Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the...
Transcript of Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the...
![Page 1: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/1.jpg)
Modulation of bacterial outer membrane vesicleproduction by envelope structure and contentSchwechheimer et al.
Schwechheimer et al. BMC Microbiology 2014, 14:324http://www.biomedcentral.com/1471-2180/14/324
![Page 2: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/2.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 DOI 10.1186/s12866-014-0324-1
RESEARCH ARTICLE Open Access
Modulation of bacterial outer membrane vesicleproduction by envelope structure and contentCarmen Schwechheimer1, Adam Kulp2 and Meta J Kuehn1*
Abstract
Background: Vesiculation is a ubiquitous secretion process of Gram-negative bacteria, where outer membranevesicles (OMVs) are small spherical particles on the order of 50 to 250 nm composed of outer membrane (OM) andlumenal periplasmic content. Vesicle functions have been elucidated in some detail, showing their importance invirulence factor secretion, bacterial survival, and biofilm formation in pathogenesis. Furthermore, OMVs serve as anenvelope stress response, protecting the secreting bacteria from internal protein misfolding stress, as well as externalenvelope stressors. Despite their important functional roles very little is known about the regulation and mechanism ofvesicle production. Based on the envelope architecture and prior characterization of the hypervesiculation phenotypesfor mutants lacking the lipoprotein, Lpp, which is involved in the covalent OM-peptidoglycan (PG) crosslinks, it isexpected that an inverse relationship exists between OMV production and PG-crosslinked Lpp.
Results: In this study, we found that subtle modifications of PG remodeling and crosslinking modulate OMVproduction, inversely correlating with bound Lpp levels. However, this inverse relationship was not found in strainsin which OMV production is driven by an increase in “periplasmic pressure” resulting from the accumulation of protein,PG fragments, or lipopolysaccharide. In addition, the characterization of an nlpA deletion in backgrounds lacking eitherLpp- or OmpA-mediated envelope crosslinks demonstrated a novel role for NlpA in envelope architecture.
Conclusions: From this work, we conclude that OMV production can be driven by distinct Lppconcentration-dependent and Lpp concentration-independent pathways.
BackgroundOuter membrane vesicles (OMVs) bud from the outermembrane (OM) of Gram-negative bacteria [1-4]. Thesespherical particles are composed of outer membraneentrapping lumenal periplasmic content [3] and have adiameter of around 50 to 250 nm, as visualized by elec-tron and atomic force microscopy [4,5]. Predominately,studies of OMV function have centered around topicsrelated to pathogenesis, such as their role in the dissem-ination of virulence factors and genetic material, as well asdegradation enzymes (proteases, hydrolases and lipases)which allow protection of an ecological niche and acquisi-tion of nutrients in addition to the nucleation of biofilms[2,6-9]. OMV production is also an envelope stressresponse and a reduction in vesiculation under stressfulconditions is harmful to the bacterial cells [10-17]. Our
* Correspondence: [email protected] of Biochemistry, Duke University Medical Center, Durham, NC27710, USAFull list of author information is available at the end of the article
© 2014 Schwechheimer et al.; licensee BioMedCreative Commons Attribution License (http:/distribution, and reproduction in any mediumDomain Dedication waiver (http://creativecomarticle, unless otherwise stated.
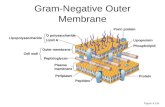
understanding of the mechanism and regulation of OMVproduction, however, remains extremely fragmented.The Gram-negative envelope consists of a cytoplasmic
or inner membrane (IM) and the OM, separated by theperiplasmic space which contains the peptidoglycan (PG)sacculus [18]. The OM of Gram-negative bacteria is asym-metric with the inner leaflet composed of phospholipidsand the outer leaflet composed of lipopolysaccharide(LPS) [19-21]. The PG is a highly dynamic polymer,especially during cell growth and growth phase transi-tions [22]. For envelope stability, the OM is tethered tothe PG sacculus via an abundant OM lipoprotein, Lpp,by covalent crosslinking [23-26].It has been long-appreciated that the OM must dissoci-
ate from the underlying PG for an OMV bud to form[27,28]. Indeed, the complete loss of envelope stabilizingfactors leads to extremely high OMV production, althoughthis is accompanied by a loss of membrane integrity andcellular leakage [4,29,30]. Since wild-type (WT) bacteria innormal and in inducing conditions, along with numerous
Central. This is an Open Access article distributed under the terms of the/creativecommons.org/licenses/by/4.0), which permits unrestricted use,, provided the original work is properly credited. The Creative Commons Publicmons.org/publicdomain/zero/1.0/) applies to the data made available in this
![Page 3: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/3.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 2 of 12
hypervesiculation mutants, produce OMVs without com-promising envelope stability [12,15,17,31-33], a moremoderate and regulated modulation of envelope struc-ture must be present that can yield OMVs.We hypothesized that alterations in the PG structure
underlying the OM could be a means by which cells maymodulate OMV production in either direction. This ideais strengthened by data demonstrating that the deletion ofthe amidase autolysin in Porphyromonas gingivalis, anenzyme that cleaves PG amide bonds, led to an increase inOMV production [34]. The opposite effect, however, thatincreased crosslinking leads to hypovesiculation, has neverbeen observed.The IM lipoprotein, NlpA is one of very few envelope
components that have been characterized and found tohave a dominant effect on OMV production. It waspreviously established that the loss of NlpA causeddecreased OMV production in an otherwise WT strain[15,31], and suppressed the protein accumulation-driven hypervesiculation phenotype of the ΔdegP mu-tant, which lacks the periplasmic protease/chaperoneDegP [15].In this study, we analyzed the effect on OMV produc-
tion of mutations that alter PG structure and Lpp cross-linking. We were also curious whether bound Lpp levelsdictate vesiculation levels for bacteria under inducingconditions, particularly those involving build-up of ma-terial in the periplasm. We investigated bound Lpp levelsfor mutants in which periplasmic misfolded protein, PGfragments, or LPS accumulation led to upregulated OMVproduction. Finally, we investigated the genetic interac-tions between nlpA and genes encoding envelope modify-ing and stabilizing proteins.
