Mineral formation in micaceous Mediterranean Red soils of ...hera.ugr.es/doi/15019044.pdf ·...
Transcript of Mineral formation in micaceous Mediterranean Red soils of ...hera.ugr.es/doi/15019044.pdf ·...

European Journal of Soil Science, June 1998,49, 253–268
Mineral formation in micaceous Mediterranean Red soils ofSierra Nevada, Granada, Spain
J . M . M A R T I N - G A R C I A a, G . D E L G A D Ob, J . F . PAR R A G A b, J . B E C Hc & R . D E L G A D O b
aDepartamento de Geologı´a, Facultad de Ciencias Experimentales, Universidad de Jae´n, Campus Universitario Las Lagunillas, 23071Jaen, bDepartamento de Edafologı´a y Quımica Agrıcola, Facultad de Farmacia, Universidad de Granada, Campus UniversitarioCartuja, 18071 Granada, andcDepartament de Biologia Vegetal, Facultat de Biologia, Universitat de Barcelona, Avda. Diagonal 645,08080 Barcelona, Spain
Summary
We investigated the processes of mineral formation in three Alfisol profiles of Sierra Nevada (southernSpain), with special emphasis on the little-studied process of mica inheritance, particularly as regardsits quantitative aspects. X-ray diffraction, conventional and high resolution transmission electronmicroscopy, selected-area electron diffraction, and geochemical analysis of the soil solution were used,and the granulometric fractions gravel, coarse sand, fine sand, silt and clay were studied, as was unalteredparent rock. Most interesting was inheritance of dioctahedral mica (illites) with small crystallochemicalchanges. The transition of mica from parent rock to clay was characterized by small crystallochemicalchanges affecting their structural formulae, reduction of the 2M1 polytype content, decrease in crystallitesize, increase in crystal defects, and other changes in particle morphology. We propose the term‘pedocrystallochemical evolution’ for the transition. The changes reverse the processes that take placein micas when rocks are formed by sedimentation, diagenesis and metamorphism. The kaolinite in thesoils is a result of neoformation, and acts as the equilibrium phase within the chemical system. Reddeningseems to have been caused by both inheritance and neoformation of iron oxides (haematite1 goethite).
Introduction
Minerals in soils result mainly from three processes, namely(i) inheritance from parent materials, (ii) neoformation bycrystallization from solutions and colloidal gels, and (iii)transformation of existing minerals into new species, a processthat most frequently affects phyllosilicates. Although inheritedminerals, also called ‘allochthonous’ or ‘detritic minerals’, areassumed not to have been transformed mineralogically in thecourse of transition from parent material to soil, it is nonethelessimportant to investigate inherited mineral phases in order tointerpret soil evolution accurately.
Micas are common in soils and most are there as a resultof inheritance (Fanninget al., 1989). Nevertheless, they canundergo a variety of changes, particularly in the soil. One suchchange is degradative transformation towards 2:1 swellingminerals, which involves changes in composition and loss oflayer charge through mechanisms that are still poorly under-stood (Fanninget al., 1989). Singer (1989) has suggested thatneoformation gives rise to micas in arid soils. However, thisprocess has not been completely elucidated. Most inheritedmicas are dioctahedral (Graf von Reichenbach & Rich, 1975)
Correspondence: R. Delgado. E-mail: [email protected] 17 December 1996; revised version accepted 4 October 1997
© 1998 Blackwell Science Ltd 253
and are fairly stable in soils. They can undergo small changesinduced by the soil medium that affects their structural units(layers and interlayers) without changing their mineral classi-fication.
The most common dioctahedral micas in soils belong to thegroup of ‘true micas’, which have a layer charge (x) per halfcell unit of 1, and to the dioctahedral subgroup, characterizedby trivalent cations in the octahedral sheet. The general formulafor a cell unit (Brownet al., 1978) is [X2][Y 4][Z8]O20(OH,F)4,where X is K1, Na1; Y is Al31, Fe31, Fe21, Mg21; and Z isSi41, Al31. Dioctahedral micas are therefore an ideal materialin which to study inheritance.
Mediterranean Red soils (mostly Xeralfs; Luvisols, Nitisols,etc.) are common in regions bordering the Mediterranean Seaand in other regions with similar climates (Torrent, 1995).They are characteristically red or reddish with stronglydeveloped argillic horizons. This soil population providesoptimal material for studies of mineral inheritance because thealteration is weak or of only moderate intensity, and the 2:1phyllosilicates are common (Pe´dro, 1987), specifically illitemicas (Torrent, 1995). Neoformation is less frequent thaninheritance and kaolinite is the most common neoformedphyllosilicate (Delgadoet al., 1990, 1994; Torrent, 1995).
Our present research aimed to characterize the principal

254 J. M. Martın-Garcıa et al.
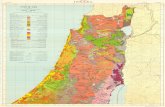
Figure 1 Location of the area sampled in the Sierra Nevada mountains,southern Spain.
processes of mineral genesis in micaceous Alfisols in southernSpain, paying particular attention to inheritance of diocta-hedral micas.
Materials and methods
We studied three profiles of red soils identified as Haploxeralfsand Cryoboralfs in Soil Survey Staff (1994) or as HaplicLuvisols and Chromic Luvisols in FAO-UNESCO (1988) inSierra Nevada (Granada, Spain) (Figure 1). The parent rocksconsist of mica schists or micaceous quartzites, which aremicaceous metamorphic rocks with green schist facies fromthe Veleta Nappe in the Nevado-Fila´bride Complex (Puga,1976; Martı´n-Garcı´a, 1994). Micas in these soils were previ-ously identified by Martı´n-Garcı´aet al. (1997) as illites (relatedin structure and composition to phengitized muscovite) andparagonites.
Colour was recorded as hue, value and chroma from theMunsell colour charts. Reddening was assessed as a colourindex, called here redness index: RI5 (25 – hue)3 chroma/value, based on the redness ratio (RR) of Torrentet al. (1983)with the constant increased from 10 to 25. Micromorphologyof the Bt horizons was characterized in thin sections.
Granulometric fractions were obtained from the fine earth(, 2 mm) by sieving and sedimentation (Robinson pipette)(Soil Conservation Service, 1972). These were clay (, 2 µm),silt (2–50µm), fine sand (50–200µm), coarse sand (200–2000µm), and gravel (. 2 mm). Organic carbon content wasdetermined by the dichromate oxidation method. Cationexchange capacity (CEC) and base saturation were measuredby the ammonium acetate method (pH 7) and the sodiumchloride method (Soil Conservation Service, 1972); pH wasmeasured in a 1:1 soil:water suspension by weight.
Soil solution from saturation pastes was used to measure pHand concentrations of Na1, K1 (flame ionization photometry),Ca21, Mg21, Fe31 and SiO2 (atomic absorption spectrometry).
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
Free forms such as iron, aluminium and silica in fine earthwere extracted with sodium citrate-dithionite (Holgrem, 1967)and ammonium oxalate-oxalic acid (McKeague & Day, 1966),and measured by atomic absorption spectrometry.
The mineralogical composition of all granulometric fractionswas determined by X-ray diffraction (XRD) in samples ofunoriented powder by using a holder filled from the side. APhilips PW 1730 diffractometer was used under the followingoperating conditions: radiation (Cu Kα) at 35 kV and 15 mA,scan speed 2° 2θ min–1, paper speed 2 cm/°2θ, time constant2 s. Oriented aggregates of silt and clay fractions were preparedby sedimentation and drying on a glass slide, which was thensubjected to solvation with ethylene glycol and dimethylsulfox-ide (Brown & Brindley, 1980). Mineralogical composition wasdetermined by XRD with the intensity factors method (Klug& Alexander, 1976), using the factors published by Delgadoet al. (1982). The types of interstratified phases in the clay andsilt fractions were determined on the basis of the position ofpeaks in oriented aggregates using the Hendricks and Tellerformula for infinite crystallites (MacEwanet al., 1961).
Crystallochemical parameters of illites were determined.The micaceous nature of the material was confirmed by theposition of the 002 and 003 peaks of illite (Srodon & Eberl,1984), and no appreciable amounts of illite–smectite interstrati-fied phases were found. X-ray diffraction was used to study theb0 parameter, from reflection 060 (µ 0.150 nm) in unorientedpowder. The experimental conditions were: angular range 59–64 °2θ, scan speed 0.2 °2θ min–1, paper speed 0.5 cm min–1,time constant 0.4 s. The illite crystallinity index (IC) wasmeasured by determining the half-peak width of the 1.0 nmmica peak on oriented clay and silt preparations (Ku¨bler,1968); IC was expressed in °2θ. Crystallite size perpendicularto 00l planes was determined using the Scherrer equation(Klug & Alexander, 1976); as a size standard (. 100 nmcrystal size) we used macroscopic, idiomorphic crystals ofphengite from pegmatitic rocks associated with granodioriticbatholith from the Pedroches area (Co´rdoba, Spain). Thepercentage of 2M1 polytypes in relation to 1M 1 2M1 wasdetermined in unoriented powder from the intensity ratios ofthe 0.258, 0.280 and 0.500 nm peaks (approximate spacing)(Tettenhorst & Corbato´, 1993). The abbreviations 2M and 1Mrefer to the numbers of layers in the unit cell (1 or 2,respectively), and the crystalline system: monoclinic (M). Sub-index 1 in 2M1 refers to the stacking angle in successivelayers (60°). The working conditions for IC and polytypedetermination were scan speed 0.5 °2θ min–1, paper speed0.5 cm min–1, time constant 2 s. Quartz in the sample was usedas an internal standard, and, when necessary, high purity quartzwas added.
The morphological characteristics of clay-sized mica par-ticles were studied by transmission electron microscopy (TEM)with a Zeiss M10C apparatus at 80 kV. Free iron forms werepreviously removed from the samples. Some samples were

