Lipids - biyokimya.vetbiyokimya.vet/documents/biyokimya/Lipids.pdf · Lipids Lipid are found in...
Transcript of Lipids - biyokimya.vetbiyokimya.vet/documents/biyokimya/Lipids.pdf · Lipids Lipid are found in...

LIPIDSSerkan SAYINER, DVM PhD. Assist. Prof.
Near East University, Faculty of Veterinary Medicine
Department of Biochemistry

Lipids Lipid are found in nature together with carbohydrates
and proteins. It is a group of organic substances that
carry the greatest importance in quantitative terms.
They are generally water-insoluble and dissolved in
organic solvents such as ether and chloroform.
They can be used by the living beings.
The most important features are their energy source
and their presence in membranes.
They may be esters of fatty acids or esterified.

Lipids have a unique place among organic molecules.
The identities of lipids are based on their physical
properties, not on the presence of specific functional
groups they contain.• For this reason, they are very wide and different, or are found,
and each has various functions.
Lipids contain very high numbers of non-polar C─C
and C─H bonds.
Lipids

In addition, most lipid molecules have several polar
bonds. • These polar bonds were made with various functional groups.
As a result, lipids are;• Nonpolar or weak polar,
• Soluble in organic solvents such as C6H14 and CCl4,
• Biomolecules that are insoluble in polar solvents such as
water.
Lipids have common properties with hydrocarbons in
many ways.
Lipids

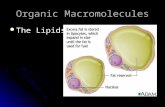
1. Lipids are cell membrane components.• The cell membrane surrounds the cell to protect it from
external influences and allows metabolic activity in the cell to
take place.
• Membranes are not a simple structure surrounding the cell, but
contain many important enzymes and transport systems in
their structure.
• Membranes contain many specific receptors on their outer
surface, helping hormones and other substances to effect.
General Function of Lipids

Carbohydrates
Hydrophobic
region
Hydrophilic
region
Choleserol Peripheral protein
Integral protein
Cytoplasm
Nucleus
Cell Membrane Cell

Polar
head
Mem
ran
phosp
holipid
Non-Polar
tail
Polar
head
Hydrophobic
region Libid bilayerİntracellular
enviroment
Extracellular
enviroment
Cell
Membrane
Simple
diffusion
Faciliated
transport
Active
transport
Integral
membrane
protein
Energy
input
needed

2. They are good source of energy (predominantly
triglycerides).• Fat molecules, a good energy store, give more energy than
carbohydrates of the same weight.
• 9 kcal/g of energy is gained with the oxidation of fatty acids.◦ Carbohydrates and proteins give 4 kcal/g of energy.
General Function of Lipids
Adipose tissue

3. Lipids are the source of some important items in organism.
• Lipoproteins
• Bile acids
• Vitamins in fat (A, D, E, K)
• Steroid hormones
4. They are effective in protecting against infections.• Eicosanoids
◦ Prostaglandins, Thromboxans, Prostacyclins, Leukotrienes, Lipoxins, Resolvins, Eoxins
General Function of Lipids

5. They are involved in cellular signaling.• There are lipid messengers.
• They bind to protein structured target receptor or enzyme
(such as kinase, phosphatase) and allows the formation of a
specific response in the cell.
• These messengers are;◦ Sfingolipid secunder messengers (Seramid, sphingosine ...),
◦ Phosphatidylinositol secunder Messenger,
◦ G-protein coupled receptor activators (Prostaglandins,
lysophosphatidic acid ...),
◦ Nuclear receptor activators (Steroid hormones ...).
General Function of Lipids

2. NON-HYDROLYZABLE LIPIDS• They are lipids that can not be
broken down into smaller
molecules when hydrolyzed with
water. The structure of such lipids
is more varied.
1. Steroids
2. Fat-soluble vitamins
3. Eicosanoids
1. HYDROLYZABLE LIPIDS• They are lipids that can be
separated into smaller
molecules when hydrolyzed
with water.
1. Triglycerides
2. Phospholipids
3. Waxes
4. Sphingolipids
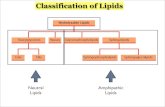
Classification of Lipids

MEMBRANE LIPIDS (POLAR)
• Phospholipids◦ Glycerophospholipids
– Glycerol + 2 fatty acids + P +
Alcohol
◦ Sphingophospholipids
– Sphingosine + fatty acid + P +
Choline
• Glycolipids◦ Sphingoglycolipids
– Sphingosine + fatty acid + mono or
oligosaccharide
STORAGE LIPIDS (NEUTRAL)
• Triglycerides◦ Glycerol + 3 fatty acids
Classification of Lipids

UNSAPONIFIED LIPIDS
• Terpenes
• Steroids
• Prostaglandins
• Alcohol and Ketone type
waxes
SAPONIFIED LIPIDS
• Glycerides
• Phosphoglycerides
• Sphingolipids
• Ester type waxes
Classification of Lipids

Complex Lipids• Phospholipids
• Sphingolipids
• Glycolipids
• Lipoproteins
• Sulfolipids
• Aminolipids
Simple Lipids• Fatty acids
• Neutral fats (Triglycerides)
• Waxes
Derived Lipids• Eicosanoids
• Steroids
• Ketone bodies
• Fat-soluble vitamins
Classification of Lipids

3. GLYCERIN-FREE LIPIDSA. Sphingolipids
i. Ceramides
ii. Sphingomyelins
iii. Glycosphingolipids
B. Aliphatic alcohols and waxes
C. Terpenes
D. Steroids
4. Lipids linked to other class compoundsA. Lipoproteins
B. Eicosonoids
C. Lisophosphoglycerides
D. Lipopolysaccharides
1. FATTY ACIDS
2. GLYCERIN CARRIED LIPIDSA. Neutral Oils
i. Mono-, di- and triglycerides
ii. Glycerin ethers
iii. Glycosylglycerols
B. Phosphoglyceridesi. Phosphatides
ii. Diphosphatidylglycerin andPhosphatidylinositol.
Classification of Lipids

Definition, Nomenclature, Numbering, Classification,
Essential Fatty Acids, Physical and Chemical Properties
Fatty Acids

Fatty AcidsThey form the most important class of lipids and are
long-chain organic acids with 4-28 carbon atoms.
It has a hydrocarbon tail and a carboxyl group in its
structure.
In other words «Fatty acids are monocarboxylic
organic acids with hydrocarbon chains»• They are obtained predominantly from the hydrolysis of
triglycerides.
They are insoluble in water and show grease character.

Fatty Acids Fatty acids are not found freely in cells and tissues.
Most of the fatty acids form complexes with other
lipid molecules.• E.g. Triglycerides, phospholipids.
They are enzymatically or chemically hydrolyzed from
the tissues or from where they are.
Free fatty acids are found in the cells at very low levels.

Unesterified forms are called free fatty acid (FFA / non-
esterified fatty acid, NEFA), and this form is
transported in the plasma with the link to the albumin.
About 100 different fatty acids have been identified
from various plants, animals and microorganisms.
The majority of the fatty acids present in the animal are
unbranched, straight-chain derivatives and have a
number of double carbon atoms.
Fatty Acids

Fatty Acids Fatty acids are separated from each other by the
number of carbon atoms in the structure or the double
bond structure and chain lengths they contain.
Almost all of the fatty acids found in nature have
double carbon atoms. 16 and 18 C atoms are mostly
found.• Palmitic acid is a common 16 C fatty acid.
• There are both polar and non-polar parts in the structure.
• The hydrophobic part of a lipid is always bigger.

Palmitic Acid(C16H32O2)
Skeletal structurePolar C─O and O─H bonds
Nonpolar C─C and C─H bonds
Hydrophilic
head
Hydrophobic
hydrocarbon chain

Fatty AcidsThere are two different types of fatty acids.
• Saturated Fatty Acids: Fatty acids which have a long
hydrocarbon tail and contain only single C─C bonds. For
example; Palmitic acid and Stearic acid.◦ This group includes short chain volatile acids (short-chain fatty acids)
obtained as a result of microbial carbohydrate fermentation.
◦ Especially in rumen this event is very important for ruminants.
◦ Acetic acid (C2) is the simplest short-chain or volatil fatty acid
(VFA).
◦ In animals, palmitic acid (C16) is obtained by lipogenesis, from which
other saturated and unsaturated fatty acids are synthesized.

Fatty Acids• Unsaturated Fatty Acids: There are one or more double
bonds (C=C) in the structure.◦ If there is only one double bond in the structure, it is called
monounsatured fatty acid.
◦ If there is more than one double bond, it is called polyunsaturatedfatty acid.
◦ Unsaturated fatty acids are dominant over saturated fatty acids, especially in animals living in cold environments and in highlyorganized plants.
◦ Because of the diversity of the double bonds, they have isomers.
◦ The geometric isomerism (cis- or trans-) is observed according to the orientations of the groups connected to the double bonds.
◦ If the groups are on the same side, it is called cis-configuration. Ifthe groups are on the opposite side, it is called trans-configuration.

Fatty Acids◦ The double bond of oleic acid is in cis-configuration and twisted at
the double bond location, so appearing L-shaped.
◦ Elaidic acid is in trans-configuration and protects the flat form
(straight chain) where double bonds are present.
◦ Most natural long chain unsaturated fatty acids have cis-
configuration. For example Arachidonic acid 4 has double bonds in the
cis-configuration and it is U-shaped.
◦ Some trans-unsaturated fatty acids (e.g. Elaidic acid) are found in
ruminants (due to rumen flora). Moreover, their presence in vegetable
oils, such as margarine, led to their health questioning. – They cause problems like hypercholesterolemia, atherosclerosis, coronary
atherosclerosis and essential fatty acids in metabolism.