ResultsOMV production and Lpp crosslinking changes inverselywith altered PG structureTo examine the relationship between modulation of PGstructure and levels of OMV production, we examined aPG hydrolase mutant, ΔmepAΔdacBΔpbpG, which lacksthree of the endopeptidases that cleave the PG peptidebonds. We observed that OMV production increased inthis triple mutant strain (Figure 1A). This strain, alongwith all other strains used in this work, were tested formembrane integrity using previously published assays[15] (Additional file 1: Table S1) so that we could besure that the measured OMV fractions were not inflatedwith the presence of membrane fragments released as aconsequence of instability.Next, we examined the Lpp crosslinking levels of the
ΔmepAΔdacBΔpbpG strain. We used an immunoblot-ting assay that allows us to distinguish between the PGcrosslinked form of Lpp, and the OM lipid-anchored butuncrosslinked form of Lpp (historically referred to as the
‘bound’ and the ‘free’ form, respectively). As expected, wefound an inverse relationship between OMV productionand bound Lpp (Figure 1B). The amount of free Lppwas comparable to WT (Figure 1C), suggesting that theobserved decrease is not a result of an overall decreasein Lpp.We also investigated the L,D-transpeptidase ΔynhG-
ΔycbB double mutant, which contains the commonD-Alanine (D-Ala)-Diaminopimelic acid (DAP) pep-tide crosslinks but lacks the minor DAP-DAP crosslinks[35]. We were especially interested in this strain in lightof its relationship to Lpp crosslinking, since it had beenshown that DAP-DAP muropeptides are enriched incovalently crosslinked Lpp [36]. Interestingly, the loss ofDAP-DAP crosslinks correlated with a strong hypovesi-culation phenotype, ~ 60% lower than WT (Figure 1D).When examining the Lpp crosslinking levels of this mu-tant we found a significant increase in covalently attachedLpp (by ~ 2.6 fold, Figure 1E), whereas the concentrationof free Lpp resembled WT levels (Figure 1C). It shouldbe noted that this was the first strain in which we ob-served an increase in bound Lpp, demonstrating thatbound Lpp levels can have a dynamic range in bothdirections. Taken together, these data suggest that themodulation of PG structure can alter the levels of OMVproduction in either direction via an inverse relation-ship to PG-Lpp crosslinking.
The inverse relationship between Lpp crosslinks and OMVproduction does not hold for mutants that accumulateperiplasmic proteinThe data presented above demonstrated that for strainscontaining mutations directly affecting PG structure,vesiculation inversely correlates with the cellular concen-tration of Lpp crosslinks. We were curious whether wewould observe a decrease in the level of covalent Lppcrosslinking when increased OMV production was in-duced by misfolded protein build-up, as is the case inthe ~40-fold OMV hypervesiculating ΔdegP mutant[15]. We quantified covalent Lpp crosslinks in the ΔdegPstrain and found that Lpp crosslinking levels were notdifferent from the WT (Figure 2A). In control experi-ments, free Lpp in the mutant were also not statisticallysignificant from WT levels (Figure 2B). These datasuggest that the accumulation of periplasmic proteincreates an increase in periplasmic pressure, which inturn leads to hypervesiculation, but that this occurswithout altering the total numbers of Lpp crosslinks.
Accumulation of PG fragments correlates with increasedOMV production without altering Lpp crosslinkingWe further examined vesiculation and envelope cross-linking in another case where envelope products accu-mulate in the periplasm. The ΔampG mutant lacks the IM
![Page 4: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/4.jpg)
Figure 1 OMV production and Lpp crosslinking changes inversely with altered PG structure. (A) Relative fold OMV production in culturesof the indicated strains grown in LB overnight at 37°C was determined by quantitating OMVs by OMPs, normalizing to OD600, and dividing byOD600-normalized OMV production in a WT culture. (B) Relative fold crosslinked Lpp in cultures of the indicated strains grown in LB to an OD600
of ~ 0.4 at 37°C was determined by immunoblotting of PG copurified Lpp, normalizing to OD600, and dividing by OD600-normalized crosslinkedLpp in a WT culture. (C) Relative fold of free Lpp in cultures of the indicated strains grown overnight in LB was determined by quantitativeimmunoblotting of Lpp in whole cell preparations, normalizing to OD600, and dividing by OD600-normalized Lpp in a WT culture. (D) Relative foldOMV production in cultures of the indicated strains grown in LB overnight at 37°C was determined as in part A. (E) Relative fold crosslinked Lppin cultures of the indicated strains grown in LB to an OD600 of ~ 0.4 at 37°C was determined as in part B. Error bars indicate standard error of themean (SEM). p values refer to comparisons with WT. *, p≤ 0.05; n ≥ 3.
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 3 of 12
permease AmpG, and this mutant is impaired in transport-ing muropeptides from the periplasm to the cytoplasm forPG recycling [37]. We utilized the ΔampGΔamiD doublemutant which also lacks the amidase AmiD, causing largePG fragments to accumulate in the periplasm because theyare also too large to fit through the porins [38]. Whenwe examined OMV production, we determined thatthe ΔampGΔamiD mutant exhibited ~ 14-fold increased
OMV production with respect to WT (Figure 2C). Thesedata supported the hypothesis that periplasmic accumula-tion of PG fragments caused their subsequent sheddinginto the medium via OMVs. Direct verification and quan-titation of PG fragments in the OMVs is extremely chal-lenging, technically, and therefore was not able to bedetermined in the scope of this study. To investigate thestate of the envelope for this strain, free and crosslinked
![Page 5: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/5.jpg)
Figure 2 Accumulation periplasmic PG fragments or protein correlates with increased vesiculation without alteration of Lpp crosslinking.(A) Relative fold crosslinked Lpp in cultures of the indicated strains grown in LB to an OD600 of ~ 0.4 at 37°C was determined as described in Figure 1B.(B) Relative fold of free Lpp in cultures of the indicated strains grown overnight in LB at 37°C was determined as described in Figure 1C. (C) Relativefold OMV production in cultures of the indicated strains grown in LB overnight at 37°C was determined as described in Figure 1A. Error bars indicateSEM. p values refer to comparisons with WT unless indicated by a bracket. *, p≤ 0.05; NS, p > 0.05; n≥ 3.
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 4 of 12
Lpp levels were measured. No difference in the levelsof bound or free Lpp were found for the ΔampGΔamiDmutant and the WT strain (Figure 2A and B). In sum, amutant in which PG fragments accumulate in the peri-plasm hypervesiculates without exhibiting altered totallevels of Lpp crosslinking, similar to the effect when proteinaccumulation drives vesiculation.