Mineral formation in micaceous soil255
Table 1 General characteristics of three Alfisols (SR1, SR2 and SR4) sampled in the Loma de la Cuna de los Cuartos area of the Sierra NevadaMountains in southern Spain
Profile SR1 SR2 SR4
Sequencea Ah, AB, Bt1, Bt2, BCt, Btb Ap, AB, Bt, BCt1, BCt2 Ah, ABt, Bt1, Bt2/CClassificationb Coarse-loamy micaceous mesic Fine-loamy micaceous mesic Loamy-skeletal micaceous cryic
Ultic Haploxeralf; Haplic Luvisol Ultic Haploxeralf; Chromic Lithic Cryoboralf; ChromicLuvisol Luvisol
Coordinates 37°99120–3°239520 37°99120–3°239520 37°89430–3°229100
Physiographic location Slightly concave slope Midslope Footslope(mountainous)
Parent material Slope deposit of micaceous Slope deposit of micaceous Mica schistsquartzite quartzite
Slope /% 37 23 10Vegetation Degraded oak stand Former farmland, thyme field Thyme fieldDrainagea Class 3 Class 3–4 Class 4Elevation /m 1420 1410 1995Mean annual soil temperature /°Cc 10.8 10.9 7.3Mean winter soil temperature /°Cc 3.8 3.9 0.3Mean summer soil temperature /°Cc 17.8 17.9 14.3Annual precipitation /mm 730 730 873Water soil reserve /mm 140.2 38.0 54.3Winter water surplus /mm 365.0 424.3 614.5Summer water deficit /mm 244.0 307.5 229.4Moisture regimed Xeric Xeric XericTemperature regimed Mesic Mesic Cryic
a FAO (1977).b Soil Survey Staff (1994); FAO-UNESCO (1988).c Depth of 50 cm.d Soil Survey Staff (1994).
also used to obtain selected-area electron diffraction (SAED)patterns.
Coarse clay (0.2–2µm) and silt were examined with highresolution transmission electron microscopy (HRTEM) andSAED to determine the stacking sequence and degree ofpolytypism, and to measure layer spacing in mica crystals.These samples were analysed (after free iron forms had beenremoved) with a Philips CM-20 apparatus equipped withan EDAX detector. The working conditions for HRTEMobservation were 200 kV and objective aperture 40µm.
Results and discussion
Morphological and analytical characteristics of the soilprofiles
The soils were identified (Table 1) as Haploxeralfs (SR1 andSR2) and Cryoboralf (SR4) in Soil Survey Staff (1994) or asHaplic Luvisol (SR1) and Chromic Luvisols (SR2 and SR4)in FAO-UNESCO (1988). The horizon sequence was basicallyA, Bt, although in fact the sequence was more complex.Horizons Ah, Ap and AB shared features with horizon E.Profile SR4, sampled at an elevation of 1995 m, marks theupper limit of appearance of these types of soil in SierraNevada (Martı´n-Garcı´a, 1994). Soil temperature decreased,and precipitation increased, with increasing elevation (meanannual soil temperature more than 7.3°C at a depth of 50 cm).
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
As expected in a Mediterranean climate, periods of droughtand high temperatures alternate during the year with periodsof water surplus and low temperatures (Figure 2). The moistureregime of all three soils was xeric. The temperature regimewas mesic in SR1 and SR2, and cryic (near frigid) in SR4.
The Bt horizons are sandy clay loam and sandy loam, andall other horizons have sandy loam texture (Table 3). Claycontent was greatest in horizons Bt and BCt in relation to theoverlying horizons (Ah, Ap and AB). In all profiles the increasein absolute value of clay was more than 3%, and therefore theBt and BCt horizons were technically argillic (Soil SurveyStaff, 1994). The ratios (% clay Bt)/(% clay Ah, Ap, AB, ABt)ranged from 1.1 to 2.5, indicating considerable clay illuviation.In addition, clay may have been formed by alteration insubsuperficial horizons. The textural change from A to Bthorizons was smallest in SR1, intermediate in SR4 and greatestin SR2, possibly because the SR4 and SR2 profiles had agreater water surplus because of their gentler slopes (Table 1).
Illuvial features (cutans on the ped faces and on pore walls)were visible macroscopically and were especially notable inhorizons Bt and BCt (Table 2). Microscopic examination ofthin sections revealed ferriargillans occupying 2–3% of thevolume of the Bt horizon. Most ferriargillans were limpid,although some were microlaminated (limpid clay alternatingwith mottled clay). There were indications of illuviation ofclay and iron forms: no disruptions were evident in the cutansand most of the pores were empty.