Fatty AcidsThe most abundant fatty acids in mammalian living
beings are oleic acid (18:1), palmitic acid (16:0) and
stearic acid. (18:0).• Fatty acids between 14-24 C can be synthesized and stored,
with the most 16-18 C in the mammals.
A saturated fatty acid
No double bonds in long chain structure
An unsaturated fatty acid
There is one cis-double bond in the long chain
structure.
Stearic AcidOleic Acid
Cis- double bond

Fatty AcidsMammalians can synthesize saturated and
monounsaturated fatty acids.
The most common unsaturated fatty acids in animal lipids are palmitoleic, oleic, linoleic and arachidonicacids.
Oleic acid is the most common fatty acid in nature. Itwas detected that all known natural oils and phospholipids have oleic acid.

Fatty AcidsHowever, nutrients must contain polyunsaturated fatty
acids (PUFAs).• These fatty acids must be taken from nutrients by mammals,
because they can not synthesize and/or synthesize enough to
meet their needs.
Linoleic acid (18:2), which can not be synthesized by
mammals, is found in vegetable oils. Linolenic acid
(18:3) is abundant in fish oils and both are essential
fatty acids.

Saturated fatty acids with a 10 or less C atom are rarely
found among animal lipids.
Bacteria contain simpler saturated fatty acids. However,
mono-saturated fatty acids are also found.
Polyunsaturated fatty acids usually contain a double
number of C atoms.• Those containing a single number of C atoms are rare in living
creatures on land (trace level). But it is found in high amounts
in sea creatures.
Fatty Acids

Each fatty acid has a generic name according to trivial naming (common names). For example Oleic acid, stearic acid etc…
According to IUPAC systematic naming, fatty acids are named according to hydrocarbons having the same number of carbon atoms.
In this system, "-e" suffix of the hydrocarbon arereplaced with the suffix "-oic asit".
Nomenclature of Fatty Acids

For this reason;• Saturated fatty acids terminate with the suffix "-anoic".
◦ A saturated fatty acid of 10 carbons; Decanoic acid = capric acid or
◦ A saturated fatty acid of 18 carbons; Octadecanoic acid = stearic acid.
• Doubly bonded unsaturated fatty acids terminate with
the suffix "-enoic acid".◦ 18 carbons, an unsaturated single double bonded, and a double bond in the
chain between the 9th and 10th carbons; 9, octadecaenoic = oleic acid or
◦ 18 carbons with 3 double bonds and double bonds in positions 9-10, 12-13
and 15-16; 9,12,15 octadecatrienoic = alpha-linolenic acid.
– 9,12,15 octadecatrienoic acid or α-linoleic acid refers to the number of carbon atoms
that double bonds start to show the position of the unsaturated bond, and to the
number of double bonds to indicate the number of double bonds.
Nomenclature of Fatty Acids

Nomenclature of Fatty AcidsThe naming of fatty acids follows the Greek
alphabetical order.
The names α (2nd C), followed by β (3hr C) and γ (4th
C) are given to the carbon atoms after the carboxyl
group (COOH - 1st C).
The farthest C atom (Terminal group, CH3 group) from
the carboxyl group is named ω.• If there are more than one double bond, these double bonds
are not conjugated (-CH=CH-CH=CH-), separated by a
methylene group. For example: -CH=CH-CH2-CH=CH-

ω αβγδ
ω α
β
γ
δ
16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1
16:0
Palmitic Acid(C16H32O2)
Skeletal structure

Nomenclature of Fatty Acids Numbering and display of unsaturated fatty acids can be
done in two ways.1. Starting from the COOH head: There is a double bond
between the 9th and 10th carbon atoms in the majority of the unsaturated fatty acids.
◦ Starting at C 'in COOH, C atoms are numbered.
◦ The carbon atom in which the unsaturated bond is located is designated Δ sign and the atomic number of the C atom to which the bond begins is written as Δ9.
◦ If it contains more than one double bond, the double atom at which the double bonds begin is written by adding a comma (Δ9,12,15).
◦ If it is to be written without specifying Δ, write C atom number followed by «:», double bond number followed by «;» and C atom numbers at which double bonds start (e.g. 18:2;9,12).

Nomenclature of Fatty Acids2. Starting from the Omega-C atom (CH3 terminal end)
◦ Unsaturated fatty acids are sometimes classified as omega-nacids.
◦ C atoms are numbered starting from ω-C or n-C atom.
◦ The C atom number of the first double bond indicates the ωgroup in which the fatty acid is present. (E.g. In Omega-3, first double bond is located at C atom number 3, or written as n-3).
◦ Then with the order; The total number of C's, «:» followed by the number of double bonds, followed by «;» and the number of C atoms at which double bonds start.
◦ As a result, if an unsaturated fatty acid is to be written according to omega classification, first the omega group is specified. Subsequently, the total number of C atoms, the number of double bonds, and the positions where double bonds are present is written (for example: ω6, C18: 2, 6,9 or n-6,18: 2;6,9).

IUPAC Numerical Multiplier
Nomenclature of Fatty Acids
Number Multiplier Number Multiplier Number Multiplier
1 mono- 11 undeca- 21 heneicosa-/henicosa-
2 di- 12 dodeca- 22 docosa-
3 tri- 13 trideca- 23 tricosa-
4 tetra- 14 tetradeca- 24 tetracosa-
5 penta- 15 pentadeca- 25 pentacosa-
6 hexa- 16 hexadeca- 26 hexacosa-
7 hepta- 17 heptadeca- 27 heptacosa-
8 octa- 18 octadeca- 28 octacosa-
9 nona- 19 nonadeca- 29 nonacosa-
10 deca- 20 eicosa-/icosa- 30 triaconta-

CH 3CH 2CH 2CH 2CH 2CH CHCH 2CH CHCH 2CH 2CH 2CH 2CH 2CH 2CH 2COOH1
Name the fatty acid starting from the COOH head.
2
α
3β
4
γ
91218
ω
• 18 Carbon atoms.
• Two double bonds on 9th and 12th carbon atoms.
• Δ9,1218:2 or,
• 18:2;9,12.• Fatty Acid is,
• Linoleic Acid (9,12-octadecadienoic acid)

18
Name the fatty acid starting from the CH3 terminal end.
171615961
ω
• 18 Carbon Atoms.
• Two double bonds on 6th and 9th carbon atoms.
• The first double bond is at 6th C so it is an ω6 or n-6.
• ω6,C18:2;6,9 or,
• n-6,18:2;6,9.• Fatty acid is,
• Linoleic Acid (9,12-octadecadienoic acid), an ω6 fatty acid.
CH 3CH 2CH 2CH 2CH 2CH CHCH 2CH CHCH 2CH 2CH 2CH 2CH 2CH 2CH 2COOH

Linoleic Acid (Unsaturated)

Naming according to COOH head
• Δ6,9,12,1518:4
• 18:4;6,9,12,15
Naming according to CH3 terminal end
• ω3,C18:4;3,6,9,12
• n-3,18:4;3,6,9,12
6,9,12,15-octadecatetraenoic acidt
Stearidonic acid

Naming according to COOH head
• Δ5,8,11,14,1720:5
• 20:5;5,8,11,14,17
Naming according to CH3 terminal end
• ω3,C20:5;3,6,9,12,15
• n-3,20:5;3,6,9,12,15
5,8,11,14,17-eicosapentaenoic acid
Timnodonic Acid

Naming according to COOH head
• Δ5,8,11,14 20:4
• 20:4;5,8,11,14
Naming according to CH3 terminal end
• ω6,C20:4;6,9,12,15
• n-6,20:4;6,9,12,15
5,8,11,14-eicosatetraenoic acid
Arachidonic Acid
5
8
11
14
6
9
12
15
ω
αβ 1
1
20

Draw the fatty acid mentioned above.
all-cis-7,10,13,16,19-docosapentaenoic acid
Clupanodonic Acid

Classification of Fatty Acids
Acetic Acid (2:0)
Propionic Acid (3:0)
Butyric Acid (4:0)
Valeric Acid (5:0)
Caproic Acid (6:0)
Caprilic Acid (8:0)
Capric Acid (10:0)
Lauric Acid (12:0)
Myristic Acid (14:0)
Palmitic Acid (16:0)
Stearic Acid (18:0)
Arachidic Acid (20:0)
Behenic Acid (22:0)
Lignoceric Acid (24:0)
Serotic Acid (26:0)
Montanic Acid (28:0)
SATURATED FATTY ACIDS

Classification of Fatty Acids
Palmitoleic Acid (16:1;9-ω7)
Oleic Acid (18:1;9 (cis)–ω9)
Elaidic Acid (18:1;9 (trans)–ω9)
Vacsenic Acid (18:1;11-ω7)
Linoleic Acid (18:2;9,12–ω6)
γ-Linolenic Acid (18:3;6,9,12–ω6)
α-Linolenic Acid (18:3;9,12,15–ω3)
Arachidonic Acid (20:4;5,8,11,14–ω6)
UNSATURATED FATTY ACIDS
Timnodonic Acid(20:5;5,8,11,14,17 – ω3)
Eruadic Acid (22:1;13 – ω9)
Klupanodonic Acid(22:5;7,10,13,16,19 – ω3)
Cervonic Acid(22:6;4,7,10,13,16,19 – ω3)
Nervonic Acid (24:1;15 –ω9)

Classification of Fatty Acids
ω3
• α-Linolenic Acid (18:3;9,12,15-ω3)
• Timnodonic Acid (20:5;5,8,11,14,17–ω3)
• Klupanodonic Acid (22:5;7,10,13,16,19–ω3)
• Servonic Acid (22:6;4,7,10,13,16,19–ω3)
ω6• Linoleic Acid (18:2;9,12–ω6)
• γ-Linolenic Acid (18:3;6,9,12–ω6)
• Arachidonic Acid (20:4;5,8,11,14–ω6)
OMEGA CLASSIFICATION of Unsaturated Fatty Acids
ω7• Palmitoleic Acid (16:1;9-ω7)
• Vacsenic Acid (18:1;11-ω7)
ω9• Eruadic Acid (22:1;13–ω9)
• Nervonic Acid (24:1;15-ω9)• Oleic Acid (18:1;9 (cis)–ω9)
• Elaidic Acid (18:1;9 (trans)–ω9)

Classification of Fatty Acids
Short-Chain FAs• Asetic acid (2:O)
• Propiyonic acid (3:0)
• Butyric acid (4:0)
• Valeric acid (5:0)
Medium-Chain FAs• Caproic acid (6:0)
• Caprilic acid (8:0)
• Capric acid (10:0
• Laurl acid (12:0)
Long-Chain FAs• Miristoleic acid (14:1)
• Miristic acid (14:0)
• Palmitoleic acid (16:1;9)
• Palmitic acid (16:0)
• Stearic acid (18:0)
• Oleic acid (18:1;9 (cis))
• Linoleic acid (18:2;9,12)
• Linolenic acid (18:3;6,9,12)
• Arachidonic acid (20:4;5,8,11,14)
• Arachidic acid (20:0)
• Lignoceric Acid (24:0)

Short-Chain Fatty AcidsAcetic Acid (2:0)
• It is major final product of symbiotic microbes.
Propiyonic Acid (3:0)• It is the end product of carbohydrate fermentation by
symbiotic microbes. It’s a gluconeogenic fatty acid.
Butyric Acid (4:0)• It is the main end product of carbohydrate fermentation
carried out by simbiotic microbes.
Valeric Acid (5:0)• It is the minor end product of carbohydrate fermentation
carried out by symbiotic microbes.