LPS accumulation also leads to hypervesiculation withoutmodulating bound Lpp concentrationWe next reasoned that accumulation of LPS fragmentscould generate a similar effect to induce OMV produc-tion as either the accumulation of PG fragments or peri-plasmic protein. Data published recently indicate thatindividual mutations that alter the sugar core structure ofLPS (ΔrfaC, ΔrfaG, and ΔrfaP) lead to periplasmic LPSaccumulation due to the disruption of LPS maturation inthe envelope of the cell [39]. Additional evidence furthersupports the concept of periplasmic LPS accumulation:rfaC and rfaG mutant strains contain an increased amount
of LPS in comparison to WT [40,41], and furthermoreincreasing LPS production leads to abnormal structures inthe periplasm, implying that LPS overproduction results ina reduction of proper, OM-localized LPS, but not a reduc-tion in the overall amount of LPS in the envelope [42].As expected, all three LPS core mutants exhibited
hypervesiculation phenotypes (Figure 3A). However, it isrecognized that mutant strains ΔrfaC, ΔrfaG, and ΔrfaPactivate the σE envelope heat shock response, a processdiscovered to require both mislocalized, periplasmic LPSas well as a misfolded outer membrane protein (OMP)component for activation [39]. Since σE activation impli-cated the presence of misfolded OMPs, and previous workfrom our lab showed that periplasmic protein accumula-tion leads to hypervesiculation, we needed to determine ifthe reason for hypervesiculation in the ΔrfaC, ΔrfaG, andΔrfaP mutants was not actually solely due to increasedperiplasmic protein levels. We measured the amountof periplasmic protein in the mutants and found thatthe ΔrfaC and ΔrfaG mutants contained WT levels
![Page 6: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/6.jpg)
Figure 3 Accumulation of periplasmic LPS correlates with increased OMV production without alteration of Lpp crosslinking. (A) Relativefold OMV production in cultures of the indicated strains grown in LB overnight at 37°C was determined using FM4-64 and normalized to CFU as inFigure 1A. (B) Protein concentrations in periplasm preparations of the indicated strains grown ~16-18 hrs in LB at 37°C were determined by BradfordAssay. (C) Relative fold crosslinked Lpp in cultures of the indicated strains grown in LB to an OD600 of ~ 0.4 at 37°C was determined as described inFigure 1B. (D) Relative fold of free Lpp in cultures of the indicated strains grown in LB at 37°C was determined as described in Figure 1C. (E) The lipidto protein ratio in the OMVs purified from cultures of the indicated strains grown in LB overnight at 37°C was determined by dividing the amount oflipid, measured using FM4-64, by the OMP concentration, measured by densitometry. (F) Relative fold OMV production in cultures of the indicatedstrains grown in LB overnight at 37°C was determined as described in Figure 1A. Error bars indicate SEM. *, p≤ 0.05; n≥ 3.
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 5 of 12
(Figure 3B), supporting the hypothesis that it was theincrease in periplasmic LPS, not protein, which led tohypervesiculation. The periplasmic protein concentra-tion in the ΔrfaP strain was significantly higher thanthat of the WT (Figure 3B), thus we could not distin-guish whether the hypervesiculation phenotype of thismutant resulted from accumulation of periplasmic proteinor LPS, or a combination of these.
Next, we determined the amount of covalently cross-linked Lpp in these LPS mutants in order to see if theseinversely correlated with the OMV phenotypes. CovalentLpp crosslinking was unchanged with respect to WT forΔrfaC; ΔrfaG and ΔrfaP exhibited a slight reduction,albeit not statistically significant (Figure 3C). In controlexperiments, the amount of free Lpp in the strains was alsonot significantly different from WT (Figure 3D). These
![Page 7: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/7.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 6 of 12
results support the hypothesis that OMV production inthese LPS mutants is predominantly driven by accumu-lated material rather than a decrease in overall covalentLpp crosslinking.To test our model that the accumulation of mislocal-
ized envelope lipid in the cell leads to its secretion viaOMVs, we assessed whether OMVs produced by theseLPS mutants were enriched in lipid. We quantified thelipids in OMVs using a lipophilic dye (FM4-64) thatbecomes fluorescent upon membrane intercalation.These values were then divided by the quantity ofOMPs in the OMVs from each of the strains. Theresults show a four-fold increase in the lipid to OMPratio for ΔrfaC OMVs, a 15-fold increase for ΔrfaG,and a 29-fold increase for ΔrfaP OMVs, with respect tothe WT OMV control, confirming lipid accumulationin the OMVs of ΔrfaC, ΔrfaG, and ΔrfaP (Figure 3E).These data strongly support the idea that accumulatedlipid, LPS, is in the secreted OMVs.
A complex role for NlpA in envelope architectureTo further investigate the envelope architecture of peri-plasmic accumulation-induced hypervesiculating mutants,we tested the effect of deleting nlpA in those strains.The loss of the IM lipoprotein, NlpA, decreases OMVproduction in an otherwise WT strain [15,31] and sup-presses the hypervesiculation phenotypes of the protein-accumulating, ΔdegP mutant [15]. Interestingly, however,the ΔnlpA mutation was not epistatic to ΔampGΔamiD,and the ΔnlpAΔrfaP double mutant still produced a sig-nificantly increased amount of OMVs (Figures 2C and 3F).These data suggested that membrane architecture differsfor hypervesiculating strains containing different accumu-lated periplasmic products.We next examined the relationship between nlpA and lpp.