256 J. M. Martın-Garcıa et al.
Figure 2 Climatic data and soil water balance in the SR2 profile. PE, potential evapotranspiration; S, surplus; U, utilization; D, deficit; R,recharge. Available water storage profile5 38 mm.
Table 2 Morphological features of the soil horizons
Munsell Colour RIa
Soil Horizon Depth /cm Dry Moist Dry Moist Structureb Illuviation cutansc
SR1 Ah 0–15 10YR 6/2 10YR 3/2 1.67 3.33 m2sbkAB 15–33 10YR 6.5/2 10YR 4/2.5 1.54 3.13 c2abk xBt1 33–88 10YR 6.5/4 10YR 4/3 3.08 6.25 c2abk xxBt2 33–88 10YR 6/4 10YR 4/3.5 3.30 4.38 c2sbkBCt 88–109 10YR 6/4 10YR 4/3 3.30 3.75 c2abk xxBtb . 109 10YR 6/5 7.5YR 4.5/4 4.17 6.67 c2sbk xxx
SR2 Ap 0–5/10 10YR 6.5/4 7.5YR 4.5/4 3.08 6.67 vc2 pLAB 5/10–12/17 7.5YR 5.5/5 7.5YR 4/4 6.82 7.50 m2sbk xBt 12/17–30/50 7.5YR 6/6 7.5YR 4/4 7.50 7.50 m2pr xxBCt1 . 30/50 5YR 5/6 2.5YR 4/6 12.00 18.75 xxBCt2 . 30/50 5YR 5.5/6 2.5YR 3.5/6 10.91 22.06 xx
SR4 Ah 0–8 7.5YR 5.5/3 7.5YR 3/3 4.09 7.50 f2sbkABt 8–21 7.5YR 7/5 7.5YR 4/5 3.21 9.38 cabk xxBt1 21–38 5YR 6/4 5YR 4/5 6.67 12.50 m2abk xxxBt2/C . 38 2.5YR 4.5/6 2.5YR 3/6 4.29 13.33 m2abk xx
a RI (Redness Index)5 [(25 – nhue) 3 chroma]/value; nhue is the numerical notation of hue (adapted from Torrentet al., 1983).b Soil Survey Staff (1951).c Illuviation cutans (visual estimation): x, scarce; xx, moderate; xxx, abundant.
The soil (Table 3) was moderately acid to neutral (5.5–7.0),and pH tended to decrease with depth. Base saturation wasless than 50% in horizon BCt1 of SR2 and in all subsuperficialhorizons of SR4 but exceeded 50% in all other horizons.Cation exchange capacity was small to moderate, because thesoil contained only small quantities of organic matter (exceptnear the surface) and because of the type of clay present(essentially illitic and kaolinitic) (see next section).
The citrate-dithionite-extractable forms of Fe (Fe cd) weremore abundant than oxalate-extractable forms (Fe ox) (Table 3)and Fe was more abundant than Al and Si in the citrate-dithioniteextract (Al cd and Si cd): these forms were more abundant in
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
horizonBt than in theoverlyinghorizons,and increased indeeperlevels of the profiles. Oxalate-extractable free forms of Fe, Aland Si were concentrated in the upper horizons. Because the freeforms extracted with oxalate were of either a non-crystalline orpoorly crystalline nature (Borggaard, 1988) we assumed that thedifference between citrate-dithionate-extractable and oxalate-extractable material represented well-crystallized forms. Thevalues of Fe cd – ox (difference between quantities of Fe cd andFe ox) were greater in horizon Bt and increased with depth(Table 3).
Total citrate-dithionite-extractable forms (Fe1 Al 1 Si) infine earth correlated significantly with clay content in the fine

Mineral formation in micaceous soil257
Tabl
e3
Ana
lytic
alch
arac
teris
tics
ofth
eso
ilho
rizon
s
Coa
rse
sand
Fin
esa
ndS
iltC
lay
Fre
efo
rms
/%c
/%B
t cla
yG
rave
lO
CC
EC
Bas
esa
t.S
oil
Hor
izon
/Acl
aya
/%b
/%pH
/cm
ol 1kg
–1/%
Fe
cdF
eox
Fe
cd–o
xF
ecd
Bt/Aa
Fe
cd–o
xB
t/Aa
Al
cdA
lox
Si
cdS
iox
SR
1A
h33
2329
15–
592.
57.
016
.666
2.34
0.47
1.87
––
0.28
0.25
0.09
0.02
AB
3823
2811
–59
0.6
6.4
8.7
562.
360.
262.
10–
–0.
130.
130.
060.
02B
t132
2426
181.
451
0.2
5.9
7.3
563.
220.
332.
891.
41.
40.
400.
150.
110.
04B
t236
2723
141.
121
0.4
6.0
7.5
563.
000.
292.
711.
31.
40.
340.
130.
110.
02B
Ct
3327
2317
1.3
370.
35.
96.
557
3.26
0.33
2.93
1.4
1.5
0.51
0.13
0.11
0.04
Btb
4322
2015
1.2
500.
36.
26.
161
3.26
0.29
2.97
1.4
1.5
0.36
0.11
0.11
0.02
SR
2A
p40
2921
10–
370.
46.
94.
555
2.46
0.14
2.32
––
0.23
0.09
0.06
0.02
AB
3724
2217
–37
0.4
6.5
7.3
583.
370.
203.
17–
–0.
360.
150.
090.
02B
t31
2422
231.
751
0.3
5.7
8.5
523.
990.
263.
731.
41.
40.
600.
190.
110.
02B
Ct1
3725
1919
1.4
690.
25.
68.
349
3.79
0.13
3.66
1.3
1.3
0.38
0.13
0.11
0.02
BC
t232
1915
342.
560
0.3
5.5
11.6
665.
690.
165.
532.
02.
00.
640.
170.
150.
02
SR
4A
h37
3121
11–
341.
76.
38.
358
2.99
0.23
2.76
––
0.28
0.21
0.04
0.02
AB
t31
3125
13–
630.
85.
96.
849
3.17
0.17
3.00
––
0.28
0.21
0.06
0.02
Bt1
3031
2415
1.2
490.
56.
06.
448
3.16
0.13
3.03
1.0
1.0
0.38
0.19
0.04
0.02
Bt2
/C24
2817
282.
371
0.4
5.9
8.3
484.
030.
103.
931.
31.
40.
430.
190.
060.
02
Per
cent
ages
refe
rto
100%
fine
eart
h(1
05°C
).cd
,ci
trat
e-di
thio
nite
;ox
,ox
alat
e;cd
–ox,
(citr
ate-
dith
ioni
te)
–(o
xala
te).
aA
,ho
rizon
Ah,
Ap,
AB
,A
Bt.
bP
erce
ntag
esre
fer
tow
hole
soil
(fine
eart
h1
grav
el)
(wt/w
t).
cP
erce
ntag
esas
oxid
es(F
e2O
3,A
l 2O
3,S
iO2)
.
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268

