/Geen Kc-
uitdrukking, korrekte substitusie: Max./Maks. 8
7

Physical Sciences P2/Fisiese Wetenskappe V2 16 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 7/ VRAAG 7 PENALISE ONCE FOR THE INCORRECT CONVERSION OF UNITS. PENALISEER EENMALIG VIR VERKEERDE OMSKAKELING VAN EENHEDE.
7.1 7.1.1
Ionises / dissociates completely (in water) Ioniseer / dissosieer volledig (in water).
(1) 7.1.2 NO3
- / Nitrate ion / Nitraatioon (1) 7.1.3
pH = -log[H3O+] / -log[H+] = -log(0,3) = 0,52
Notes/Aantekeninge: • If no/incorrect formula/Indien geen/foutiewe
formule: Max./Maks: 3
2
• If no substitution step: 2 marks for correct answer./Indien geen substitusie stap: 2 punte vir korrekte antwoord.
(3) 7.2 7.2.1
1,0n
=2
Vn
=c
∴ n(HCℓ) = 0,2 mol
(3) 7.2.2 Burette / Buret (1) 7.2.3 B
Titration of strong acid and strong base. Titrasie van sterk suur en sterk basis. OR/OF The endpoint will be approximately at pH = 7 which is in the range of the indicator. Die eindpunt sal ongeveer by pH = 7 wees wat in die gebied van die indikator is.
(3) 7.2.4 The number of moles of acid in the flask remains constant.
Die getal mol van die suur in die fles bly konstant.
(1)
-

Physical Sciences P2/Fisiese Wetenskappe V2 17 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
7.2.5
021,0n
=2,0
Vn
=c
n = 4,2 x 10-3 mol n(HCℓ in excess/in oormaat): n(HCℓ) = n(NaOH)
= 4,2 x 10-3 mol
(3) 7.2.6 POSITIVE MARKING FROM QUESTION 7.2.1 AND 7.2.5.
POSITIEWE NASIEN VAN VRAAG 7.2.1 EN 7.2.5.
Marking criteria / Nasienriglyne: • n(HCℓ reacted) = initial (from Q7.2.1) – excess (from Q7.2.5).
n(HCℓ reageer) = begin (van Q7.2.1) – oormaat (van Q7.2.5). • Use mol ratio of acid: base = 2 : 1. Gebruik molverhouding suur : basis = 2 : 1.
• Substitute / Vervang 40 into / in Mm
n =
• 1005,4
)/reactedMgO(m ×reageer.
• Final answer / Finale antwoord: 87,11 %
OPTION 1 / OPSIE 1
n(HCℓ reacted/gereageer): 0,2 – 4,2 x 10-3 = 0,196 mol n(MgO reacted/gereageer): ½n(HCℓ) = ½(0,196) = 9,8 x 10-2 mol
n(MgO reacted/gereageer) = Mm
∴ 0,098 = 40m
∴m = 3,92 g
% purity/ suiwerheid = 100×5,4
92,3
= 87,11% (Accept range: 87 - 87,11 %.) (Aanvaar gebied: 87 – 87,11 %)
OPTION 2 / OPSIE 2 n(HCℓ reacted/gereageer): 0,2 – 4,2 x 10-3 = 0,196 mol
n(HCℓ reacted/gereageer) = Mm
0,196 = 5,36
m
∴m(HCℓ reacted/gereageer) = 7,154 g 40 g MgO .............. 73 g HCℓ x g MgO ................... 7,154 g ∴x = 3,92 g
% purity/suiwerheid = 100×5,4
92,3
= 87,11% (Accept range: 87 - 87,11 %.) (Aanvaar gebied: 87 – 87,11 %)
(5) [21]