We initially constructed and characterized a ΔnlpAΔlppdouble mutant. We found that ΔnlpA appeared to have noeffect on the phenotype of the Δlpp strain (Additional file 1:Figure S1), however the high level of “vesicles” pro-duced by either the lpp mutant or the double mutantare probably not true OMVs. The loss of Lpp, the mostabundant E. coli protein [18,24], makes the envelope ofthis strain quite fragile [23,29,30], and it is likely thatany additional structural stress (e.g. from the nlpA dele-tion) would be negligible. Therefore, we turned to thetriple L,D-transpeptidase mutant ΔycfSΔybiSΔerfK whichlacks the three enzymes which from the covalent crosslinkbetween Lpp and PG [43] and exhibits a hypervesiculationphenotype (43-fold) [4] (Figure 4A). The addition ofthe nlpA mutation to the triple mutant led to an in-crease in vesiculation (Figure 4A). Since we previouslydemonstrated that the loss of nlpA does not manifestits OMV phenotype until stationary phase [15], wewere curious to examine whether OMV production in
the triple mutant was unaffected by the lack of nlpA dur-ing log phase growth. To test this, we quantified OMVs inthe supernatant of log-phase cells and found that the dele-tion of nlpA increased vesiculation in the ΔycfSΔybiSΔerfKL,D-transpeptidase mutant (Figure 4B). These resultssupported the concept that nlpA plays a critical role instabilizing the envelope under particular conditions.We also examined whether the loss of nlpA showed
genetic interactions with other envelope stabilizingfactors. Previous genetic and crosslinking studies haveshown that there is an interaction between Lpp andOmpA [30,44,45], an OM β-barrel protein with a peri-plasmic PG-interaction domain [46,47]. A deletion inompA, which encodes OmpA, resulted in ~26-foldhypervesiculation (Figure 4C), consistent with the phe-notypes of the ΔompA Salmonella and Vibrio choleraemutants [29,48]. We tested if the nlpA deletion wasepistatic to ΔompA. Similar to the results for the tripleL,D-transpeptidase mutant, the ΔnlpA mutation alsoexacerbated the ΔompA hypervesiculation phenotypesin both overnight (Figure 4C) and log phase cultures(Figure 4D). Together, these data support a complexrole for NlpA, depending on envelope conditions. Spe-cifically, NlpA is critical to the ability to increase OMVsin conditions of protein, but not PG fragment and lipidaccumulation, and the loss of NlpA increases hypervesi-culation when levels of envelope stabilizing factors aredecreased.
DiscussionDespite investigations revealing that OMVs function incritical areas such as pathogenesis, bacterial survival,and envelope stress, our knowledge of the mechanismand regulation of OMV production has remained quitecryptic. To gain mechanistic insight into OMV produc-tion, we analyzed the effect of specific gene mutationson OMV phenotypes and their relationship to cell en-velope structure. The results begin to reveal a complexrelationship between envelope remodeling, crosslink-ing, periplasmic content, and OMV production. Wehave shown here that multiple routes modulate vesicu-lation: one that is dependent on and one that is inde-pendent of the overall concentration of bound Lpp.Both of these pathways appear to be stimulated by mul-tiple factors: cellular covalent Lpp crosslinking can bealtered by changes in PG structure, whereas envelopeaccumulation of material (protein, PG fragments andLPS, or a combination of these), as well as the loss ofthe IM lipoprotein NlpA in a background lackingbound Lpp or OmpA, result in hypervesiculation withminimal or no contribution from overall changes inbound Lpp levels. The data are summarized in a set ofworking models in Figure 5.
![Page 8: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/8.jpg)
Figure 4 The effect of ΔnlpA on vesiculation phenotypes. Relative fold OMV production in cultures of the indicated strains grown in LB at37°C overnight (A, C) or to an OD600 of ~ 0.4 (B, D) was determined as described in Figure 1A. p values refer to comparisons with WT (A, C) orindicated background strain (B, D). Error bars indicate SEM. Statistical comparisons are with WT (A, C) or mutant control strains (B, D) unlessdenoted by a bracket. *, p≤ 0.05; n ≥ 4.
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 7 of 12
Fine-tuning of OMV production through PG biosynthesisand structureThe level of Lpp crosslinking was investigated for mu-tants with moderate PG structure effects that also exhib-ited increased and decreased levels of vesiculation. Herewe report that the triple endopeptidase deletion mutanthypervesiculates and exhibits a decrease in covalent Lppcrosslinking (Figure 1B). This hypervesiculation pheno-type was notably consistent with the previous P. gingivalisendopeptidase mutant [34]. The opposite situation wasfound with the loss of the genes responsible for the minorDAP-DAP PG crosslinks (ΔynhGΔycbB). In this mutant,OMV production is lower than WT (Figure 1D), with aconcomitant increase in bound Lpp (Figure 1E). These datasupport a model in which PG dynamics directly modulatethe number of covalent envelope crosslinks and, thereby,indirectly modulate OMV production (Figure 5A).The increase in bound Lpp for the ΔynhGΔycbB strain
was particularly interesting in light of a previous reportwhich showed that PG-Lpp crosslinks are enriched atsites of DAP-DAP crosslinks [36]. We hypothesize thateither DAP-DAP crosslinks could serve as “locationmarkers” for crosslinking of Lpp and that in the absence
of these markers, Lpp crosslinking to the PG is morerandom and more distributed across the PG sacculus, oralternatively, that the residues typically involved in theDAP-DAP crosslinks may be utilized for Lpp crosslink-ing in this mutant.
OMV production relieves stress caused by the accumulationof diverse, potentially harmful products in the envelopeHere we demonstrate that the accumulation of periplas-mic PG fragments and LPS leads to an increase in OMVproduction. These data are consistent with the previ-ously described role of OMVs in relieving protein-mediated envelope stress induced by a σE-stimulatingmodel misfolded polypeptide and the lack of the DegPprotease [12,15]. In addition, we detected increased ra-tios of lipid:protein in LPS mutant strain OMVs, whichindicates accumulated LPS cargo enrichment in OMVs.Similarly, the σE-stimulating model misfolded polypep-tide was enriched in OMVs, and misfolded DegP sub-strates were present in OMVs purified from the DegPprotease-deficient strain [12,15]. PG in OMVs from PGaccumulating strains could not be detected due to tech-nical limitations, however it should be mentioned that
![Page 9: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/9.jpg)
Figure 5 Mechanistic working models of OMV production and modulation. Models of how changes in envelope structure lead tomodulation of OMV production are based on the data presented here and in prior studies, as described in the text. (A) Modulation of PGstructure and metabolism can up- and downregulate OMV production through levels of bound Lpp (circled in red). (B) Periplasmic bulk accumulation(red aggregates) leads to hypervesiculation without altering bound Lpp levels. (C) The IM anchored lipoprotein NlpA contributes to envelope integrityin conjunction with Lpp and OmpA.