258 J. M. Martın-Garcıa et al.
Table 4 Characteristics of the soil parent rock
CrystallochemicalMineralogy /% parameters of micas Munsell Coloura
Soil Rock Qz Fd Phyll. Iron oxides b0 /nm %2M1 Dry Moist
SR1 Micaceous quartzite 54 2 42 2 0.9001 100 5Y 5/1 5Y 2.5/1SR2 Micaceous quartzite 52 1 43 4 0.9003 100 5Y 5.5/1 5Y 2.5/1SR4 Mica schist 19 , 1 76 5 0.9006 100 5Y 4/1 5Y 2.5/1Mean 42 1 53 4 0.9003 100 5Y 4.8/1 5Y 2.5/1
Qz, quartz; Fd, felspars (K-felspars and plagioclases); Phyll., phyllosilicates (K-mica, paragonite and chlorite); iron oxides, haematite and goethite.a Crushed rock (, 0.5 mm).
earth (r 5 0.93). This finding suggests that the clay fractionis the most highly enriched in citrate-dithionite-extractablefree forms.
The ratios of Fe Bt/A for Fe cd and Fe cd – ox (Table 3)indicate that iron forms in the profiles were illuviated towardsdeeper zones (values greater than 1).In situprecipitation mightalso account for this. The type of cutan (ferriargillan) indicatesthat iron moves with the clay. Iron oxyhydroxides are boundto clay particles partly because of the negative charge on thelatter (mainly illites), which favours the nucleation of ironoxides (Boero & Franchini-Angela, 1992). However, when wecompared the ratios of free iron illuviation with those for clay(Table 3) we noted a small discrepancy in soils SR2 and SR4,attributable to the greater mobility of clay fractions with respectto iron oxides, as suggested by Bornand (1978).
Total oxalate-extractable Fe, Al and Si were related to theorganic phase of the soil and were found in greater proportionin organic-mineral horizons (Ah). Organic carbon was linearlycorrelated with the sum of oxalate-extracted oxides divided bythe sum of citrate-dithionite-extracted oxides (r 5 0.79).
Mineralogical composition and outline of mineral formation
The parent rock (Table 4) was composed mainly of quartz andphyllosilicates (K-mica, paragonite and chlorite), with smallerproportions of felspars (albite and orthoclase) and iron oxides(haematite and goethite). According to Puga (1976), haematiteis formed during metamorphism in these rocks and goethite isa postmetamorphic mineral which fills tectonic fissures. InSR1 and SR2 the parent rock was micaceous quartzite whereasin SR4 it was a mica schist in which phyllosilicates weremore common.
The minerals in the soils (Tables 5 and 6) were quartz,phyllosilicates (illite, paragonite, interstratified phases, chloriteand kaolinite), iron oxides (goethite and haematite), and felspars(albite and orthose). In addition, smectite was detected in theclay fraction. In gravel and sands, quartz was the most abundantmineral. In silt and clay, phyllosilicates (mainly micas) werethe most common minerals. The micas consisted of illites(K-micas with some degree of phengitation) and paragonite
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
(Na-mica). Illite predominated, with a mean illite/paragoniteratio of 12:1 in silt and 10:1 in clay (Table 6).
The presence of micas, chlorite, quartz, felspars and ironoxides in both the parent material and the soil suggests thatthey are inherited. However, goethite and haematite also occurin these soils, appearing to result from neoformation, becausetheir percentages (Tables 4 and 5) were greater in soil than inrock and they were concentrated in the fine earth fractions.
Kaolinite in the soils also seems to have resulted fromneoformation since it is absent from the parent rock but presentin all granulometric fractions of the soil. Kaolinite is notcharacteristic of the facies of these mica schists and quartziteswith moderate degrees of regional metamorphism (Puga, 1976).The XRD diagrams (Figure 3) confirm this finding: thereflection of kaolinite at 0.358 nm (002) was absent from thetrace diagram of the parent rock, but appeared in all the soilfraction diagrams, becoming more intense with decreasingparticle size. Quantitative data on the silt and clay fractions(Table 6 and Figure 3) support this view since in some caseskaolinite accounted for nearly 30% of all phyllosilicates in theclay fraction. Moreover, the amount of kaolinite in clay andsilt increased with increasing depth in all profiles (horizons Btand BCt). The TEM images of kaolinite crystal morphologyin silt and clay fractions provided evidence for neoformationin all horizons, showing flat, pseudohexagonal, idiomorphicparticles larger than 0.5µm, that were equidimensional in thea–b plane, with clearly defined edges. Geochemical studies ofthe soil solution further support our view of neoformation.
Interstratified minerals seemed to originate from the trans-formation of micas and were found exclusively in the silt andclay fractions (Table 6). They were probably a random mixtureof phases that swell at 1.0 nm and others that swell at 1.4 nm.This composition was suggested by XRD signals that indicateda wide band between 1.1 and 1.2 nm, which was displacedslightly towards larger spacings by ethylene glycol solvation.The 1.1 nm:1.4 nm ratio wasµ 3:1.
Smectites were not found in parent rocks but were presentexclusively in the clay fraction. These minerals may haveoriginated from neoformation, from transformation (frommicas), or both.

Mineral formation in micaceous soil259
Tabl
e5
Min
eral
ogic
alan
alys
is(X
-ray
diffr
actio
n)of
grav
elan
dfin
eea
rth
frac
tions
;al
lva
lues
are
%
Fin
eea
rth
Gra
vel
Coa
rse
sand
Fin
esa
ndS
iltC
lay
Soi
lH
oriz
onQ
zF
dP
hyll.
Iron
oxid
esQ
zF
dP
hyll.
Iron
oxid
esQ
zF
dP
hyll.
Iron
oxid
esQ
zF
dP
hyll.
Iron
oxid
esQ
zF
dP
hyll.
aIr
onox
ides
SR
1A
h60
137
260
233
567
426
344
251
312
284
2A
B60
236
251
141
761
333
340
155
410
385
2B
t159
237
254
335
856
336
547
248
314
278
6B
t246
249
347
245
660
233
545
349
312
182
5B
Ct
551
422
572
365
554
356
443
503
131
797
Btb
402
553
512
407
542
377
393
553
93
817
SR
2A
p57
140
258
232
864
231
332
264
211
383
3A
B55
240
355
135
955
139
541
352
410
382
5B
t46
248
445
148
659
234
539
355
37
185
7B
Ct1
501
463
572
365
633
304
353
575
71
848
BC
t255
240
354
239
566
428
234
259
55
185
9
SR
4A
h33
260
541
149
972
223
335
356
610
285
3A
Bt
602
353
452
4211
692
263
353
566
101
854
Bt1
502
444
472
456
742
204
383
545
71
875
Bt2
/C53
242
356
135
878
216
439
252
76
187
6
Qz,
quar
tz;
Fd,
fels
pars
(K-f
elsp
ars
and
plag
iocl
ases
);P
hyll.
,ph
yllo
silic
ates
(K-m
ica,
para
goni
te,
kaol
inite
,in
ters
trat
ified
min
eral
san
dch
lorit
e);
iron
oxid
es,
haem
atite
and
goet
hite
.a
Phy
llosi
licat
esin
clud
ing
smec
tite.
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268