Caproic Acid (6:0)• It is the minor end product of carbohydrate fermentation
carried out by symbiotic microbes.
Caprilic Acid (8:0)• Also known as octanoic acid.
• It is found in vegetable oils.
Lauric Acid (12:0)• It's found in sperm.
Medium-Chain Fatty Acids

Miristic Acid (14:0) • It is found in vegetable oils.
Palmitic Acid (16:0)• It is found in vegetable and animal oils.
Stearic Acid (18:0)• It is found in vegetable and animal oils.
Arachidic Acid (20:0)• It is found in high amounts in peanut oil.
Behenic Acid (22:0) • It is found in high amounts in the seeds.
Long-Chaind Fatty Acids

Palmitoleic Acid (16:1;9-ω7)• It is found in most lipids.
Oleic Acid (18:1;9 (cis)–ω9)• It is widely found in neutral lipids.
Elaidic Acid (18:1;9 (trans)–ω9)• Ruminant lipids.
Vacsenic Acid (18:1;11-ω7)• It is created by the bacteria.
Linoleic Acid (18:2;9,12–ω6)• It is found in plants and animals.
Unsaturated Fatty Acids

γ-Linolenic Acid (18:3;6,9,12–ω6)• It is found in plants and animals.
• Linoleic acid derivative.
α-Linolenic Acid (18:3;9,12,15–ω3)• It is commonly found in fish fats.
Arachidonic Acid (20:4;5,8,11,14–ω6)• It is also called Eicosatetraenoic acid.
• It is found in animal phospholipids.
Timnodonic Acid (20:5;5,8,11,14,17–ω3)• Also known as Eicosapentaenoic acid.
• It is commonly found in fish fats.
Unsaturated Fatty Acids

Eruadic Acid (22:1;13 – ω9)• It is found in mustard seed oil.
Clupanodonic Acid (22:5;7,10,13,16,19 – ω3)• It is named as Docosapentaenoic acid.
• It is found in fish fat and in the brain phospholipids.
Cervonic Acid (22:6;4,7,10,13,16,19 – ω3)• It is named as Docosahexaenoic acid.
• It is found in fish fat and in the brain phospholipids.
Nervonic Acid (24:1;15 – ω9)• It is named as Tetracosenoic acid.
• It is found in the cerebrocytes.
Unsaturated Fatty Acids

They were first detected ~60 years ago.
Because they can not be synthesized by the organism, they are fatty acids which must be taken with nutrients.
Linoleic acid, which is one of the unsaturated fatty acids, is considered to be a true essential fatty acid. It can not be synthesized by mammals.
Linoleic acid is found in the structure of triglycerides and glycerophospholipids by 10-20%.
Essential Fatty Acids

Linoleic acid, arachidonic acid and timnodonic acid
can be synthesized by mammals over linoleic acid.
However, since this synthesis is not at a level that meets
the needs, these fatty acids must also be taken with
foods.
Thus, linoleic acid, linolenic acid, arachidonic acid and
timnodonic acid are essential fatty acids.
Essential Fatty Acids

Some disorders arise as a result of inadequate intake with food or no intake.• Deficiencies are characterized by skin problems such as
lesions, dryness and a decline in growth.
Essential fatty acids are required for the synthesis of eicosanoids (prostaglandins, prostacyclins, thromboxanes, leukotrienes, lipoxins).• These compounds have hormone-like effects and are present in
trace amounts, but their physiological effects are very important.
Essential Fatty Acids

Both physical and physiological properties of fatty acids depend on the length of the carbon chain and on the number of double bonds in the molecule (the degree of unsaturation of the fatty acid).
Fatty acids are amphipatic and have both hydrophobic and hydrophilic domains. This binary structure generally plays a key role in the function of biological lipids against water.• Hydrocarbon groups try to combine to form a minimum surface with
the carboxyl groups forming a contact surface around the mass of water.
• The chain length of the hydrocarbon group is determined by superior behavior. Hydrophobic structures are very strong inpalmitic acid.
Physical Properties of Fatty Acids

Fatty acids are not water-soluble, but their Na+ and K+ salts are soluble in water.
The Na+ and K+ salts of fatty acids emulsify oils and water-insoluble fatty substances.• Na and K soaps dissolve in water and they are cleaners. Others are
not soluble in water and are not cleaners. Commercial soaps are Na salt of palmitic, stearic and oleic acid.
Ca+2 or Mg+2 soaps of fatty acids are very difficult to dissolve in water and thus can not emulsify fatty layers. When K soaps are used in hard water (calcareous water) containing Ca and Mg, they do not dissolve in the water and precipitate to the bottom.
Physical Properties of Fatty Acids

The polar head of soap forms a hydrophilic cover around
fatty part and the fat droplets are reduced into smaller
pieces, so the lipid layer is removed.
Bath soaps are a general mixture of fatty acids with
potassium soaps. Sodium or potassium soaps are
amphipathic. Ionized carboxyl head is polar and tail part
is nonpolar.
Physical Properties of Fatty Acids

Physical Properties of Fatty Acids All saturated fatty acids with a carbon number up to 10
are liquid and volatile at ordinary temperature. Fatty acids with a carbon number greater than 10 are solid at body temperature.
As the carbon chains of the fatty acids increase, the fatty acid hardens and the melting point begins to rise.
Unsaturated fatty acids are liquid at room temperature due to double bonds in the structure. Also, as the number of double bonds increases, the melting point begins to fall.• For example: The 18:2 unsaturated fatty acids are liquid at 0° C.
Unsaturated fatty acids have high reactivity due to the double bonds they carry.


In practice, natural acyl glycerols contain fatty acid mixtures which are shaped to fit their functional roles. For example, membrane lipids that must be liquid in
all environmental temperatures contain more unsaturated fatty acids than storage lipids. In tissues encountering cold, eg. Lipids found in pigs or
hibernating animals or on the extremities of animals are more unsaturated. • In other words, living beings living in cold regions have more
unsaturated fatty acids in membrane lipids than living in warm regions. The glycerides, which are rich in saturated fatty acids, are solid.
Physical Properties of Fatty Acids

Almost all of the naturally occurring long chain
unsaturated fatty acids are in the cis- configuration
However, in the case of unsaturated fatty acids, the
isomeric forms of the double bonds are the cis- and
trans- isomers which appear as isomeric forms,
depending on the sequence around the double bond.• For example, oleic acid has a melting point of +13°C and cis.
When oleic acid is treated with nitric acid, the trans-form of
elaidic acid occurs. The melting point of elaidic acid is +45°C.
Physical Properties of Fatty Acids

Doubly bonded fatty acids are oxidized by strong
oxidizing agents such as hydrogen peroxide (H2O2) and
superoxide anion radicals (˙OH, O2˙).
These substances are toxic to the cell.
Accordingly, peroxidation of lipids in the cell membrane
leads to deterioration of membrane protein structure.
Chemical Properties of Fatty Acids

Salts of Fatty Acids• The salts of fatty acids whose carbon number is higher than
6 are called soap.
• The ionic head of soaps form a polar group to bind with water
by hydrogen bonding.
• Non-polar tail are assembled together. So soap molecules that
are dispersed in water form mycelia.
• Na and K soaps dissolve in water and are cleaners. Others are
not soluble in water and are not cleaners.
Chemical Properties of Fatty Acids

• Potassium soaps are softer than sodium soaps and melt faster.
The soaps of unsaturated fatty acids are more soluble in water
and alcohol than in saturated ones.
• The soaps sold in the market are the sodium salts of the same
fatty acids. There are sodium carbonate and sodium silicate to
make the water softer.
• Potassium salts of palmitic, stearic or oleic acid are known
as arabic soap (soft soap).
Chemical Properties of Fatty Acids

• Calcium soaps of long chain fatty acids are found in the
addition of engine oils.
• Aluminum soaps have been used in the industry since they
have formed a durable gel.
• Cleaners called detergents have been developed. These
substances are usually alkylbenzenesulfonates, a family of
compounds that are similar to soap but are more soluble in
hard water.
Chemical Properties of Fatty Acids

Detergent Formation• Detergents are metal (such as Na, K, Ca, Mg) salts of sulfuric
acid esters of higher alcohols which are reduction products of fatty acids.
• Fatty acid first turns into alcohol, which is done with high pressure and heat.
• Detergents can be used more easily in hard water as they form Ca+2 and Mg+2 salts. Cleaning ability is more.
• Since they are sulfuric acid esters, their salts are superior cleaning agents due to their not breaking up in acid solutions.
• Even with acid, they do not break down and cause environmental pollution.
Chemical Properties of Fatty Acids

