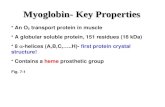
Myoglobin Site: muscles Structure: Monomer (one globin chain), tertiary structure Function: oxygen...
-
Upload
harry-tyler -
Category
Documents
-
view
220 -
download
0
Transcript of Myoglobin Site: muscles Structure: Monomer (one globin chain), tertiary structure Function: oxygen...

Myoglobin
•Site: muscles•Structure: Monomer (one globin chain) , tertiary structure
•Function: oxygen storage
Binding : - binds oxygen more tightly
-1 heme group and binds 1 O2

Hemoglobin
Site: red blood cellsStructure :
Tetramer (4 globin chains); 4 heme groups and bind 4 O2 quaternary structure
Function :
oxygen carrier fromthe lung to tissues
•Binding: binds oxygen less tightly•) HbA=α2β2(


Types of Hb:
Hb A or HbA1 (Adult Hb): is the normal Hb in adults represents about 97% of total Hb. it is composed of 2 α and 2 β chains.
HbA2: minor adult Hb, comprised 3% of normal adult Hb. Composed of 2 α and 2 δ chains
HbF(fetal Hb, α2γ2 Hb): is the main Hb during fetal life and in newborns then disappear gradually where it is replaced by Hb A shortly after birth.It is composed of 2α and 2 γ chains.
Hb F has greater affinity for O2 than HbA so ensure O2 transfer from maternal circulation to fetus RBCs through placenta (through umbelical vein) .
Note: The overall hemoglobin composition in a normal adult is approximately 97.5% HbA1, 2% HbA2 and 0.5% HbF .

Mutations in hemoglobin (hemoglobinopathies):
Sickle cell anemia (Hb S disease):It is a genetic disorder of blood caused by mutation in β-globin chain resulting in the formation of Hb S. The mutation occurs in 6th position of β-chain where glutamic acid is replaced by valine (non polar). Valine residues aggregate together by hydrophobic interactions leading to precipitation of Hb within RBCs. RBCs assume sickle-shaped leading to fragility of their walls and high rate of hemolysis.


Such sickled cells frequently block flow of blood in narrow capillaries and block blood supply to tissue (tissue anoxia) causing pain and cell death.Note: The life time of erythrocyte in sickle cell is less than 20 days, compared to 120 days for normal RBCs.


Patients may be : Heterozygotes (HbAS):
There are two copies of the gene that synthesize β globin chain. In Hb AS, mutation occurs only in one copy of the gene so patients have some normal Hb A. These patients have sickle cell trait with no clinical symptoms and can have normal life span.
Or: Homozygotes (Hb SS): mutation occurs in both
copies of gene that synthesize β-globin chain with apparent anemia and its symptoms

Genes responsible for synthesis of Hb molecule (α and β globin chains)

Electrophoresis:
It is the movement of charged molecules in an electrical field towards the oppositely charged electrode.It is used for separation of proteins for diagnosis of some diseases such as immune disease , genetic diseases (such as Hb S and Hb C diseases. The rate of movement of charged molecules depends on the of charge on the molecule
Technique of electophoresis:In the standard method, protein samples are applied to plate coated with gel close to the cathode end. The plate is then immersed in alkaline buffer (pH 8.6), so proteins in this pH will be negatively charged.

2) The plate is connected to two
electrodes and a current is passed
through the plate to separate
proteins.
3) As proteins are negatively
charged, they will move toward
anode (positive electrode). They
move according to charge on
protein.

Electrophoresis

Electrophoresis apparatus

Use of Protein Electrophoresis to diagnose sickle cell
HbA moves faster than HbS because it is more negatively chareged (glutamic acid (negatively charged) in Hb A is replaced by valine (non polar in HbS). Carriers have both HbA and HbS so the sample will be separated as two bands (one for HbA , the faster) and the second for HbS.

Hb C disease: Like HbS, Hb C is a mutant Hb in which glutamic
acid in 6th position of β-chain is replaced by lysine. RBCs will be
large oblong and hexagonal leading to mild anemia.

Hb A, S, and C after electrophoresis