260 J. M. Martın-Garcıa et al.
Table 6 Mineralogical analysis (X-ray diffraction) of phyllosilicates in the silt and clay fractions (oriented aggregates); all values are %
Silt Clay
Soil Horizon Chl Int Ill Par Ka I/(N1 T)a Chl Int Ill Par Ka Sm I/(N1 T)a
SR1 Ah 6 5 75 11 3 11.5 13 9 44 8 22 4 1.9AB 6 7 77 7 3 9.0 20 15 29 9 24 3 1.4Bt1 5 4 79 7 5 10.1 10 6 50 5 26 3 1.9Bt2 5 3 81 7 4 13.3 14 8 48 6 22 2 2.1BCt 4 3 81 7 5 11.5 10 4 55 4 23 4 2.2Btb 4 4 80 8 4 11.5 12 6 44 9 26 3 1.9
SR2 Ap 6 5 80 6 3 11.5 8 7 68 6 10 1 4.6AB 6 5 77 8 4 10.1 9 8 63 4 15 1 3.2Bt 5 7 72 10 6 6.7 7 9 61 8 13 2 3.2BCt1 6 8 69 8 9 4.9 7 9 59 7 17 1 2.7BCt2 7 9 67 4 13 3.5 9 8 51 5 26 1 1.9
SR4 Ah 4 3 83 6 4 13.3 9 7 55 6 22 1 2.3ABt 4 4 84 5 3 13.3 8 7 59 4 21 1 2.4Bt1 3 4 83 6 4 11.5 8 7 57 4 23 1 2.2Bt2/C 2 2 84 6 6 11.5 7 7 48 8 29 1 1.7
Chl, chlorite; Int, interstratified minerals; Ill, illite; Par, paragonite; Ka, kaolinite; Sm, smectite.a I/(N 1 T) 5 (inherited)/(neoformed1 transformed) ratio5 (chlorite 1 illite 1 paragonite)/(kaolinite1 interstratified minerals1 smectite).
In view of the different origins attributed to phyllosilicates(inherited 5 chlorite, illite and paragonite; neoformed5kaolinite; and transformed5 interstratified minerals and smect-ite), we calculated the mineral evolution index for the silt andclay fractions as: inherited/(neoformed1 transformed) (Table6). Neoformed and transformed phyllosilicates in the soil weremore abundant in the clay than in the silt. However, the indexdid not exceed 1 in any case, suggesting that inherited phaseswere abundant in both silt and clay. In addition, the mineralevolution index decreased with depth, showing that deephorizons contained larger amounts of neoformed or transformedphyllosilicates as a result of illuviation from higher horizons,in situ neoformation or transformation, or both.
Finally, we should consider the possibility that some of theminerals arrived as dust from the Sahara and were depositedin the region (see Yaalon & Ganor, 1973). Barrioset al. (1987)found quartz, kaolinite, smectite, felspars, micas, calcite andiron oxides in an aeolian dust collected in Andalucı´a (southernSpain), all of which except calcite are present in the Alfisolsthere. It seems unlikely that wind-blown dust has contributedsignificantly to the mineral assemblage in the profiles weexamined.
Mica inheritance
The values ofb0 measured in micas (Tables 4 and 7) rangedfrom 0.9001 to 0.9030 nm, indicating a dioctahedral structure(Griffen, 1992). The mean values ofb0 tended to increase withdecreasing particle size (rock→ gravel→ coarse sand→ fine
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
sand→ silt → clay). However, the increments inb0 betweenfractions were not constant (Figure 4A) and the greatestchanges occurred in early stages, when rock was incorporatedinto the soil as gravel, and in final stages when soil particlesreached colloidal sizes (clay).
According to Guidottiet al. (1989) and Smoliar-Zviagina(1993) the variations in the values ofb0 are related to micacomposition. We have calculated the mean structural formulaefor our micas (Table 8) using the correlation equations forb0
and atomic content published in Martı´n-Garcı´a et al. (1997).The crystallochemical changes in the structural formulae ofmica when the mineral is incorporated into clay from theparent material are small (Table 8), as expected for inheritance(i.e. ∆SiIV 5 0.11). The decrease in the laminar charge(– 0.18) and the increase in the degree of phengitation (1 0.09)should be noted.
Observations with HRTEM ofc–bsections of mica crystalsfrom silt particles (Figure 5) revealed the presence of 2M1,d(001)5 2.0 nm, and 1M, d(001)5 1.0 nm, polytypes. Thetwo polytypes were mixed both along thec axis and laterally.The interstratification was in segregated domains (Figure 5A)and other more heterogeneous types (Figure 5B), with patchyand irregular mixing. We observed domains of relatively perfectlayer stacking along thec axis and laterally, and other domainsof more irregular geometry (Figure 5B) where layers wereslightly curved or disrupted. We also observed lenticular layerseparation (Figure 5A), caused in part by cation diffusioninduced by the electron beam (Peacor, 1992). In domainswhere irregular layers predominated (Figure 5B) some cases of

Mineral formation in micaceous soil261
Figure 3 Powder X-ray diffraction pattern of parent rock and soilgranulometric fractions, in region 23–25 °2θ (Cu Kα).
spacings (2.0–3.0 nm) appeared, possibly reflecting crystallinedefects, local changes in composition, or both, caused byalterations in the mica.
The relative spatial distribution of 2M1 and 1M polytypesobserved with HRTEM (Figure 5) gave rise to some hypothesesregarding the 2M1 → 1M transition. Interstratifications insegregated domains may have been caused by 2M1 → 1Mpolytype changes influenced by large discontinuities in thecrystal structure, favoured by 00l planes of exfoliation (Figure5A). Irregular mixtures (2M1 1 1M) may have been producedby polymorphic changes arising from smaller or highly local-ized structural defects (Figure 5B).
The study of the SAED patterns (Figures 6 and 7) confirmedthe presence of 2M1 and 1M mica polytypes and suggested
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
that a proportion of the 1M polytypes would be 1Md. Withorientation parallel to theb*–c* axes (Figure 6) the SAEDpatterns corresponded to 2M1 polytypes, since under theseconditions it is not possible to distinguish between 2M1 and1M when they coexist. However, the electron diffractionpatterns in coarse clay (Figure 6B) were less well defined thanthe patterns for silt (Figure 6A), with larger, more diffuse spotsthat probably indicate greater stacking disorder and a largerproportion of 1M polytypes (1Md). The presence of concentricrings, indicating turbostratic stacking, in orientations parallelto the a*–b* axes (Figure 7) revealed the existence of 1Mdstacking.
The percentage of 2M1 polytypes in relation to 1M 1 2M1,measured with XRD (Tables 4 and 7), showed values of 100%2M1 in mica from the parent rock and which graduallydecreased with decreasing particle size. As with theb0 para-meter, the variations between fractions were not constant(Figure 4B). However, in this case the granulometric sequenceshowed three steps: rock to gravel, gravel to coarse sand, andsilt to clay.
The TEM images of mica particles from the clay fractionshowed flat crystals in thea–b plane (Figure 7). Roundededges and corrosion gulfs were observed (see explanation inthe next section). Two types of mica particles were distinguish-able: (i) particles approaching 1000 nm in mean diameter,containing larger numbers of layers, and formed by disorderedstacks of several crystals (Figure 7A), and (ii) small particleseach apparently comprising a single thin, spindle-shaped crys-tal, with a mean maximum diameter in thea–b plane ofµ 500 nm (Figure 7B). The TEM images suggested somegeneral principles of mica inheritance. The larger particles(Figure 7A) decreased in size through thea, b and c axes,eventually reaching the size of the smallest particles(Figure 7B). The fact that crystallite size (measured with XRD)in the direction perpendicular to 00l planes decreased from thesilt to clay supports this hypothesis (Table 7). Romeroet al.(1992) also considered physical disintegration as clear evidenceof mica weathering.
We suggest that changes in structural formulae, stackingorder and morphological features in mica crystals whichundergo a process of inheritance take place in parallel. This isevident when Figure 4(A,B) are examined together and themicas from the silt and the clay fractions (Tables 7 and 8) arecompared. The changes in structural formulae probably resultedfrom chemical exchanges between mica crystals and thesurrounding medium, favoured by the loss of stacking order,decreasing crystal size and increasing numbers of crystaldefects, all of which would provide a more reactive surface.
Moreover, the crystallochemical evolution of micas in soilappears to proceed in parallel with the process of pedogenesisand the micas in the clay fraction are those which undergo thegreatest transformations. Consequently, we propose the termpedocrystallochemical evolution to designate the process whichtake place in dioctahedral micas from the soils we analysed.