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 8 of 12
the ΔampGΔamiD mutant strain releases large PG frag-ments into the cell-free medium [38], and since theseare too large to diffuse through the OM porins, thisobservation is consistent with their secretion via OMVs.We have previously found that vesiculation enhances
survival in cases of periplasmic protein accumulation[12,15] and can now extend this model to include theshedding of LPS via OMVs. Very recently, YciM was iden-tified as a negative regulator of LPS biosynthesis, and anexcess of LPS was confirmed to be responsible for the
death of yciM mutants [49]. Interestingly, they report thatsuppressor mutations include those that either downregu-late LPS biosynthesis via other routes, or they are part ofa group of genes that is involved in OM assembly ororganization (lpp, rfaP, ybcN, galU). Notably, all the mu-tants from the second group hypervesiculate (A. Kulp,A. Manning, B. Sun, T. Ai, D. Rodriguez, A. Schmidt, andM. Kuehn, unpublished data) [29,50].These results further establish the general and import-
ant role OMV production plays in bacterial well-being,
![Page 10: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/10.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 9 of 12
but we considered why bacteria do not simply expandtheir periplasm to accomodate the excess material with-out the concomitant loss of “macromolecular energy” thatresults from OMV release. In fact, it has been shown thatthe eukaryotic endoplasmic reticulum membrane expandsto adapt to an increase in misfolded protein [51]. Theanswer is straightforward when considering the bacterialenvelope architecture: The OM and PG are connected byLpp, a finite covalent crosslink. With such a constraint,either the concentration of misfolded/mislocalized enve-lope material could increase, the level of crosslinks coulddecrease, or the membrane could bulge out. High concen-trations of material could become toxic to the properfunction of the envelope cells [15,52], therefore this isnot a viable option. Unlike the situation for PG struc-tural mutants, overall bound Lpp levels do not changeunder conditions of periplasmic accumulation, suggest-ing more localized changes in the envelope architecturewere responsible for OMV generation (see model,Figure 5B). Apparently, the trapped periplasmic materialcannot prevent the formation of bound Lpp, but insteadpushes the OM outward, either by taking advantage of“nanoterritories” of OM containing locally decreasedlevels of bound Lpp, or by displacing bound Lpp to siteson the periphery of the outwardly bulging OM. Subse-quent spontaneous membrane fusion events, could thenresult in OMV budding and release.
The contribution of NlpA to envelope architectureThe data demonstrating that the loss of nlpA increasedOMV production in strains that were also missing the en-velope stabilizing factors, bound Lpp and OmpA, (Figure 4)led us to hypothesize a structural role of the IM lipid-anchored protein, NlpA, within the envelope thatdepended on these other factors: NlpA could providean IM-based scaffolding site to stabilize the sites ofLpp- and OmpA-based envelope crosslinks as depictedin our working model (Figure 5C). This is supported bythe observations that NlpA is most critical during sta-tionary phase [15], at a time when Lpp-PG crosslinkinghas been shown to increase [36]. But, if NlpA helps tostabilize crosslinks, why would the ΔnlpA strain thenhave a hypovesiculation phenotype? We propose thatother factors in the envelope that depend on boundLpp or OmpA are overcompensating for the loss ofnlpA in this mutant, creating a more tightly crosslinkedenvelope. Interestingly, the undervesiculation pheno-type of the ΔnlpA strain is manifested in stationaryphase, whereas the phenotypes presented in this workare already present in log phase, suggesting that thefactor in the ΔnlpA strain that can (over)compensatefor NlpA only appears late in the cell cycle. Notably,vesiculation levels did not change when nlpA was de-leted in mutants that directly affect PG components
(ΔampGΔamiD and ΔnlpI) (Figure 2C and Schwechheimeret al, [58]). Further work is necessary to fully elucidate theaccessory role of NlpA in the envelope and in OMVbiogenesis.
ConclusionsImplications for regulated OMV production by WT bacteriaIn sum, these data reveal that OMV levels are not solelydictated by Lpp crosslinking; at least two mechanisms canalter OMV budding, one dependent on and the other inde-pendent of overall levels of Lpp crosslinking. Our resultshelp us to understand how WT bacteria might regulateOMV levels in different situations and times in their lifecycle. In the first, cells could use localized or cell-cycle(temporal) modulation of the PG structure by modifyingthe equilibrium between PG synthesis and degradation toaffect overall bound Lpp and, consequently, OMV levels. Inthe other, bulk deposition of envelope material within theperiplasm, as a result of a localized secretion apparatus or astress response, could allow outward bulging of the OMand ultimately OMV release at areas with locally-reducedamounts of bound Lpp or by relocating bound Lpp. As acomplex entity whose integrity must be preserved for theviability of the cell, the envelope is modulated by numerousother factors, such as OmpA and NlpA, which contributein specific ways to the modulation of the envelope architec-ture. Although many of the envelope components studiedhere are conserved amongst other Gram-negative bacterialspecies, further investigation is required to understandwhether these principles regarding the modulation ofOMV production are also conserved in other species.
MethodsGrowth conditions and reagentsStrains used in this work are summarized in Table 1.Bacteria were grown in liquid culture in Luria–Bertani (LB)broth (EM Science) or on plates of solid LB agar supple-mented with 50 mg/mL kanamycin or 100 mg/ mL ampi-cillin (Sigma). The single gene mutants originate from theKeio Collection [53]. To create mutants with multiple dele-tions, the kanamycin resistance marker was removed fromthe single mutant [54]. The additional mutation was thenadded by transduction of the marked gene deletion usingP1 phage [55] from the donor single Keio mutant straininto the unmarked Keio recipient mutant strain. The singleKeio deletion strains, as well as the mutants constructed forthis work were either sequenced with a primer upstreamand downstream of the deleted gene or PCR amplified withprimers upstream/ downstream of the deleted gene and thekanamycin cassette to confirm the genotypes.