• Since some of the detergents contain K+ , such detergents are
more environmental pollutant.
• Because K+ is a good growth factor for the growth of green
algae living in sweet water (fresh water).
• Along with the mixing of detergents into the surrounding
waters, algae growth in water is increased and therefore the
amount of oxygen in the water decreases.
• Decreasing oxygen in the water will cause aquatic organisms to
die especially fish.
Chemical Properties of Fatty Acids

Ester formation.• The carboxyl groups of fatty acids are reversibly associated
with alcohols.
• The esterification is self-slow, but is rapid in the presence of
heat or hydrogen ions.
Features of Double Bond• Hydrogenation: It is the saturation of the ethylene bond (-
CH=CH-) in the structure of unsaturated fatty acids with
hydrogen. For example: When oleic acid is hydrogenated,
stearic acid is formed, meaning the unsaturated fatty acid is
converted to saturated fatty acid.
Chemical Properties of Fatty Acids

• Addition a halogen to double bond: They are saturated by
adding halogen to the double bonds. This process takes place
in the presence of catalysts such as Br, Pt, Ni, F, Cl, I and Cu at
high pressure.
• Oxidation: Oxidation of ethylene bonds.◦ KMNO4 is used as the oxidant in the oxidation of fatty acids. Since the
oxidation process is very complex, many intermediate metabolites
may form during oxidation.
◦ Ozonoide is an any of a class of unstable cyclic compounds formed by
the addition of ozone to a carbon–carbon double bond.
• These reactions are used to determine which carbon atoms are
joind to double bongin in an any unsaturated fatty acids.
Chemical Properties of Fatty Acids

• Peroxidation: Peroxidation of polyunsaturated fatty acids,
which is formed in vivo and is called lipid peroxidation, is a
chain reaction.
◦ During the reaction, very reactive free radicals are formed
which contain unpaired electrons.
◦ Free radicals enter into wide-ranging reactions in living
organisms.
◦ However, the defense system (antioxidative system) in the
organism prevents or controls the harmful effects of these
compounds.
Chemical Properties of Fatty Acids

Glycerin
Neutral Lipids (Mono-, di- and triglycerides, Glycerin ethers, Glycosylglycerols)
Phosphoglycerides (Phosphatides, Diphosphatidylglycerin and Phosphatidylinositols)
Lipids carrying Glycerin

Glycerin Also known as glycerol. It is a trivalent polyol with a
sweet, thick, colorless, odorless and liquid character.
It forms the backbone of glycerolipids.• Glycerin may be miscible with water and ethyl alcohol in any case
and will not dissolve in ether, chloroform and benzene.
• If it is oxidized with hydrogen peroxide under the catalytic action
of iron salts in a slightly alkaline medium, a mixture of
glyceraldehyde and dihydroxyacetone occurs.
• Due to its water-absorbing and moisturizing properties, it is a
desirable ingredient in cosmetic and pharmaceutical products. It is
used in the production of some explosive substances (nitroglycerin).
• It is easily ingested by animals.

Glycerol(1,2,3-propanetriol)
Dihydroxyacetone Glyceraldehyde
1
2
3
1
2
3
sn-1
sn-2
sn-3
Chirality?

Neutral lipids are esters of fatty acids with glycerol.
They form the most common class of lipids.
Among the vegetable oils; olive, cottonseed, linseed,
coconut, peanut, soybean and poppy oils are the most
important and common.
It is found in all animal tissues in more or less amounts.
Neutral Lipids

Lipid storages have three important functions.1. They are substitute nutrients.
2. They serve as insulators against heat loss from the body.
3. They serve as cushions to protect the internal organs from
external impacts.
Classification;• Mono-, di- and triglycerides
◦ Fats (animal) and Oils (herbal)
• Glycerin ethers
• Glycosyl glycerines
Neutral Lipids

When an alcohol group of glycerin is esterified with a
one mole fatty acid, monoglyceride occurs.
When two alcohol groups of glycerin is esterified with a
two mole fatty acids, diglyceride occurs.
When three alcohol groups of glycerin is esterified with
three mole fatty acids, triglyceride occurs.
Mono-, di- and triglycerides

Triglycerols (Triacylglycerol) or triglycerides
(Triacylglycerines) are triesters which are formed by
esterification of 3 fatty acids and glycerol.
In general, the structure of the fat is triglyceride. Often
triglycerides are used for fats.• The lipids in animal and vegetable oils are mostly
triglycerides.
• These are a concentrated metabolic energy source of 9 kcal/g.
Mono-, di- and triglycerides

Glycerol Triglyceride
(Ester groups are shown
with red color)
Diagram of
Triglyceride
Gly
cero
l
Fatty Acids
R group contains 11-10
C atoms.
Fatty Acid
Fatty Acid
Fatty Acid

Palmitic Acid
Oleic Acid
α-Linolenic Acid
Gly
cero
l
C55H98O6
Triacylglycerol (TAG-TG)(1-palmitoil-2-oleil-3-α-linolenoil-glycerol/POL)

Both animal fats and vegetable oils, if necessary, are highly
complex esters of fatty acids with glycerin. These esters are
called glyceride.
The fatty acids in glycerides can also be the same or
different.• If all three of the esterified fatty acids are the same, simple
(homogeneous) glycerides form. For example, if three moles of
stearic acid is bound to a glycerol, it is called stearin/tristearin. If
3 moles of oleic acid is bound, it is called olein.
• When fatty acids esterified to glycerol to form glyceride are
different from each other, mixed (heterogeneous) glycerides
occur.
Mono-, di- and triglycerides

Physical Properties of Triglycerides
The fatty acids in the triglycerides may be saturated
and/or unsaturated. Animal fats and vegetable oils are
triglycerides with different physical properties.
Animal fats have a high melting point. They are solid at
room temperature.
Vegetable oils have a low melting point. They are liquid
at room temperature.

To understand that a triglyceride is a fat or an oil, fatty
acids in its structure must be taken care.
As the number of double bonds in the fatty acid chain
increases, the melting point of triglyceride decreases.
While animal fats are composed of low double bond
fatty acids, vegetable fats are composed of high double
bond fatty acids.
Physical Properties of Triglycerides

Solid fats have saturated fatty acids in relatively high proportions and are generally of animal origin.• Pork oil, butter and whale shell high in saturated fat.
• Without double bonds, the three side chains of saturated lipid extend parallel to each other and have a high melting point.
Liquid oils have a higher percentage of unsaturated fatty acids and are usually of vegetable origin.• Fats extracted from corn, soybean oil and olives contain more
unsaturated lipids.
• Unsaturated lipids, a cis- double bond, forms a bend in the side chain and makes it more difficult to pack efficiently in solid form. This leads to a lower melting point.
Physical Properties of Triglycerides

Physical Properties of Triglycerides
Melting points are higher than the melting point of the
fatty acid they carry.
Short chain fatty acids are soluble in both water and
organic solvents. Long chain fatty acids do not dissolve
in water, but dissolves in organic solvents.
Except for the lipids of hydroxy-fatty acids, other lipids
dissolve in boiling petroleum ether.

Physical Properties of Triglycerides
The specific gravity of oils and fats is lower than water.
Pure glycerides are colorless, odorless, tasteless. If a
glyceride has color, odor and taste, it is derived from
foreign substances mixed with glycerides.
The yellow color of the butter comes from the presence
of herbal pigments called carotene and xanthophylls.
Unsaturated fatty acids of liquid oils can be solidified by
saturating with hydrogen. Structures that are solidified
by hydrogenation are called margarine.




Hydrolysis• Triglycerides are hydrolyzed by boiling with acids or bases
under normal pressure or with water under high pressure or
by catalytic action of certain enzymes; lipase.
• The formation of unpleasant odor and taste in oils known as
aggravation is partly due to the release of fatty acids.
• Hydrolysis of triglycerides yields 1 mole of glycerol and 3 mole
of fatty acid.
Chemical Properties of Triglycerides

Hydrolysis cleaves the three single bonds between the carbonyl carbons
and the oxygen atoms of the esters. Since tristearin contains three
identical R groups on the carbonyl carbons, three molecules of a single
fatty acid, stearic acid, are formed.
The three
bonds drawn
in red are
broken in
hydrolysis.
Glycerol
Stearic acid
Tristearin

Glycerol
Stearic acid
Palmitic acid
Palmitoleic acid

Saponification• Soap is prepared by the basic hydrolysis (saponification) of a
triacylglycerol. Heating an animal fat or vegetable oil with
aqueous base hydrolyzes the three esters to form glycerol
and sodium salts of three fatty acids.
• Saponification Value: It is expressed by potassium hydroxide
(KOH) in mg required to saponify one (1) gram of fat.◦ The saponification value is regarded as an index showing the average
molecular weight of fatty acids.
◦ Some soaps, especially Na and K soaps, are easily soluble in water
because the dissolution of the carboxylic groups is quicker.
Chemical Properties of Triglycerides

Sodium stearate is the sodium salt of stearic acid, an
18-carbon saturated fatty acid.
Sodium stearate ionic endpolar head long hydrocarbon chain
nonpolar tail

TrioleinGlycerol
Sodium oleat
soap

Chemical Properties of Triglycerides
Separation of fatty acids• It is the phenomenon of separating fatty acids in an fat or oil.
• This reaction makes it possible to remove fatty acids in an oil
or fat in free form.
Hydrogenation• Unsaturated bonds in fats are saturated with hydrogen.
• The unsaturated bonds of fatty acids in the oils are saturated
with hydrogen to yield margarines.

Chemical Properties of Triglycerides
Halogenation• The unsaturated bonds of fatty acids are saturated with
halogens such as Cl, Br, I.
• Iodine Value: It is the mass of iodine in grams that is consumed by 100 grams of a fatty acid.
• The iodine value is an indication of the relative unsaturation of oils.
Oxidation• It occurs when O3 or O2 is added to the double bonds of the
double acids of unsaturated fatty acid. The aggravation of the oil is partly due to the hydrolysis and the partial oxidation.