262 J. M. Martın-Garcıa et al.
Figure 4 Variation inb0 parameter (A) and percentage 2M1 (B) in micas from the parent rock–clay transition (mean values per profile): (a) parentrock–gravel transition; (b) gravel–coarse sand transition; (c) silt–clay transition.
Two points raised in earlier studies on micas in geologicalsettings support our findings.1 Similar changes to those observed by us in the numbers ofatoms in the formula, but in the opposite direction, take placeas the degree of diagenesis and metamorphism increases(Pearce & Clayton, 1995).2 The process of sedimentation→ diagenesis→ initial meta-morphism involves the 1Md → 1M → 2M1 sequence (Kisch,1983). This sequence is basically an inverted version of theone we found in pedological settings: 2M1 → 1M(1Md). The1M polytype is characteristic of alteration settings and lowtemperature sedimentary settings, such as soils (Graf vonReichenbach & Rich, 1975; Kisch, 1983).
Geochemical study of the soil solution
The concentration ranges (mmoles dm–3) of the solutes investi-gated in the soil solution were: Si41 (0.07–0.28), Na1 (0.10–0.22), K1 (0.06–0.73), Ca21 (0.11–2.24), Mg21 (0.07–0.77)
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
and Fe31 (, 0.01–0.03). The pH varied between 5.2 and 7.6.All the points representing soil solution in the SiO2–Al2O3–K2O–H2O system (Garrels, 1984) (Figure 8) fall outside thefields of stability of felspars and micas, and fall within thefield of kaolinite. This could be because the mineral phasesfrom the parent rock are not in equilibrium with the soilsolution and are consequently unstable. The kaolinite is stablein this medium and arises from neoformation, which would fitin with the mineralogical composition. On the basis of stabilitydiagrams, Torrent (1995) concluded that kaolinite was thestable mineral phase of Mediterranean soils, in which silicaactivities are small.
Morphological evidence of the instability of micas includedthe appearance of corrosion gulfs under TEM: these appearedto arise from the preferential dissolution at the edges of theparticle where the structure is weakened by bond rupture andcharge decompensation. We found a highly significant linearcorrelation between silica content in the soil solution and thepercentage of illite in clay particles from fine earth (r 5 0.80).

Mineral formation in micaceous soil263
Tabl
e7
Cry
stal
loch
emic
alpa
ram
eter
sof
mic
asfr
omgr
anul
omet
rical
frac
tions
inso
ilho
rizon
s
Fin
eea
rth
Gra
vel
Coa
rse
sand
Fin
esa
ndS
iltC
lay
Soi
lH
oriz
onb 0
/nm
%2M
1b 0
/nm
%2M
1b 0
/nm
%2M
1b 0
/nm
%2M
1IC
aC
ryst
allit
esi
ze/n
mb 0
/nm
%2M
1IC
aC
ryst
allit
esi
ze/n
m
SR
1A
h0.
9013
840.
9009
370.
9007
640.
9010
890.
4747
.30.
9022
160.
5825
.0A
B0.
9009
630.
9004
510.
9017
780.
9012
650.
5135
.80.
9016
340.
5726
.0B
t10.
9013
820.
9006
100
0.90
1146
0.90
1580
0.47
47.3
0.90
1436
0.48
43.5
Bt2
0.90
0870
0.90
1335
0.90
2582
0.90
1266
0.51
35.8
0.90
1133
0.50
39.3
BC
t0.
9013
100
0.90
1057
0.90
1190
0.90
1034
0.40
78.5
0.90
2123
0.47
47.3
Btb
0.90
0582
0.90
0333
0.90
0427
0.90
0547
0.46
50.0
0.90
2619
0.47
47.3
Mea
nS
R1b
0.90
1080
0.90
0753
0.90
1362
0.90
1162
0.47
47.3
0.90
1827
0.50
39.3
SR
2A
p0.
9019
600.
9016
100
0.90
2387
0.90
1939
0.41
75.0
0.90
2819
0.44
57.0
AB
0.90
1394
0.90
1265
0.90
0953
0.90
1239
0.36
100.
00.
9027
290.
5135
.8B
t0.
9026
580.
9003
320.
9014
300.
9030
420.
4843
.50.
9022
180.
4941
.0B
Ct1
0.90
1844
0.90
0136
0.90
1057
0.90
1245
0.40
78.5
0.90
2625
0.45
52.3
BC
t20.
9010
910.
9008
480.
9011
380.
9011
160.
3610
0.0
0.90
2721
0.45
52.3
Mea
nS
R2b
0.90
1966
0.90
0647
0.90
1346
0.90
1936
0.42
66.5
0.90
2521
0.47
47.3
SR
4A
h0.
9016
820.
9017
620.
9013
380.
9013
570.
4266
.50.
9016
180.
5135
.8A
Bt
0.90
0510
00.
9010
370.
9011
540.
9013
480.
4362
.50.
9028
140.
4941
.0B
t10.
9003
840.
9005
560.
9011
830.
9014
590.
4552
.30.
9021
220.
5135
.8B
t2/C
0.90
0671
0.90
1165
0.90
0869
0.90
1462
0.43
62.5
0.90
1323
0.58
25.0
Mea
nS
R4b
0.90
0683
0.90
1056
0.90
1065
0.90
1457
0.43
62.5
0.90
1920
0.53
32.5
Tota
lm
ean
0.90
1278
0.90
0954
0.90
1260
0.90
1353
0.44
57.0
0.90
2123
0.50
39.3
Sta
ndar
dde
viat
ion
0.00
0616
0.00
0522
0.00
0621
0.00
0519
0.05
20.7
0.00
067
0.05
10.0
Mea
nin
crea
ses
from
pare
ntro
ckto
clay
:∆b
05
0.00
18nm
,∆%
2M1
5–
77%
.a
IC,
illite
crys
talli
nity
inde
x(°
2θ).
bP
rofil
em
eans
wei
ghte
dto
horiz
onth
ickn
ess.
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268