OMV purification and quantitationMedia (250 mL) was inoculated (1:250 dilution) from 37°Covernight cultures, and the bacterial cultures grown to an
![Page 11: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/11.jpg)
Table 1 Strains used in this study
Strains Genotype Source/reference
BW25113 rrnB3 ΔlacZ4787 hsdR514 Δ(araBAD)567Δ(rhaBAD)568 rph-1 WT of Keio collection (Baba et al. 2006 [53])
Keio collection single mutants BW25113 with indicated single mutations: ΔnlpA::Kan, ΔdegP::Kan,ΔrfaC::Kan, ΔrfaG::Kan, ΔrfaP::Kan
(Baba et al. 2006 [53])
MK1277 BW25113 ΔycfS, ΔybiS, ΔerfK::Kan (Schwechheimer et al. 2013 [4])
MK1334 BW25113 ΔampG, ΔamiD::Kan This Work
MK1335 BW25113 ΔampG, ΔnlpA, ΔamiD::Kan This Work
MK1336 BW25113 ΔpbpG, ΔdacB, ΔmepA::Kan This Work
MK1337 BW25113 ΔynhG, ΔycbB::Kan This Work
MK1352 BW25113 ΔycfS, ΔybiS, ΔerfK, ΔnlpA::Kan This Work
MK1353 BW25113 ΔnlpA, ΔompA::Kan This Work
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 10 of 12
OD600 ~ 0.4 (for log phase) or grown overnight at 37°C(~16 h). Cells were pelleted with the Beckman Avanti J-25centrifuge (JLA-10.500 rotor, 10 000 g, 10 min, 4°C) andthe resulting supernatants filtered [low protein bindingDurapore membrane, 0.45 μm polyvinylidene fluoride,Millipore]. Filtrates were centrifuged again with the Beck-man Avanti J-25 centrifuge (JLA-16.250 rotor, 38 400 g,3 h, 4°C) followed by another step of centrifugation withthe Beckman Optima TLX Ultracentrifuge if the pelletswere not visible. In these cases, most of the supernatantwas poured off, and the region where pelleted materialshould be was “resuspended” in the residual supernatantand re-pelleted (TLA 100.3 rotor, 41 000 g, 1 h, 4°C).Pellets were resuspended in Dulbecco’s phosphate buff-ered saline with added salt (0.2 M NaCl) (DPBSS), andfilter-sterilized through 0.45 μm Ultra-free spin filters(Millipore). A portion of the filtrate was plated on LBagar and incubated at 37°C overnight to verify that thesuspensions were free of bacteria.To quantitate OMV yield, OMV preparations were boiled
for 6 min in 2× Laemelli buffer, separated by 15% SDS-PAGE, and stained with SYPRO Ruby Red (MolecularProbes) overnight in the dark. Prior to and after stain-ing, the gel was fixed for 1 h in a solution of 10% MeOHand 7% acetic acid. Ruby-stained proteins were detectedunder UV light (Additional file 1: Figure S2 showsrepresentative gels samples). E. coli Omps F/C and A werequantified by densitometry (NIH Image J software). TheOMP density values were divided by the OD600 of theoriginal culture to calculate OMV production and thisvalue was divided by the OMV production of the WT oruntreated control strain to determine relative fold OMVproduction. Measurements of OMV yield using FM4-64was as described previously [31].
PG purification, digestion and quantitation of covalentlycrosslinked LppUnless otherwise indicated, media (500 mL) was inoculated(1:250 dilution) from overnight 37°C bacterial cultures and
cultures grown at 37°C until they reached OD600 ~ 0.4. PGwas isolated from broth cultures based on the protocolby Lam et al. [56]. Briefly, cells were pelleted and resus-pended in PBS after which the ice-cold suspensionswere dropped in an equal volume of vigorously stirring,boiling 10% SDS. Samples were boiled for 4 h and thenincubated at 37°C, continuously shaking, overnight. Thefollowing day, the PG was pelleted with the BeckmanOptima TLX Ultracentrifuge (TLA 100.3 rotor, 80 000 g,15 min, 30°C), resuspended in 1% SDS followed byanother 2 h of boiling. PG was washed four times withdeionized water and finally resuspended in equal volumesof deionized water.Equal fractions of the purified sacculi were digested
with 15 mg/mL chicken egg lysozyme (Sigma-Aldrich)in 10 mM Tris–HCl, pH 8, at room temperature for2 days. Lysozyme digested PG was separated by 15%SDS-PAGE and Lpp was detected by immunoblottingand quantified by densitometry (NIH Image J software).The Lpp density values were divided by the OD600 ofthe original culture to calculate the amount of Lpp thatwas covalently crosslinked to PG, and this value wasdivided by the PG-crosslinked Lpp of the WT strain todetermine relative fold of bound Lpp. We chose to usecell density as the denominator for these experimentsrather than the traditional total PG, since this calcula-tion rather provides insights into the budding dynamicsof the OM.
Quantitation of free LppThis method was adapted from Cowles et al. [57]. A5 ml culture was grown overnight (~16 hrs) in LB at 37°C.1 ml of this culture was spun down in a microfuge (10000 g, 4 min, room temperature), resuspended in 50 μl 1%SDS in PBS and 50 μl 2× Laemelli buffer. Samples wereboiled for 10 min and separated by 15% SDS-PAGE. FreeLpp was detected by immunoblotting and quantified bydensitometry (NIH Image J software). The free Lppdensity values were divided by the OD600 of the original
![Page 12: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/12.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 11 of 12
culture to calculate the amount of free Lpp, and thisvalue was divided by the free Lpp of the WT controlstrain to determine relative fold of free Lpp.
Periplasmic protein contentPeriplasm was isolated and quantified after overnightgrowth (37°C, 16–18 h) using a previously publishedprotocol [15].
FM4-64 lipid analysis of OMVsTo determine the lipid to OMPs ratio within OMVs, oneportion of the purified WT, ΔrfaC, ΔrfaG, and ΔrfaPOMVs were incubated with FM4-64 (Invitrogen), 3.3 g/mlin phosphate-buffered saline (PBS) for 10 min at 37°C.FM4-64 incubated in PBS was used as a negative control.The fluorescence signal was measured with a MolecularDevices SpectraMAX GeminiXS fluorometer (excitation:506 nm, emission: 750 nm). To determine the OMPsconcentration, a second portion of OMVs was treatedas explained above under OMV purification and quanti-tation. Lastly the lipid value was divided by the OMPvalue and normalized to the WT strain.
StatisticsParameters used for the T-test are equal variance due tothe comparison of identical experimental repetitions orunequal variance due to different experimental repeti-tions and a two-tail distribution. For direct sample sizecomparison, the paired T-test was used, and for foldcomparison, the unpaired. The T-test value of ≤ 0.05 wasconsidered statistically significant; if the value was lowerthan 0.05, the significance value is given under the cor-responding data. The number of times each experimentwas repeated (n) is stated in the figure legends.
Additional file
Additional file 1: Supporting information.
Competing interestsThe authors declare that they have no competing interests.
Authors’ contributionsCS conducted experiments, developed and modified the assays, and draftedthe manuscript. AK conducted the rfa mutant vesicle phenotype experiment.MJK helped conceive the study, participated in the experimental design andcoordination, and helped to draft, edit, and finalize the manuscript. All havegiven final approval to this work and have no conflicts of interest to report.
AcknowledgementsThis work was supported by NIH grant R01GM099471. We are grateful forthe generous contributions of Tom Silhavy (Lpp antibody) and the NationalBioResource Project (NIG, Japan) for the E.coli Keio Collection.
Author details1Department of Biochemistry, Duke University Medical Center, Durham, NC27710, USA. 2Department of Molecular Genetics and Microbiology, DukeUniversity Medical Center, Durham, NC 27710, USA.