Chemical Properties of TriglyceridesAcetylation
• Fats or oils which carry fatty acids with free hydroxyl groups
are acetylated with acetic anhydride.
• Acetyl Number: A measure of free hydroxyl groups in fats or
oils determined by the amount of potassium hydroxide used
to neutralize the acetic acid formed by saponification of
acetylated fat or oil.
• The acetyl number gives the average amount of oxy-acids in
the fat or oil.

Chemical Properties of TriglyceridesRancidification
• It is the process which causes a substance to become rancid, that is, having a rank, unpleasant smell or taste.
• Acid Value◦ It is the mass of potassium hydroxide (KOH) in milligrams that is
required to neutralize one gram of free fatty acid. It is regarded as an index of rancidity caused by free fatty acids.
• Reichert Value (Reichert-Meissl-Wollny Value)◦ It is an indicator of how much volatile fatty acid can be extracted
from fat through saponification. It is equal to the number of milliliters of 0.1 normal hydroxide solution necessary for the neutralization of the water-soluble volatile fatty acids distilled and filtered from 5 grams of a given saponified fat.

Chemical Properties of TriglyceridesCauses of rancidification;1. Hydrolytic rancidity refers to the odor that develops when
triglycerides are hydrolyzed and free fatty acids are released.
2. Oxidative rancidity is associated with the degradation by
oxygen in the air. Via a free radical process, the double bonds
of an unsaturated fatty acid can undergo cleavage, releasing
volatile aldehydes and ketones. Oxidation primarily occurs
with unsaturated fats.
3. Microbial rancidity refers to a process in which
microorganisms, such as bacteria or molds, use their enzymes
such as lipases to break down fat.

Glycerin Ethers: They are lipids in which one or more
of the carbon atoms on glycerol is bonded to an alkyl
chain via an ether linkage, as opposed to the usual
ester linkage. It is found in animal tissues (shark and
whale fat).
Glycosylglycerols: A class of glycolipids structurally
analogous to phospholipids; they are the major
glycolipids of plants and microorganisms but are rare in
animals. Contain high levels of linoleic acid.
Neutral Lipids

Phospholipids: They are lipids containing a phosphate
group in its molecular structure together with fatty
acids and alcohols. There are 2 classes.
• Phosphoglycerides (Glycerophospholipids): Glycerides which
carry phosphoric acid esterified with glycerin/gycerol
(glycerophospholipids).
• Sphingophospholipids: Glycerides which carry phosphoric acid
esterified with sphingosine alcohol.
Phospholipids

Phospholipids are both structurally and functionally
important.• They are the basic building blocks of cell membranes.
• They are important components of lipoproteins.
• They function in intracellular signal transduction pathways like
lipid signaling (LPA, S1P, PAF, PIP ...).
• They are involved in blood coagulation (phosphotidylserine).
• They are found in the bile secretion (phosphatidylcholine).
• They are involved in the structure of the pulmonary
surfactants (phospholipoprotein).
Phospholipids

Phosphoglycerides are much more than sphingolipids and form a family with different structures.
Fatty acid is present at the C1 and C2 positions of glycerol. Usually;• The fatty acid in C1 is saturated,
• Fatty acid in C2 is an unsaturated fatty acid.
• The most common fatty acids are palmitic, stearic, oleic, linoleic and arachidonic acids.
In the C3 position, the phosphate ester is present, which is esterified with the hydroxy group of the İnositol or with the hydroxy group of one of the 3 nitrogenous bases; Choline, Serine or Ethanolamine.
Phosphoglycerides (Glycerophospholipids)

They are common in all animal and plant cells.
They are mostly found in eggs, brain, liver, kidney,
pancreas, lung and heart.
They are separated from other lipids by their dissolution
in acetone.
They are separated into 3 groups as Phosphotides,
Diphosphatidylglycerol (Cardiolipin) and
Phosphoinositides.
Phosphoglycerides (Glycerophospholipids)


Phosphatides are widely found in nature.Their glycerin is esterified with only 2 fatty acids.The 3rd -OH group of glycerin is a phosphodiester
moiety. This phosphodiester is attached to an alkyl group (R) derived from a low molecular weight alcohol.They are glycerophosphate derivatives and often
carry a nitrogenous base. This group contains lecithin, cephalin, phosphatidylserine, plasmalogens andplatelet-activating factor (PAF).
Phosphatides

Phosphatides Lecithin (Phosphatidylcholine)
• Glycerophosphate derivatives. It's a nitrogenous base.
• Except for the choline, the remainder is called phosphatidic
acid.
• Lecithin is therefore also called phosphatidylcholine.
• The fatty acids in the structure may be saturated or
unsaturated.
1 mole Glycerine + 2 moles Fatty acids + 1 mole H3PO4 + 1 mole Choline
LECITHIN

Phosphatides• Choline are also considered as vitamin.
• Inadequacy leads to some disorders such as fatty liver and
hemorrhage in kidneys.
• The phosphorylase A enzyme can partially hydrolyze lecithin.
This enzyme can be found in snake venom, hornet venom and
some microorganisms.◦ When hydrolyzed with phosphorylase A, 1 mole of fatty acid is
removed and the remaining part is called lysolecithin or
lysophosphatidylcholine. This molecule has a strong hemolytic effect.
• They are insoluble in water, but have a great deal of water.
Perhaps it is one of the most important elements of
protoplasm.

PhosphatidesCephalin (Phosphatidylethanolamine)
• They are similar with lecithin.
• The only difference between them is the presence of
ethanolamine (colamine) instead of choline.
1 mole Glycerine + 2 moles Fatty acids + 1 mole H3PO4
+1 mole Ethanolamine
CEPHALIN

Phosphatides• Fatty acids in the structure are usually one saturated and an
unsaturated.
• In lecithins, two fatty acids can be saturated or unsaturated.
This is a difference between the two.
• It is found in all body tissues, especially in the brain.
• Especially they are located in the structure of cellular
membranes.
• Cephalin is less soluble in alcohol than lecithin.

Lecithin Cephaline
EthanolamineCholine


PhosphatidesPhosphatidylserine
• They are similar to lecithin.
• The only difference between them is that serine is found
instead of choline, which is esterified with phosphoric acid.
• It is located on the structure of cell membranes.
• Cells function in signal pathways and coagulation.
1 mole Glycerine + 2 moles Fatty acids+ 1 mole H3PO4 + 1 mole Serine
PHOSPHATIDYLSERINE

PhosphatidesPlasmalogens
• When hydrolyzed, they give a mixture of high molecularaldehydes and esters of α- or β-glycerophosphoric acid with colamine or choline. It is an ether phospholipid.
• It is mostly found in brain-nerve cells (myelin) and muscle (cardiac) tissues.◦ It has been determined that it is present in excess amount in the
plasma membrane of cancer cells. It is thought to be involved in metastasis.
1 mole Glycerol + 1 mole High Fatty acid aldehyde (palmitaldehyde or
stearylaldehyde) + 1 mole Fatty acid + 1 mole H3PO4 + 1 mole Colamine/Choline
PLAZMALOJEN

PhosphatidesPlatelet-Activating Factor (PAF)
• It is an ether phospholipid. In the structure there is an ether-linked to long alkyl chain at the C1 position and an ester-linked to acetyl residue at the C2 position. It is synthesized by leukocytes.
• In many tissues, they have various effects. Ex. Platelet aggregation and relaxation of vascular smooth muscles. It acts in the inflammation and immune system.
1 mole Glycerol + 1 mole Fatty acid alkyl group (16C) + 1 mole Acetyl residue
+ 1 mole H3PO4 + 1 mole Choline
PAF

Two phosphatidic acid moieties connect with a glycerol backbone in the center to form a dimeric structure. It is found in all plants and animals.
• It is found on bacterial membranes and on the inner mitochondrial membrane of mammals. It is the only known antigenic phospholipid molecule.
It was first isolated from the heart muscle.Helps the formation of quaternary structure of proteins. It acts as a proton trapping agent for oxidative
phosphorylation.
Diphosphatidylglycerine (Cardiolipin)

The structure contains a group of inositol instead of
nitrogenous bases.• Inositol is a polyol and sugar alcohol (glucose).
• Inositol is involved in the myo-İnositol structure, myo-
conformer.
Phosphatidylinositols form a minor component on the
cytosolic side of eukaryotic cell membranes. But their
functions are great.
Phosphorylated forms of phosphatidylinositol are
called phosphoinositides.
Phosphatidylinositols

Phosphainositide;• 1 mole glycerin + 1 mole myo-İnositol + 2 moles of fatty
acids + 1-3 phosphoric acid.
• They are widely found in nature.
• They are involved in lipid signaling, cell stimulation and
membrane trafficking.◦ Phosphatidylinositol-3-phosphate, Phosphatidylinositol-4-phosphate,
Phosphatidylinositol-5-phosphate
◦ Phosphatidylinositol-3,4-biphosphate, Phosphatidylinositol-3,5-
biphosphate, Phosphatidylinositol-4,5-biphosphate
◦ Phosphatidylinositol-3,4,5-triphosphate
Phosphotidylinositols

Sphingolipids (Ceramides, Sphingomyelins, Glycosphingolipids [Cerebrosides,
Sulfatides, Globosides/Ceramide Oligosaccharides, Gangliosides]),
Aliphatic Alcohols and Waxes, Terpenes, Steroids
Glycerin-Free Lipids

The first group of Glycerin-free lipids are sphingolipids.
Sphingolipids carry sphingosine (18-carbon alcohol)
instead of glycerin.• They were discovered in brain extracts in the 1870s and were
named after the mythological Sphinx because of their
enigmatic nature
Sphingolipids are important structural components in
the membranes of animal and plant cells.• There is a lot in the brain and nerve tissue but store in trace
amount.
Sphingolipids

The sphingosine is an alcohol having 18 C atom, a
double bond and an amino group, so it’s also an amino
alcohol.
These compounds are regarded as derivatives of the
sphingosine base (4-sphingenine) or
dihydrosphingosine (sfinganine).• Fatty acids are linked by forming amide bond with amine
groups of sphingosine.
• Different sphingolipids come into being by binding different
substances to OH group of sphingosine.
Sphingolipids