264 J. M. Martın-Garcıa et al.
Figure 5 High resolution transmission electron microscopy (HRTEM) images of two mica particles from the silt fraction of horizon BCt2 inprofile SR2. (A) 2.0 nm spacing (2M1 polytype) is indicated by the A arrows; lateral change at 1.0 nm (1M polytype), and patchy mixing ofpolytypes. Perfect stacking with 1.0 nm spacing (1M polytype) is indicated by the B arrows. Lenticular layer separation is indicated by the Carrow, and shows the change from 1M to 2M1, illustrating irregular mixing of polytypes in segregated domains. (B) 2.0 nm spacing (2M1 polytype)is indicated by the A arrows. Greater than 2.0 nm spacing is indicated by the B arrows. Disrupted and curved layers in an area of irregulardomains are indicated by the C arrow.
Table 8 Crystallochemical parameters of the structural formula of micas (mean values) from parent rock and soil granulometric fractions (halfunit-cell)
Structural formula
Fraction Tetrahedral sheet Octahedral sheeta xIV b xVI b xtotal b
Parent rock (Si3.13Al 0.87) Al 1.77 (Fe, Mg)0.22 – 0.87 – 0.14 – 1.01Gravel (Si3.18Al 0.82) Al 1.74 (Fe, Mg)0.26 – 0.82 – 0.13 – 0.95Coarse sand (Si3.16Al 0.84) Al 1.75 (Fe, Mg)0.25 – 0.84 – 0.13 – 0.97Fine sand (Si3.18Al 0.82) Al 1.74 (Fe, Mg)0.26 – 0.82 – 0.13 – 0.95Silt (Si 3.19Al 0.81) Al 1.74 (Fe, Mg)0.27 – 0.81 – 0.11 – 0.92Clay (Si3.24Al 0.76) Al 1.72 (Fe, Mg)0.31 – 0.76 – 0.07 – 0.83
Variation from parent rock to clay:∆SiIV 5 0.11, ∆Al IV 5 – 0.11, ∆AlVI 5 – 0.05, ∆(Fe, Mg)5 1 0.09, ∆xIV 5 – 0.11, ∆xVI 5 – 0.07, and∆xtotal 5 – 0.18.a Fe as Fe31.b xIV, tetrahedral sheet charge;xVI, octahedral sheet charge;xtotal, total layer charge.
This correlation suggests that a large portion of the silica canbe attributed to alterations in the mica.
The coexistence in these soils of the dissolution andinheritance of mica can be explained by the changes in thepedogenic medium as a result of seasonal changes in thesoil moisture (Figure 2). During droughts, solutes are
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
concentrated, leading to increased potassium and silicaactivity and increased pH, and reach equilibrium with mica,thus favouring inheritance. When the soil is saturatedwith water, dissolution is favoured and kaolinite is thephase in equilibrium with the solution (Figure 8). Also,when the soil is wet, ions move between mica crystals

Mineral formation in micaceous soil265
Figure 6 Selected area electron diffraction (SAED) patterns of mica crystals. Orientation parallel tob*–c*. (A) Horizon BCt2, profile SR2, siltfraction. (B) Horizon BCt2, profile SR2, coarse clay fraction.
and the solution, whereas, on drying, the internal modifica-tions within the mica layer are consolidated andstabilized, favouring inheritance. Singer (1989) noted that
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
the equilibrium of illite in arid soils was favoured byseasonal increases in solute concentration in the soilsolution.

266 J. M. Martın-Garcıa et al.
Figure 7 Transmission electron microscope bright-field images and selected area electron diffraction (SAED) patterns (orientation parallel toa*–b*) of mica crystals in the clay fraction of horizon ABt in profile SR4. The SAED patterns show concentric rings with periodicities of 0.45 nm(020 planes) and 0.225 nm (040 planes) (polytypes 1Md). (A) Coarse clay particle formed by crystals stacking. The SAED pattern shows multiplespots. (B) Small clay particle. The SAED pattern shows a hexagonal morphology.
Soil reddening
The redness index (RI) was most intense in Bt horizons (e.g.Bt in SR4) and deeper levels of the profiles (e.g. BCt in SR2)(Table 2). Redness depended primarily on the haematite1
goethite content of the clay fraction (Table 5), as shown bythe significant linear correlation between RI and the percentageof these oxides determined by XRD in clay (r 5 0.73, RI dry;r 5 0.76, RI moist). The correlations between RI and freeiron also support this relation. We found significant linearcorrelations between:1 RI and crystalline forms of free iron (Fe2O3cd – ox)(r 5 0.79, RI dry;r 5 0.85, RI moist);
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
2 RI and total free iron (Fe2O3cd) (r 5 0.77, RI dry;r 5 0.81, RI moist), and3 RI and the relative content of crystalline iron forms(Fe2O3cd – ox/Fe2O3cd) (r 5 0.64, RI dry; r 5 0.73, RImoist).
Reddening also correlated significantly with clay content(r 5 0.61, RI dry; r 5 0.70, RI moist), a logical finding inview of the fact that this size fraction is associated withfinely divided iron forms that are responsible for thered colour.
Another factor that can influence reddening is pH,which showed a significant inverse correlation with RI(r 5 – 0.62, RI dry and RI moist). The most acid horizons

Mineral formation in micaceous soil267
Figure 8 Stability relations of soil solutions and some mineral phases(25°C, 1 atm) in the SiO2–Al2O3–K2O–H2O system as a function ofpH-pK1 against pH4SiO4 (adapted from Garrels, 1984).
(Bt and BCt) were also the reddest. The low pH (acidity)might have caused increased iron release andreddening.
As occurs with the process of mica inheritance, the Mediter-ranean soil climate (alternating wet and dry seasons) favoursreddening: Fe is released when the soil is wet and crystallizesto red oxides on drying in summer.
Conclusions
We have identified several important processes of mineralformation in Alfisols in the Sierra Nevada mountains ofsouthern Spain. Most minerals are inherited from the soil’sparent material. There is a moderate pedochemical weathering,with the formation of new mineral phases in all granulometricfractions. The last is most intense in the deepest horizons ofthe profiles (horizons Bt and BCt). Illuviation of clay withiron oxides was responsible for an argillic horizon. Reddeningof the soil mass, produced by crystalline iron oxides, was alsomore intense with increasing depth in the profiles.
The most interesting minerals of inheritance are the diocta-hedral micas illite and paragonite. Mica inheritance is accom-panied by crystallochemical evolution. The clay fraction, asthe most changed pedogenic fraction, contains the micas thatare the most evolved in crystallochemical terms. We thereforepropose the term pedocrystallochemical evolution to designatethe changes that occur in mica in the soil.
The changes that take place in dioctahedral micas in Alfisolsduring inheritance are minor atom substitutions (increasedtetrahedral silicon and increased phengitation), small reductionsin layer charge, increasedb0 values, changes in stackingpatterns (increased proportion of 1M polytypes relative to 2M1
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
polytypes), increased number of crystal defects, and reducedcrystal size.
Kaolinite is the most abundant neoformed phase. Otherneoformed phases in the soil are haematite and goethite.
Climate (periods of water deficit alternating with periods ofwater surplus) appears to have an important influence oninheritance of mica and reddening.
The processes involved in mica inheritance from the parentrock to the different size fractions of the soil could be thesame sequence of events that sedimentary illites and smectitesundergo in the geological processes of diagenesis and meta-morphism, but in reverse order. In this context, the soil canbe considered as one more link in the geological cycle ofthese minerals.
Acknowledgements
This study was supported by the Spanish Ministry of Educationand Science projects no PB94-0787 ‘El Proceso de Herenciade Micas en Suelos Rojos Mediterra´neos: Cuantificacio´n yEcologıa’. X-ray diffraction measurements and conventionaland high resolution transmission electron microscopy werecarried out by the staff of the Centro Instrumental de Apoyoa la Investigacio´n of the University of Granada. We thankProfessors J. Torrent (Universidad de Co´rdoba) and R. Webster(Rothamsted Experimental Station) for their comments on themanuscript and their valuable suggestions.
References
Barrios, J., Barro´n, V., Pena, F. & Torrent, J. 1987. Particle size andmineralogical composition of aeolian dusts collected in Spain. In:The Sixth Meeting of the European Clay Groups(eds E. Gala´n,J.L. Perez-Rodrı´guez & J. Cornejo), pp. 101–103. Sociedad Espan˜olade Arcillas, Sevilla.
Boero, V. & Franchini-Angela, M. 1992. Crystallization of haematiteand goethite in the presence of soil minerals.European Journal ofMineralogy, 4, 539–546.
Borggaard, O.K. 1988. Phase identification by selective dissolutiontechniques. In:Iron in Soils and Clay Minerals(eds J.W. Stucki,B.A. Goodman & U. Schwertmann), pp. 83–98. Reidel, Dordrecht,The Netherlands.
Bornand, M. 1978.Alteration des mate´riaux fluvio-glacieres, ge´neseet evolution des sols sur terrasses quaternaires dans la moyennevalleedu Rhone. These d’etat, Universite´ de Montpellier.
Brown, G. & Brindley, G.W. 1980. X-ray diffraction procedures forclay mineral identification. In:Crystal Structures of Clay Mineralsand X-ray Identification(eds G.W. Brindley & G. Brown), pp. 305–359. Monograph No 5, Mineralogical Society, London.
Brown, G., Newman, A.C.D., Rayner, J.H. & Weir, A.H. 1978. Thestructures and chemistry of soil clay minerals. In:The Chemistryof Soil Constituents(eds D.J. Greenland & M.H.B. Hayes), pp. 29–178. John Wiley & Sons, Chichester.
Delgado Calvo-Flores, R., Barahona, E., Huertas, F. & Linares, J.1982. Los Mollisoles de la cuenca alta del rı´o Dılar (Sierra Nevada).Anales de Edafologı´a y Agrobiologı´a, 41, 59–82.