Received: 27 August 2014 Accepted: 11 December 2014
References1. Berleman J, Auer M: The role of bacterial outer membrane vesicles for
intra- and interspecies delivery. Environ Microbiol 2013, 15(2):347–354.2. Deatherage BL, Cookson BT: Membrane vesicle release in bacteria,
eukaryotes, and archaea: a conserved yet underappreciated aspect ofmicrobial life. Infect Immun 2012, 80(6):1948–1957.
3. Kulp A, Kuehn MJ: Biological functions and biogenesis of secretedbacterial outer membrane vesicles. Annu Rev Microbiol 2010, 64:163–184.
4. Schwechheimer C, Sullivan CJ, Kuehn MJ: Envelope control of outermembrane vesicle production in Gram-negative bacteria. Biochemistry2013, 52(18):3031–3040.
5. Beveridge TJ: Structures of gram-negative cell walls and their derivedmembrane vesicles. J Bacteriol 1999, 181(16):4725–4733.
6. MacDonald IA, Kuehn MJ: Offense and defense: microbial membranevesicles play both ways. Res Microbiol 2013, 163(9–10):607–618.
7. Ellis TN, Kuehn MJ: Virulence and immunomodulatory roles of bacterialouter membrane vesicles. Microbiol Mol Biol Rev 2010, 74(1):81–94.
8. Schooling SR, Beveridge TJ: Membrane vesicles: an overlooked componentof the matrices of biofilms. J Bacteriol 2006, 188(16):5945–5957.
9. Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, Hanawa T,Kamiya S: Outer membrane vesicles of Helicobacter pylori TK1402 areinvolved in biofilm formation. BMC Microbiol 2009, 9:197.
10. McMahon KJ, Castelli ME, Vescovi EG, Feldman MF: Biogenesis of outermembrane vesicles in Serratia marcescens is thermoregulated and canbe induced by activation of the Rcs phosphorelay system. J Bacteriol2012, 194(12):3241–3249.
11. Maredia R, Devineni N, Lentz P, Dallo SF, Yu J, Guentzel N, Chambers J,Arulanandam B, Haskins WE, Weitao T: Vesiculation from Pseudomonasaeruginosa under SOS. Sci World J 2012, 2012:402919.
12. McBroom AJ, Kuehn MJ: Release of outer membrane vesicles byGram-negative bacteria is a novel envelope stress response. Mol Microbiol2007, 63(2):545–558.
13. Manning AJ, Kuehn MJ: Contribution of bacterial outer membranevesicles to innate bacterial defense. BMC Microbiol 2011, 11:258.
14. Manning AJ, Kuehn MJ: Functional advantages conferred by extracellularprokaryotic membrane vesicles. J Mol Microbiol Biotechnol 2013,23(1–2):131–141.
15. Schwechheimer C, Kuehn MJ: Synthetic effect between envelope stressand lack of outer membrane vesicle production in Escherichia coli.J Bacteriol 2013, 195(18):4161–4173.
16. Tashiro Y, Sakai R, Toyofuku M, Sawada I, Nakajima-Kambe T, Uchiyama H,Nomura N: Outer membrane machinery and alginate synthesis regulatorscontrol membrane vesicle production in Pseudomonas aeruginosa.J Bacteriol 2009, 191(24):7509–7519.
17. Macdonald IA, Kuehn MJ: Stress-induced outer membrane vesicle productionby Pseudomonas aeruginosa. J Bacteriol 2013, 195(13):2971–2981.
18. Silhavy TJ, Kahne D, Walker S: The bacterial cell envelope. Cold Spring HarbPerspect Biol 2010, 2(5):a000414.
19. Galloway SM, Raetz CR: A mutant of Escherichia coli defective in the firststep of endotoxin biosynthesis. J Biol Chem 1990, 265(11):6394–6402.
20. Raetz CR: Biochemistry of endotoxins. Annu Rev Biochem 1990, 59:129–170.21. Raetz CR, Whitfield C: Lipopolysaccharide endotoxins. Annu Rev Biochem
2002, 71:635–700.22. Vollmer W, Bertsche U: Murein (peptidoglycan) structure, architecture
and biosynthesis in Escherichia coli. Biochim Biophys Acta 2008,1778(9):1714–1734.
23. Cascales E, Bernadac A, Gavioli M, Lazzaroni JC, Lloubes R: Pal lipoproteinof Escherichia coli plays a major role in outer membrane integrity.J Bacteriol 2002, 184(3):754–759.
24. Braun V: Covalent lipoprotein from the outer membrane of Escherichiacoli. Biochim Biophys Acta 1975, 415(3):335–377.
25. Braun V, Rehn K: Chemical characterization, spatial distribution andfunction of a lipoprotein (murein-lipoprotein) of the E. coli cell wall: thespecific effect of trypsin on the membrane structure. Eur J Biochem 1969,10(3):426–438.
26. Wang Y: The function of OmpA in Escherichia coli. Biochem Biophys Res Commun2002, 292(2):396–401.
![Page 13: Modulation of bacterial outer membrane vesicle …...Outer membrane vesicles (OMVs) bud from the outer membrane (OM) of Gram-negative bacteria [1-4]. These spherical particles are](https://reader033.fdocuments.us/reader033/viewer/2022060305/5f0965c97e708231d426a4d6/html5/thumbnails/13.jpg)
Schwechheimer et al. BMC Microbiology (2014) 14:324 Page 12 of 12
27. Mashburn-Warren LM, Whiteley M: Special delivery: vesicle trafficking inprokaryotes. Mol Microbiol 2006, 61(4):839–846.
28. Hoekstra D, van der Laan JW, de Leij L, Witholt B: Release of outer membranefragments from normally growing Escherichia coli. Biochim Biophys Acta1976, 455(3):889–899.
29. Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S,Cookson BT: Biogenesis of bacterial membrane vesicles. Mol Microbiol2009, 72(6):1395–1407.
30. Sonntag I, Schwarz H, Hirota Y, Henning U: Cell envelope and shape ofEscherichia coli: multiple mutants missing the outer membranelipoprotein and other major outer membrane proteins. J Bacteriol 1978,136(1):280–285.
31. McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ: Outer membranevesicle production by Escherichia coli is independent of membraneinstability. J Bacteriol 2006, 188(15):5385–5392.
32. Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM:Gene transfer potential of outer membrane vesicles of Acinetobacterbaylyi and effects of stress on vesiculation. Appl Environ Microbiol 2014,80(11):3469–3483.