Glycerol Sphingosine

Ceramides• They are N-acyl derivatives of sphingosine.
• Ceramides (Cer) are composed of sphingosine, which is an amide
linked to a fatty acyl chain (linked with amino group of
sphingosine), varying in length from 14 to 26 carbon atoms.◦ Synthesized in endoplasmic reticulum.
◦ There are two nonpolar tails in ceramides which are characteristic in all
sphingolipids.
◦ Ceramides form the basic structure of all sphingolipids.
• When they are hydrolyzed they give 1 mole of sphingosine and 1
mole of fatty acid.
• They are abundant in animal and plant tissues.
Sphingolipids

* There are two -OH groups at the 1st and 3rd carbon atoms.
* In particular, -OH at the 1st C atom is involved in the formation
of sphingomyelins and glycosphingolipids.
* Fatty acids in the structure are characteristic of very long
chains. Ex. Nervonic acid in brain tissue (24: 1, 15).
Sphingolipids
R: Region of fatty acid binding or Alkyl group
of fatty acid.
Sphingosine Alcohol

The Importance of Ceramides• They have been implicated in a variety of physiological functions
including apoptosis, cell growth, cell differentiation, aging, cell migration and cell adhesion.
• It is the main component of the stratum corneum in the epidermis.◦ They provide waterproofing with cholesterol and saturated fatty acids.
◦ It forms a barrier for microorganisms.
◦ Prevents excessive water loss by evaporation.
◦ With aging, the amount of ceramide and cholesterol in the stratum corneum is reduced.
• It is thought that ceramide or its metabolites play a role in the emergence of pathological conditions such as cancer, neurodegeneration, diabetes mellitus (insulin resistance), microbial pathogenesis, obesity (leptin resistance) and inflammation.
Sphingolipids

Sphingomyelins• They are phosphocholine or phosphoethanolamine
derivatives of ceramides. However, their general
characteristics are similar to those of phosphatidylcholines.◦ Endoplasmic reticulum, intestine and plasma membrane are
involved in synthesis.
• When hydrolyzed they give;◦ 1 mole of sphingosine alcohol, 1 mole of fatty acid, 1 mole of
choline and 1 mole of phosphoric acid.
◦ They are also phospholipids because they contain phosphoric acid.
Also classified as sphingophospholipids.
Sphingolipids

Examples of Sphingomyelins
The phosphodiester group is located at the terminal carbon

• Sphingomyelins are found especially in the membranes (e.g.
erythrocytes) and in the myelin sheath surrounding certain
nerve cells. ◦ The myelin sheath plays a vital role in normal nerve stimulation. For
example, in MS, impaired myelin sheath causes neurological problems.
• The fatty acids in the sphingomyelins structure vary
depending on where the sphingomyelin is found.◦ In the central nervous system, sphingomyelin contains stearic acid,
lignoseric acid and nervonic acid; in the spleen sphingomyelin contains
palmitic acid and lignoceric acid.
◦ Sphingomyelins often contain lignoceric acid as fatty acid.
Sphingolipids



Glycosphingolipids• They are carbohydrate derivatives of ceramides.
• They do not carry phosphoric acid and choline in their structure. Instead, they carry hexoses such as galactose,glucose and their N-acetyl derivatives.
• Depending on the accumulation of glycosphingolipids metabolic disorders occur.
• They are divided into 4 classes according to carbohydratesthey carry.◦ Cerebrosides
◦ Sulphatides
◦ Globosides (Ceramide oligosaccharides)
◦ Gangliosides
Sphingolipids

Sphingolipids• Cerebrosides
◦ When hydrolyzed they give 1
mole sphingosine + 1 mole
fatty acid + 1 mole galactose
or glucose. Mostly galactose.
◦ The carbohydrates are linked to
the C1 position of the
sphingosine.
◦ Galactocerebrosids are found abundantly in neuronal membranes in
the myelin sheaths of the brain and nerves.
◦ Glycoserbrosides are found mostly in extraneuronal membranes and
function as intermediates in the synthesis of complex glycolipids.

Sphingolipids◦ Cerebrosides are differentiated according to fatty acid carrying. They
carry fatty acids containing 24 C atoms. – The structure is similar to sphingomyelins. They contain carbohydrates instead of
phosphocholine.
◦ For example, if fatty acid is;– Lignoceric acid, it is called kerasin,
– Cerebronic acid, it is called cerebron (phrenosin),
– Nervonic acid, it is called nervon,
– Hydroxynervonic acid, it is called hydroxinervon.
◦ Cerebroside synthesis for the development of the nervous system
in the young is possible with the presence of carbohydrates,
especially galactose. For this reason, it is obvious how important it is
to take lactose.

• Sulphatides◦ They are sulfated galactocerebrosides.
◦ They are formed by the attachment of a sulfate residue to the 3rd carbon atom of galactose found in cerebrosides.
◦ Sulphatides are multifunctional molecules.
◦ As a cell membrane component; they have functions such as proteintrafficking, cell aggregation and adhesion, neural plasticity andmemory.
◦ It is involved in the nervous system, immune system, insulin secretion, blood clotting, viral and bacterial infections.
◦ Abnormal metabolism of sulphatides is also seen in many known metabolic diseases and is thought to be related to these pathologies.– E.g. Alzheimer's Disease, Parkinson's Disease, Diabetes mellitus.
Sphingolipids

Sulphatide
Sphingosine
Fatty acid
Galactose
Sulphate

Sphingolipids
• Globosides (Ceramide Oligosaccharides)◦ Ceramide oligosaccharides are sphingolipids that contain multiple serially
linked sugar units.
– Generally, D-glucose (GIc), D-galactose (Gal) or N-acetyl-D-galactosamine (GalNAc).
– Cerebrosides and globocytes are also called neutral glycolipids. They are neutral at pH 7.
◦ They are named according to the number of ceramide-linked sugar units, such
as Ceramide disaccharides, ceramide trisaccharide.
◦ They are important components of erythrocyte membranes and determine the
differentiation of blood groups.
– Human blood groups are the determinants of A, B and O, sugar groups in some glycolipids.
◦ Cytolipin H: Sphingosine + Fatty Acid + Lactose
– Cytolipin H is a ceramide disaccharide with immunological effect.
◦ Cytolipin K: Sphingosine + Fatty Acid + Lactose + N-acetyl galactosamine
– Cytolipin K is a ceramide trisaccharide derived from the kidney, which is very similar to
the globoside substance found in the human erythrocyte.

Reference: Nelson ve Cox, 2012

Sphingolipids• Ganglioside
◦ Complex sphingolipids containing a large number of sugar unitslinked to ceramide.
◦ In addition to the hexose found in the cerebrosides, gangliosidescontain several more carbohydrates.
◦ The carbohydrate may be at least one mole of N-acetyl galactosamineor N-acetylglucosamine and at least 1 mole of N-acetylneuraminic acid(sialic acid).
◦ When hydrolyzed they give 1 mole sphingosine + 1 mole fatty acid+ 1 mole hexose + 1 mole N-acetyl galactosamine/glycosamine + 1 mole N-acetylneuraminic acid (NANA-sialic acid).
◦ Depending on the type and number of carbohydrates and/orcarbohydrate derivatives they contain (especially NANA), there aredifferent types of gangliosides.


Sphingolipids◦ They are found abundantly in the nerves and spleen.
◦ They are effective in the delivery of nerve impulse, taking part in the
construction of receptors (hormones) in cell membranes.
◦ They have functions in Cell-to-cell communication.
◦ They are located at the end of the nerve and bind to the
neurotransmitter molecules, acting as impulse chemical transmissions
in the passage of one nerve from one nerve.
◦ They are also related to carcinogenesis; cell growth and
differentiation. – Tumoral cells produce different type of gangliosides, thus cells can be
differentiated.

Sphingolipids• In addition to contributing to the structural integrity of the
membranes, glycosphingolipids are associated with many
cellular functions at the cell surface level.◦ They provide antigenic chemical markers for cells.
◦ They act as chemical markers that describe the various stages of cell
differentiation.
◦ They regulate the normal growth pattern of the cells.
◦ Cells are allowed to react with other bioactive substances such as
bacterial toxins (GM1 ligands), glycoprotein-forming hormones,
interferons, and viruses.

Sphingosine
Sphingosine Ceramide Sphingomyelin Sphingomyelin Cerebroside Ganglioside
PhosphacholinePhosphaethanolamin
One sugar residue Oligosaccharide residue
Sialic acid

Aliphatic Alcohols and WaxesAliphatic alcohols can be obtained in significant amounts
from many lipid sources. • The reason for this is that they are esterified with fatty acids.
Waxes are the simplest hydrolysable lipids. Waxes are
esters (RCOOR') formed from a fatty acid (RCOOH) and
a high molecular weight alcohol (R'OH).
The length of these acids and alcohols may be C16-C30.