268 J. M. Martın-Garcıa et al.
Delgado, R., Pa´rraga, J.F., Delgado, G., Huertas, F. & Linares, J.1990. Ge´nese d’un sol fersiallitique de la formation Alhambra(Granada-Espagne).Science du Sol, 28, 53–70.
Delgado, R., Aguilar, J. & Delgado, G. 1994. Use of numericalestimators and multivariate analysis to characterize the genesis andpedogenic evolution of Xeralfs from southern Spain.Catena, 23,309–325.
Fanning, D.S., Keramidas, V.Z. & El-Desoky, M.A. 1989. Micas. In:Minerals in Soil Environments, 2nd edn (eds J.B. Dixon & S.B.Weed), pp. 551–634. Soil Science Society of America, Madison, WI.
FAO 1977.Guidelines for Soil Profile Description. FAO, Rome.FAO-UNESCO 1988.Soil Map of the World. Revised Legend. FAO,
Rome.Garrels, R.M. 1984. Montmorillonite/illite stability diagrams.Clays
and Clay Minerals, 32, 161–166.Graf von Reichenbach, H. & Rich, C.I. 1975. Fine-grained micas in
soils. In: Soil Components. Volume 2. Inorganic Components(ed.J.E. Gieseking), pp. 59–95. Springer-Verlag, Berlin.
Griffen, D.T. 1992.Silicate Crystal Chemistry. Oxford UniversityPress, New York.
Guidotti, C.V., Sassi, F.P. & Blencoe, J.G. 1989. Compositionalcontrols on thea-cell and b-cell dimensions of 2M1 muscovite.European Journal of Mineralogy, 1, 71–84.
Holgrem, G.G.S. 1967. A rapid citrate-dithionite extractable ironprocedure.Soil Science Society of America Proceedings, 31, 210–211.
Kisch, H.J. 1983. Mineralogy and petrology of burial diagenesis(burial metamorphism) and incipient metamorphism in clastic rocks.In: Developments in Sedimentology 25B: Diagenesis in Sedimentsand Sedimentary Rocks, 2(eds G. Larsen & G.V. Chilingar), pp.289–493. Elsevier, Amsterdam.
Klug, H.P. & Alexander, L.E.C. 1976.X-ray Diffraction Proceduresfor Polycrystalline and Amorphous Material.John Wiley & Sons,New York.
Kubler, B. 1968. Evaluation quantitative du me´tamorphisme par lacristallinite de l’illite. Bulletin Centre de Recherche Pau-SNPA, 2,385–397.
MacEwan, D.M.C., Ruiz Amill, A. & Brown, G. 1961. Interstratifiedclay minerals. In:The X-Ray Identification and Crystal Structuresof Clay Minerals (ed. G. Brown), pp. 393–445. MineralogicalSociety, London.
Martın-Garcı´a, J.M. 1994.La genesis de Suelos Rojos en el macizode Sierra Nevada. Tesis Doctoral, Universidad de Granada.
Martın-Garcı´a, J.M., Delgado, G., Sa´nchez-Maran˜on, M., Parraga, J.F.& Delgado, R. 1997. Nature of dioctahedral micas in Spanish redsoils. Clay Minerals, 32, 107–121.
© 1998 Blackwell Science Ltd,European Journal of Soil Science, 49, 253–268
McKeague, J.A. & Day, J.H. 1966. Dithionite- and oxalate-extractableFe and Al as aids in differentiating various classes of soils.CanadianJournal of Soil Science, 46, 13–22.
Peacor, D.R. 1992. Diagenesis and low-grade metamorphism of shalesand slates. In:Minerals and Reactions at the Atomic Scale:Transmission Electron Microscopy(ed. P.R. Buseck), pp. 335–380.Reviews in Mineralogy 27, Mineralogical Society of America,Washington, DC.
Pearce, R.B. & Clayton, T. 1995. Tschermak substitution as a indicatorof palaeotemperature in Silurian K-bentonites from the SouthernUplands of Scotland and Northern Ireland.Clay Minerals, 30,15–25.
Pedro, G. 1987. Ge´ochimie, mine´ralogie et organisation des sols.Aspects coordonne´s des proble`mes pe´dogenetiques. CahiersORSTOM, Se´rie Pedologie, 23, 169–186.
Puga, E. 1976.Investigaciones petrolo´gicas en Sierra NevadaOccidental. Tesis Doctoral, Universidad de Granada.
Romero, R., Robert, M., Elsass, F. & Garcı´a, C. 1992. Evidence bytransmission electron microscopy of weathering microsystems insoils developed from crystalline rocks.Clay Minerals, 27, 21–33.
Singer, A. 1989. Illite in the hot-aridic soil environment.Soil Science,147,126–133.
Smoliar-Zviagina, B.B. 1993. Relationships between structuralparameters and chemical-composition of micas.Clay Minerals, 28,603–624.
Soil Conservation Service 1972.Soil Survey Laboratory Methodsand Procedures for Collecting Soil Samples. US Department ofAgriculture, Washington, DC.
Soil Survey Staff 1951.Soil Survey Manual. US Department ofAgriculture, Washington, DC.
Soil Survey Staff 1994.Keys to Soil Taxonomy. SMSS TechnicalMonograph No 19, 6th edn. US Government Printing Office,Washington, DC.
Srodon, J. & Eberl, D.D. 1984. Illite. In:Micas (ed. S.W. Bailey), pp.495–544. Reviews of Mineralogy 13, Mineralogical Society ofAmerica, Washington, DC.
Tettenhorst, R.T. & Corbato´, C.E. 1993. Quantitative-analysis ofmixtures of 1M and 2M1 dioctahedral micas by X-ray-diffraction.Clays and Clay Minerals, 41, 45–55.
Torrent, J. 1995.Genesis and Properties of the Soils of theMediterranean Regions. Pubblicazione Universita` di NapoliFederico II, Napoli.
Torrent, J., Schwertmann, U., Fechter, H. & Alfe´rez, F. 1983.Quantitative relationships between soil color and hematite content.Soil Science, 136,354–358.
Yaalon, D.H. & Ganor, E. 1973. The influence of dust on soils duringthe Quaternary.Soil Science, 116,146–155.