33. Henry R, Lo M, Khoo C, Zhang H, Boysen RI, Picardeau M, Murray GL,Bulach DM, Adler B: Precipitation of iron on the surface of Leptospirainterrogans is associated with mutation of the stress responsemetalloprotease HtpX. Appl Environ Microbiol 2013, 79(15):4653–4660.
34. Hayashi J, Hamada N, Kuramitsu HK: The autolysin of Porphyromonasgingivalis is involved in outer membrane vesicle release. FEMS Microbiol Lett2002, 216(2):217–222.
35. Magnet S, Dubost L, Marie A, Arthur M, Gutmann L: Identification of the L,D-transpeptidases for peptidoglycan cross-linking in Escherichia coli.J Bacteriol 2008, 190(13):4782–4785.
36. Glauner B, Holtje JV, Schwarz U: The composition of the murein ofEscherichia coli. J Biol Chem 1988, 263(21):10088–10095.
37. Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT: Bacterial cell wallrecycling provides cytosolic muropeptides as effectors for beta-lactamaseinduction. EMBO J 1994, 13(19):4684–4694.
38. Uehara T, Park JT: An anhydro-N-acetylmuramyl-L-alanine amidase withbroad specificity tethered to the outer membrane of Escherichia coli.J Bacteriol 2007, 189(15):5634–5641.
39. Lima S, Guo MS, Chaba R, Gross CA, Sauer RT: Dual molecular signalsmediate the bacterial response to outer-membrane stress. Science 2013,340(6134):837–841.
40. Gmeiner J, Schlecht S: Molecular organization of the outer membrane ofSalmonella typhimurium. Eur J Biochem 1979, 93(3):609–620.
41. Klein G, Kobylak N, Lindner B, Stupak A, Raina S: Assembly oflipopolysaccharide in Escherichia coli requires the essential LapB heatshock protein. J Biol Chem 2014, 289(21):14829–14853.
42. Ogura T, Inoue K, Tatsuta T, Suzaki T, Karata K, Young K, Su LH, Fierke CA,Jackman JE, Raetz CR, Coleman J, Tomoyasu T, Matsuzawa H: Balancedbiosynthesis of major membrane components through regulateddegradation of the committed enzyme of lipid A biosynthesis by theAAA protease FtsH (HflB) in Escherichia coli. Mol Microbiol 1999,31(3):833–844.
43. Magnet S, Bellais S, Dubost L, Fourgeaud M, Mainardi JL, Petit-Frere S,Marie A, Mengin-Lecreulx D, Arthur M, Gutmann L: Identification of the L,D-transpeptidases responsible for attachment of the Braun lipoproteinto Escherichia coli peptidoglycan. J Bacteriol 2007, 189(10):3927–3931.
44. Pautsch A, Schulz GE: Structure of the outer membrane protein Atransmembrane domain. Nat Struct Biol 1998, 5(11):1013–1017.
45. Choi DS, Yamada H, Mizuno T, Mizushima S: Trimeric structure andlocalization of the major lipoprotein in the cell surface of Escherichiacoli. J Biol Chem 1986, 261(19):8953–8957.
46. Smith SG, Mahon V, Lambert MA, Fagan RP: A molecular Swiss army knife:OmpA structure, function and expression. FEMS Microbiol Lett 2007,273(1):1–11.
47. Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, Kim SI, Lee JC:Acinetobacter baumannii outer membrane protein A modulates thebiogenesis of outer membrane vesicles. J Microbiol 2012, 50(1):155–160.
48. Valeru SP, Shanan S, Alossimi H, Saeed A, Sandstrom G, Abd H: Lack of outermembrane protein A enhances the release of outer membrane vesiclesand survival of vibrio cholerae and suppresses viability of Acanthamoebacastellanii. Int J Microbiol 2014, 2014:610190.
49. Mahalakshmi S, Sunayana MR, Saisree L, Reddy M: yciM is an essential generequired for regulation of lipopolysaccharide synthesis in Escherichia coli.Mol Microbiol 2013, 91(1):145–157.
50. Schwechheimer C, Sullivan CJ, Kuehn MJ: Envelope control of outermembrane vesicle production in gram-negative bacteria. Biochemistry 2013,52(18):3031–3040
51. Sriburi R, Jackowski S, Mori K, Brewer JW: XBP1: a link between theunfolded protein response, lipid biosynthesis, and biogenesis of theendoplasmic reticulum. J Cell Biol 2004, 167(1):35–41.
52. Strauch KL, Johnson K, Beckwith J: Characterization of degP, a generequired for proteolysis in the cell envelope and essential for growth ofEscherichia coli at high temperature. J Bacteriol 1989, 171(5):2689–2696.
53. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA,Tomita M, Wanner BL, Mori H: Construction of Escherichia coli K-12in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol2006, 2:2006 0008.
54. Cherepanov PP, Wackernagel W: Gene disruption in Escherichia coli: TcRand KmR cassettes with the option of Flp-catalyzed excision of theantibiotic-resistance determinant. Gene 1995, 158(1):9–14.
55. Silhavy TJ, Berman ML, Enquist LW, Cold Spring Harbor Laboratory:Experiments With Gene Fusions. Cold Spring Harbor, N.Y: Cold Spring HarborLaboratory; 1984.
56. Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK:D-amino acids govern stationary phase cell wall remodeling in bacteria.Science 2009, 325(5947):1552–1555.
57. Cowles CE, Li Y, Semmelhack MF, Cristea IM, Silhavy TJ: The free and boundforms of Lpp occupy distinct subcellular locations in Escherichia coli.Mol Microbiol 2011, 79(5):1168–1181.
58. Schwechheimer C, Rodriguez DL, Kuehn MJ: NlpI-mediated modulation ofouter membrane vesicle production through peptidoglycan dynamics inE.coli. MicrobiologyOpen, In press.
Submit your next manuscript to BioMed Centraland take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution
Submit your manuscript at www.biomedcentral.com/submit















![Protection from Hemolytic Uremic Syndrome by Eyedrop … · 2019-09-04 · Outer membrane vesicles (OMVs) are spherical membrane blebs shed by Gram-negative bacteria [4]. They carry](https://static.fdocuments.us/doc/165x107/5edf9c93ad6a402d666af19e/protection-from-hemolytic-uremic-syndrome-by-eyedrop-2019-09-04-outer-membrane.jpg)