The general formula of waxes is purely simple esters of
the general formula (R-CO-O-R).
Many plant and animal bodies are covered with wax
layers.
Wax layers prevent water from penetrating from one
side and from the other side. They are highly
hydrophobic molecules.
Both plants and animals bring natural waxes to the
field.
Aliphatic Alcohols and Waxes


Thay melt at wide temperature range (35-100°C) and
insoluble in water. They are very soluble in organic
solvents.
Most common alcohols found in waxes are Lauryl
alcohol, cetyl alcohol, seryl alcohol and myristyl
alcohol.
Most common fatty acids found in waxes are myristic
acid, palmitic acid, serotic acid and melissic acid.
Aliphatic Alcohols and Waxes

Aliphatic Alcohols and WaxesWaxes are common in nature.
• They are found in the secretions of insects,
• They are found in the skin, hair and fur of animals as a protective layer
• They are found on the leaves of plants, fruits and shells.
Important Waxes;• Bee Wax (Beeswax – Cera alba) In the bee's secretion
• Carnauba Wax In plants
• Spermaceti Wax Whale
• Lanolin Wool

Beeswax• It is a mixture of ester which formed by palmitic acid and C26-
C34 fatty alcohols.• Beeswax, a complex mixture of over 200 different compounds,
contains the wax myricyl palmitate as its major component.• It melts at 62-65 ° C.• It is used in shoe polish, candle and wax paper making.
Carnauba Wax• Carnauba wax, a wax that coats the leaves of the Brazilian palm
tree, is used for hard, high-gloss finishes for floors, boats, and automobiles. Its main composition is ester myricyl cerotat.
• It melts at 80-87°C.• It is used to make duplicate paper (shoe polish, ground polish),
varnish, wax and waxy paper.
Aliphatic Alcohols and Waxes

Whale Wax (Spermaceti wax)• Spermaceti wax isolated from the heads of sperm whales, is largely
cetyl palmitate, an ester; palmitic acid + cetyl alcohol• It melts at 42-45°C.• It is mostly used in ointments and cosmetics.
Lanolin (Wool wax)• It is a fatty acid ester of lanosterol. Contains free and esterified
cholesterol.• It forms a protective layer over the woolen fleece.• It has a very complex structure (long chain esters, hydroxy esters,
diesters, lanolin alcohols, and lanolin acids).• Lanolin has a very large amount of water retaining ability. For this
reason it is used for the preparation of ointments and various cosmetic products.
Aliphatic Alcohols and Waxes

Aliphatic Alcohols and Waxes Places where waxes are used:
• Bee wax (beeswax) is used in honeycomb construction.
• Lanolin is used in the preparation of various ointments and creams, in the preparation of cosmetics.
• The oil layers on the hair, face and leaves are also composed of waxes.
• Spermaceti waxes are used in making candles.
• Carnauba wax is used in shoe polish, floor polish, varnish and candle making.
• The planktons in the seas also store significant amounts of waxesand use them as an energy source.
• The waxes secreted from the gland found in the tail parts of some birds indicate that the fur does not get wet from the water.

Terpenes are lipids composed of repeating five-carbon
units called isoprene units. An isoprene unit has five
carbons: four in a row, with a one-carbon branch on a
middle carbon.• There are double bonds in the molecule and these bonds are
conjugated.◦ Thus, they have high reaction ability.
• The polymerization of molecules of the same material with one
another is called polymerization.
• The isoprene molecules must dehydrate before being polymerized.
• When the dehydrated 5 C isoprene molecules are polymerized,
compounds called terpenes form.
Terpenes

(C5H8)n
Isoprene Unit Isoprene Unit

Most terpenes are hydrocarbons, others are alcohol,
ether, aldehyde, ketone and acid.
Many terpenes smell good.
It can be separated from other herbal substances by
lightly heating and by distillation.
Some of the terpenes are used in perfumes, sweeteners
and in medicine.
Lycopene, Carotene, Vitamin A and Squalene are some
of the most important biological terpenes.
Terpenes

The most important group of terpenes is carotenoids(tetraterpenoids).• They are in different colors; ranging from light yellow to red violet.
• Some of them are acyclic, that is, they have no ring, they have a chain structure, and some have hydroaromatic rings on both ends of the chain.
Carotinoids carrying hydroaromatic rings are called carotenes (carotins).• Carotenoids are substances that give yellow color to many natural
oils.
Because of carrying double bonds, they easily and rapidly oxidized by oxygen and ultraviolet rays.
Terpenes

Terpenes Carotinoids in aliphatic form
• Lycopene: It is tetraterpene assembled from eight isoprene unitsand is insoluble in water. It is a bright red carotenoid pigment found in tomatoes and other red fruits.
• Squalene: It is triterpene assembled from six isoprene units. All plants and animals produce squalene as a biochemical intermediate. It is a natural and vital part of the synthesis of all plant and animal sterols, including cholesterol, steroid hormones, and vitamin D in the human body.
Carotinoids with Alcohol group in Aliphatic Structure• Phytol: Phytol is an acyclic diterpene alcohol that can be used as a
precursor for the manufacture of synthetic forms of vitamin E and vitamin K1. It consist of 4 isoprene units. It is a constituent of chlorophyll.

Terpenes Carotinoids in the aliphatic structure with a carboxyl
group• α-Crocetin: It consists of 4 isoprene units. There are two carboxyl
groups at each end of the chain. It is the substance that gives the yellow color of bile.
Carotinoids with hydroaromatic rings• The substances in the building are called as carotenes. The
hydroaromatic rings are located at both ends of the four isopreneunits.
• Hydroaromatic rings are called ionone rings and there are threetype; α-ionone, β-ionone and pseudoionone rings.◦ α-ionone and β-ionone rings are closed and carry one single bond. The place
of C=C is different.◦ The pseudoionone ring is open and two double bonds are carried.◦ There are 3 types of carotene in the nature. α-, β- and γ-carotene.

α-Ionone Ring β-Ionone Ring Pseudoionone Ring

Terpenes Carotinoids with hydroaromatic rings, Carotenes;
• α-Carotene: It consists of α-ionone + 4 isoprene unit + β-iononering. It is a precursor (inactive form) to one mole vitamin A.
• β-Carotene: It consists of β-ionone + 4 isoprene unit + β-iononering. It is a precursor (inactive form) to two mole vitamin A.
• γ-carotene: β-ionone + 4 isoprene unit + pseudoionone ring. It is a precursor (inactive form) to one mole vitamin A.
Carotenes are found especially in green plants. The yellow and orange colored fruits are rich in β-carotene, such as carrot, mangoes, pumpkin and papayas.

Isoprene Unit Isoprene Unit
β-Carotene
β-Ionone
Ring
β-Ionone
Ring

Interconversion of Retinoids
β-Carotene
Retinal
(Vitamin A1)
11-cis-Retinal
Retinol
(Vitamin A)
Retinoic Acid
(Retin A)
Retinal
isomerase
Retinal
reductase

Carotinoids with an alcohol group in hydroaromatic
structure• Xanthophylls: It is a Dihydroxy α-carotene.
◦ Both ionone rings carry hydroxyl groups.
◦ Gives color to chicken fat, egg yolk and chicks' feathers.
• Cryptoxanthin: One of the ionon rings has OH group. It is a
monohydroxy β-carotene.◦ It is found in corn grains and red pepper.
◦ Since it has β-ionic ring, 1 molecule Vitamin A can be synthesized.
Terpenes

They are very common in animals and plants.
Since they have a lot of physiological activities, they are always remarkable compounds.
All steroids always carry the sterane ring (cyclopentanoperhydrophenanthrene ring).
The numbering and lettering of the steran ring is very important. Because these carbons come in and out of groups and different type steroids occurs.
Steroids

Sterane ring(cyclopentanoperhydrophenanthrene ring)
A B
C D
• The phenanthrene ring consists of 3
benzene rings.
• If this ring is saturated with
hydrogen, the double bond opens
and the perhydrofenanthrene ring
occurs.
• If a cyclopentane ring is added to
the perhydrofenanthrene ring,
cyclopentanoperhydrophenanthrene
(sterane) ring is formed.

In the formation of different steroids, the side chain
attached to 17th C atom is mostly changed.• New steroids are formed by changing the side chain.
• Steroids are derivatives of the sterane ring which carry some
functional groups such as carbon side chain, alcohol, aldehyde,
ketone and double bond.
Biologically important molekules that carry the
sterane ring are;
Steroids
• Sterols
• Bile acids
• Sex hormones
• Adrenal cortex hormones
• Vitamin D group substances

Sterols
• They carry a sterane ring and a side chain. All sterols
contain an alcoholic hydroxyl group in the 3rd
carbon atoms. There are 3 subgroups.
1. Zoosterols
2. Mycosterols
3. Phytosterols
Steroids

1. Zoosterols• It is found only in animal tissues and the most important
member is cholesterol.
• Cholesterol is found in all animal tissues, in the membranes of many animal cells, in the lipoproteins of blood plasma, and is synthesized in the liver.◦ It is found mostly in the brain, nervous tissue, adrenal glands and
egg yolk.
• They are not found in plants.
• It has an antihemolytic effect. Because of this property, it is effective against hemolytic effects of bacterial toxins, snake venom, bile salts and other hemolytic substances.
Steroids

Cholesterol Sterane ring in cholesterol;
• 1 mole OH group on the 3rd
carbon atom,
• A double bond between
carbons 5 and 6,
• Methyl groups on 10th and
13th carbon atoms,
• Carbon 17 carries a side
chain of 8 carbons.

• Properties of cholesterol;◦ It is found only in animal tissues, not in plants.
◦ It is tasteless and odorless.
◦ Oxidized if exposed to air and light.
◦ When oxidized, 7-hydrocholesterol is formed, which is the precursor
of Vitamin D3.
◦ It is the precursor of the adrenal cortex hormones, the sex hormones,
and the precursor of cholic acid (one of the bile acids).
• Cholesterol is present in tissues in two ways; Free Cholesterol
and Ester Cholesterol.
• The amount of cholesterol in the tissues is in wide range.
Steroids

• The dried form of brain white matter contains 14% cholesterol.
• Cholesterol has an important role in the lipid metabolism, the
transport of lipids.
• It is the precursor in the synthesis of bile acids, sexual
hormones and other steroids.
• In lipid metabolism disorders and aging, cholesterol fatty acid
esters are deposited in the vascular walls and adhere to the
arteries.
• It is accepted that 90% of the existing cholesterol in the body
is used in the synthesis of bile acids and 10% in steroid
hormones.
Steroids

• The chemical properties of cholesterol come from the group of
seconder alcohols and the double bond they carry. They can
esterify with fatty acids at the 3rd C atom which carries OH
group. These esters are common in blood and tissues.◦ Again, this hydroxyl group is ketonized with oxidants, such as
cholestenone.
• Another molecule of Zoosterols is the abundant amount of
lanosterol in the sheep wool.◦ Lanosterol is similar in structure to cholesterol. There is a double
bond in the side chain. Carbon-4 has two molecules of methyl groups.
There are not double bond between 5-6 Cs but there are double bond
between 8-9 Cs.
Steroids