Physical Sciences P2/Fisiese Wetenskappe V2 18 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
QUESTION 8 / VRAAG 8 8.1 • Pressure: 1 atmosphere (atm) / kPa3,101 / 1,013 x 105 Pa
Druk: 1 atmosfeer (atm) / kPa3,101 / 1,013 x 105 Pa • Temperature/Temperatuur: 25 °C / 298 K
(2) 8.2 • Platinum is inert / does not react with the H+ ions OR acid.
Platinum is onaktief / reageer nie met die H+-ione OF suur nie. • Platinum is a conductor (of electricity). Platinum is 'n geleier (van elektrisiteit).
(2) 8.3 8.3.1
Salt bridge / Soutbrug
(1)
8.3.2 -0,31 V (1) 8.3.3 2H+ + 2e- → H2 Marking guidelines / Nasienriglyne:
• 2H+ + 2e- H2 21 H2 2H+ + 2e- 2
0
H2 ← 2H+ + 2e- 22 H2 → 2H+ + 2e- 2
0
(2) 8.4 8.4.1 POSITIVE MARKING FROM QUESTION 8.3.2.
POSITIEWE NASIEN VAN VRAAG 8.3.2 .
θθθ −= oxidationreductioncell EEE
2,05 = -0,31 – θ+2M/M
E θ
+2M/ME = -2,36 (V)
M is magnesium/ Mg.
Option 2/ Opsie 2
M→ M2+ + 2e- E° = 2,36 (V) X2+ + 2e- → X E° = -0,31 (V) E° = 2,05 V Thus/Dus: E θ
reduction = - 2,36 (V) M is magnesium/ Mg.
Notes / Aantekeninge: Accept any other correct formula from the data sheet. Aanvaar enige ander korrekte formule vanaf gegewensblad.
Any other formula using unconventional abbreviations, e.g. θθθ −= RAOAcell EEE followed
by correct substitutions: 5
4
Enige ander formule wat onkonvensionele afkortings gebruik bv. θ
RM
θ
OM
θ
sel EEE −= gevolg deur korrekte vervangings: 5
4
Notes / Aantekeninge Give mark for Mg / magnesium ONLY if concluded from -2,36 V. Ken punt vir Mg / magnesium slegs toe indien afgelei uit -2,36 V
(5) 8.4.2 Exothermic / Eksotermies (1)

Physical Sciences P2/Fisiese Wetenskappe V2 19 DBE/November 2014 NSC/NSS - Memorandum
Copyright reserved/Kopiereg voorbehou Please turn over/Blaai om asseblief
8.5 The cell reaction reaches equilibrium.
Die selreaksie bereik ewewig.
Notes / Aantekeninge: Accept: One or more of reactants are used up. / The cell reaction has run to completion. Aanvaar: Een of meer van reaktanse word opgebruik. / Die selreaksie het volledig verloop.
(1) [15] QUESTION 9 / VRAAG 9
9.1 Electrolytic / Elektrolities (1)
9.2 Q & T Cu2+ + 2e → Cu
Notes / Aantekeninge: IF more than TWO electrodes, mark first two. Indien meer as TWEE elektrodes, sien eerste twee na.
Marking guidelines / Nasienriglyne
Cu2+ + 2e Cu ( 21 ) Cu → Cu2+ + 2e- ( 2
0 )
Cu ← Cu2+ + 2e ( 22 ) Cu Cu2+ + 2e- ( 2
0 )
(4)
9.3 9.3.1
Cℓ2 / chlorine (gas) / chloor(gas)
(1)
9.3.2 Cu2+ (ions) / copper(II) ions / CuCℓ2 / copper(II) chloride Cu2+ (ione) / koper(II)-ione / CuCℓ2 / koper(II)chloried
(1)
9.4 Cu is a stronger reducing agent than Cℓ- (ions) and Cu will be oxidised (to Cu2+). Cu is 'n sterker reduseermiddel as Cℓ-(-ione) en Cu sal geoksideer word (na Cu2+). OR/OF Cℓ- (ions) is a weaker reducing agent than Cu and Cu will be oxidised (to Cu2+). Cℓ-(-ione) is 'n swakker reduseermiddel as Cu en Cu sal geoksideer word (na Cu2+).
(3) [10]
-

Physical Sciences P2/Fisiese Wetenskappe V2 20 DBE/November 2014 NSC/NSS – Memorandum
Copyright reserved/Kopiereg voorbehou
QUESTION 10 / VRAAG 10 10.1 10.1.1
Nitrogen / N2 / Stikstof Hydrogen / H2 / Waterstof
(2) 10.1.2 NH3 + HNO3 → NH4NO3 Bal.
Notes / Aantekeninge: • Reactants Products Balancing: Reaktanse Produkte Balansering • Ignore double arrows. / Ignoreer dubbelpyle. • Marking rule 6.3.10. / Nasienreël 6.3.10.
(3) 10.2 Marking criteria / Nasienriglyne:
• Use ratio / gebruik verhouding: 93
• x 20 kg • x 36 / 36 % • Final answer / Finale antwoord: 2,4 kg
OPTION 1 / OPSIE 1:
% N =93 (x 36)
= 12 %
∴m(N) : )kg20(10012 ×
= 2,4 kg
OPTION 2 / OPSIE 2: m(nutrients/voedingstowwe):
10036 (x 20) = 7,2 kg
∴m(N) 93= x 7,2
= 2,4 kg
OPTION 3 / OPSIE 3: m(N):
kg4,2)10036
()20(93 =×××
(4) [9]
TOTAL/TOTAAL : 150