2. Mycosterols• They are sterols found in yeast and fungi.
• The most important member is the ergosterol. It turns into ergocalciferol under UV light. This is Vitamin D2.
• Ergocalciferol is not found in animal tissue, but it is indicated that it can be used instead of cholecalciferol.
3. Phytosterols• They are vegetable sterols and there are two important
members. Stigmasterol and sitosterol. Stigmasterol is the procursor of progesterone. Sitosterol is especially abundant in grain grains.
Steroids

Bile Acids• Bile acids are the substances that carry the Sterane ring. They
contan 24 Carbon atoms.
• It is synthesized by humans and animals using cholesterol as a
precursor. ◦ Research has shown that 85% of the body's injected cholesterol is
turned into bile acids by the liver.
• The last three carbon atoms in the side chain of cholesterol
are broken down by oxidation and the carboxyl group forms
and the bile acids occur.
• Bile acids are mainly synthesized by the liver.
Steroids

• Bile acids are oxy derivatives of cholanic acid.◦ Cholic Acid ---------------------------3,7,12-Trihydroxycholanic Acid
◦ Deoxycholic Acid -----------------------3,12-dihydroxycholanic Acid
◦ Lithocholic Acid --------------------------------3-Hydroxycolanic Acid
◦ Hyodeoxycholic acid----------------------3,6-dihydroxycolanic Acid
◦ Chenodeoxycholic Acid ----------------3,7-Dihydroxycholanic Acid
• The most commonly found bile acids are cholic acid and
chenodeoxycholic acid.
• Bile acids are not free. They are all conjugated. It is called
conjugated bile acids and is coupled with glycine and taurine
via carboxyl groups.
Steroids


• Main properties and functions of bile acids◦ It has emulsifying properties to reduce surface tension.
◦ They play an important role in the absorption of fatty acids from the
small intestine.
◦ By reducing surface tension, enzymes such as lipase can make better
effect on lipids.
◦ Because of these properties, salts of bile acids facilitate absorption of
water-insoluble cholesterol, fats, fat soluble vitamins and
phosphatides.
◦ About 5-10 grams of bile passes into the intestines.
◦ A portion of the biles found in bowel is absorbed and transfered to the
liver via vena porta and then passes through the intestines again. This
is called enterohepatic circulation.
Steroids

Lipoproteins, Eicosanoids, Lysophosphoglycerides, Lipopolysaccharides
Lipids linked to other
class compounds

Lipoproteins play an important role in the transport of
phospholipids, cholesterol and triglycerides in the
plasma.
Some lipids combine with specific proteins to form
lipoproteins.
Blood plasma lipoproteins are classified according to the
particles of lipids and their concentrations which they
contain.
There are mainly 4 groups (+1 intermediate)
lipoprotein, which contain 50-90% lipid.
Lipoproteins

Chylomicrons (CM): Triacylglycerols are carried to the tissues.
Very Low Density Lipoproteins (VLDL): They contain
triacylglycerols synthesized in the liver.
Intermediate Density Lipoproteins (IDL): Intermediate
lipoproteins.
Low Density Lipoproteins (LDL): They allow the transport of
cholesterol from liver to tissues , and the lipoprotein that
contains the most cholesterol in the body.
High Density Lipoproteins (HDL): Proteins and phospholipids are
present in excess in the structure. It allows cholesterol to be
transported from the various tissues to the liver.
Lipoproteins

Source: Engelgink, 2014

LipoproteinsThe proteins involved in the structure of lipoproteins
are called apolipoproteins or apoproteins.
These are classified as Apo-A, Apo-B, Apo-C and Apo-E.
They have also sub-fractions.
The protein components of lipoproteins organize the
entry and exit of particulate lipids into specific locations.

Lipoprotein Structure
Source: Engelgink, 2014 Source: Uni.Wisconsin

Eicosanoids Eicosanoids, are a group of biologically active
molecules which derived from arachidonic acid (20C atom).
They are molecules that are found in very low concentrations in the cell, yet have very strong effects.
Prostanoids (Prostaglandins, Thromboxanes,Prostacylcins), Leukotrienes and Lipoxins are examples.

EicosanoidsThey have a similar effect to hormones. But like
hormones, they are not synthesized and sent to the
target tissues via blood.
Depending on the external stimuli, they are synthesized
as needed and act on the tissues they synthesize.
They are not stored in the cell.
Eicosanoids are effective in events such as
reproduction, inflammatory events, wound, fever,
induction of blood clotting, and regulation of blood
pressure.

Prostaglandins (PG)• They are a subclass of eicosanoids and of the prostanoid
class of fatty acid derivatives.
• Prostaglandins have types such as PGA with acid character, PGB with basic character, PGE extracted with ether, PGF extracted with phosphate buffer. Subtypes of each type are also defined.◦ The capital letters A, B, D, E, F, G, H, I in the prostaglandin symbol
indicate the ring type of the molecule.
◦ The small letter in the prostaglandin symbol after the capital letter and written in alta shows the number of unsaturated bonds in the molecule.
◦ For the F series, the α Greek letter is used to indicate the state of the -OH group at C9.
Eicosanoids

• Prostaglandins mediate autocrine and paracrine regulatory
functions.
• Prostaglandins have numerous functions. Some of them give
the impression of the opposite of each other.
• Prostaglandins affect a wide range of cell and tissue functions.
Different types of prostaglandin types on a cell, tissue or
system may have different effects; Gastrointestinal, Pain,
Fire, Vascular, Respiration, Reproduction.◦ PGF2α is released by stimulation of the uterine oxytocin, causing the
corpus luteum to lyse.
◦ PGF2α and PGD2 act as constructors in the smooth muscles of the
trachea and bronchi, while PGE2 and PGI2 act as relaxants.
Eicosanoids

Prostacyclins (PGI2)• Prostacyclin is the primary prostaglandin synthesized by vascular
endothelium.
• Prostasicline is a vasodilator. The vein relaxes the smooth muscle.
• Prostasicline prevents platelet aggregation and adhesion to the endothelium surface.
Thromboxans• It is an eicosanoid associated with the prostaglandins, containing an
ether group in the six-membered ring.
• Thromboxanes consist of arachidonic acid in platelets; are effective in the formation of blood clots and in reducing blood flow instead of clot.
Eicosanoids


Leukotrienes• They are eicosanoids that contaion three conjugated double
bonds and not a ring structure in molecular structures.
• Leukotrienes can cause contractions in the muscles of the
airways of the lungs, resulting in asthma attack; The cysteinyl
types of leukotrienes are known as the slow-reacting substance
of anaphylaxis (SRS-A).
• Leukotrienes cause local vasodilatation, increase permeability
of the capillaries, enhance the effects of chemical mediators
and ultimately cause pain and discomfort. In other words, they
facilitate chemotaxis, inflammation and allergic reactions.
Eicosanoids

• There are four types1. Cysteinyl Leukotrienes (LTC4, LTD4, LTE4, LTF4)
2. LTB4
3. LTG4
4. LTB5
• LTC4 and LTD4 cause smooth muscle contraction and are 1000
times more effective than histamine in narrowing the lung
airways.
• At the same time, they increase fluid infiltration from small
blood vessels and constrict coronary arteries.
• LTB4 attracts neutrophils and eosinophils instead of
inflammation.
Eicosanoids

Lipoxins (LX)• Lipoxins are trihydroxy derivatives of arachidonic acid .
• Two types have been identified; Lipoxin A4 (LXA4) and B4
(LXB4).
• Although lipoxins are synthesized in very small quantities, they
are powerful inflammatory mediators and play an important
role in the resolution of inflammation.◦ By inhibiting infiltration of the polymorphonuclear leukocyte (PMN)
into the inflammatory zone, vasculary permeabilisation returns to
normal levels. It enhances non-inflammatory migration of
mononuclear cells and accelerates resolution by allowing apoptotic
PMNs to accumulate in macrophages.
Eicosanoids

Lisophosphoglycerides• They are found in the membrane and the cell. They occur by
the hydrolytic removal of one of the two fatty acids of the
glycerophospholipids. They serve as intermediate metabolites.
Lipopolysaccharides• They are found on the outer membranes of Gram-negative
bacteria. It is the main building component.
• Also known as lipoglycans and endotoxins.
• In animals,they cause strong immun reactions.
Others

Görsel Kaynak: WikiMedia

Altınışık M. Ders Notları. İnternet Erişim:
http://www.mustafaaltinisik.org.uk/89-1-08.pdf
Ası. T. 1999. Tablolarla Biyokimya, Cilt 1
Engelking LR. 2014. Textbook of Veterinary Physiological Chemistry. 3rd
edn. Academic Press.
Fidancı UR. Ders Notları. İnternet Erişim:
http://80.251.40.59/veterinary.ankara.edu.tr/fidanci/
King M. İnterner Erişim: http://themedicalbiochemistrypage.org/#nogo
Smith JG (2010). Organic Chemistry, 3rd Edition, McGraw-Hill.
Smith JG (2012). General, Organic, & Biological Chemistry 2nd Edition,
McGraw-Hill.
Sözbilir Bayşu N, Bayşu N. 2008. Biyokimya. Güneş Tıp Kitapevleri, Ankara
References

Question 1
Answer: a
1. ................ are monocarboxylic organic acids with
hydrocarbon chains.
a. Fatty acids
b. Terpenes
c. Bile acids
d. Hydrocloric Acid
e. Phosphoric Acid

Question 2
Answer: c
2. Which of the following is an essential fatty acid?
a. Palmitic Acid
b. Stearic Acid
c. Linoleic Acid
d. Lignoceric Acid
e. Myristic Acid

Question 3
Answer: d
3. Which of the following terpenes is the precursor of
Vitamin A?
a. Xanthophyll
b. Cryptoxanthin
c. α-crocetin
d. β-carotene
e. Lycopene

Your questions?

Next title;
Amino Acids, Peptides
and Proteins