Lipids
description
Transcript of Lipids

LIPIDS
-Soluble in organic solvents but not in water.-Lipid from the Greek word means Fat or lard.-Important feature in cell membranes and steroid hormones.
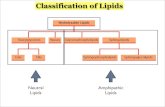
2. Different types of lipids:•Triglycerides•Phospholipids•Wax•Steroids
LipidsPhysical properties
Insoluble in water and polar organic solvents.Their densities are always less than water.They have high viscosity
Chemical properties
Their chemical structures are not very similar between different categories.Generally, they are esters formed from fatty acid and alcohol.

GLYCEROL
F.A
F.A
F.A
TRIGLYCERIDES
ESTER BONDS
Produced by ESTERIFICATION REACTION. This means there is a condensation process between an alcohol group and carboxylic group.
Group of lipids:Triglycerides ( triesters of glycerol) :
1. In the body, Fatty acids are stored as fats and oil, known as Triglycerides.-General formula:

2. Esterification is also called lipogenesis which one molecule of glycerol reacts with three molecules of fatty acids as shown below. The reaction is catalysed by lipase of a ligase.
Fig. 1 Esterification

Distribution • found and this process happen at the adipose tissue under the skin, can happen also in the liver.• outer layer of the organs such as heart, liver and digestive ducts.• Membrane neuron (myelin).• in egg shell of the birds and the insects.• The 3 fatty acids in triglycerides are usually the same type forming products such as tristearin, tripalmitin and triolein.
Physical properties
• insoluble in water, but dissolve in organic solution such as far, oils or non-polar groups.• high molecular masses.• has specific gravity is less than water, therefore they float on water.• form an emulsion if shaken with alcohol.

Chemical properties
• react with atmospheric oxygen and become rancid if kept for too long a period.• React with Sudan III reagent to form a dark red complex.• Can be hydrolysed by lipase with dilute alkali to form glycerol and fatty acid.
Physiological roles
• act as energy source and energy reserves.• They can be used for the formation of other chemicals including glucose to maintain blood glucose level and amino acids to make proteins. They can be used to form all other chemicals especially in plants and herbivores after dormancy in winter.• protect organ from physical damage if it is packed in them such as kidney.• fat at myelin layer works as the electric insulator.• fat is the important solvent for vitamin A, D, E and K and other hormones.• Saturated fat is the basis substance to synthesis cholesterol.

Differences between carbohydrates and lipids.
• The ration between H and O in carbohydrate is respectively 2:1 but the ration of H and O is relatively high.
• Carbohydrate dissolves in water but lipids do not dissolve in water.
• Carbohydrate is composed of an aldehyde or a ketone sugar lipid is an ester.
• Carbohydrate can exist in polymer such as starch but lipid does not form polymer.
• Lipid produces more energy than carbohydrates.

1. Glycerol :-is trihydroxyl-propane that is a polyhroxyl alcohol.-It is a small molecule, which can be respired in the liver or kidney to liberate energy.
2. Fatty acids:-The simplest type of lipids and are found as components in more complex lipids.- Have an even number of carbon atoms, usually between 10 and 20.- Contains a long carbon chain attached to a carboxylic acid group at one end-COOH.

3. Fatty Acids: divided into saturated F.A and unsaturated F.A.a. Saturated fatty acids:
I. Contain only single bond with no double bonds among their carbon linkages. Therefore, saturated F.A contain only CH2
groups with more hydrogen atom per carbon atom.
examples of F.A as follows:
• Palmitic acid, CH3(CH2)14 COOH
• Stearic acid, CH3(CH2)16 COOH as shown below:
Draw the linear structures for stearic and palmitic acid.

Physical properties
• readily solidify when cooled.• usually found in animals and in coconut oil.
Chemical properties
• Not readily metabolised compared to the unsaturated ones.• are readily deposited on inner layer of arterial walls especially with cholesterols increasing the chances of getting cardiovascular diseases.

b. Unsaturated fatty acids:I, contain at least one double bond among their hydrocarbon chain.ii, the ratio of the number of atoms of hydrogen per carbon atom is lower.iii, examples of unsaturated fatty acids are as follows:• Oleic acid and Linoleic acid and arachidonic acid.
Draw the structures of Oleic acid:

Physical properties
• Usually found in plants oils especially from corn and linseed and palm oil (oleic acid).• More readily metabolised than the saturated ones in our body.• Not so solidified when cooled.
Physiological roles
• Unsaturated fatty acids especially those polysaturated ones are recommended for our intake because they are readily metabolised to cut down the risk of contracting cardiovascular diseases.•

2. Phospholipids
a, are lipids that contain phosphorus usually in phosphate form that can be removed by hydrolysing with phosphatase.
b, they are two major types, the derivatives of phosphotidic acid or sphingolipid, for which the former is more common.
Phospholipids symbol
Hydrophilic head
Hydrophobic tails

c, an example is LECITHIN, which is phosphotidylcholine.its molecular structure is shown on fig.2 below:
fig.2

Lecithin as the membrane cell :
The specific properties and structure in lecithin enable it to form the cell membrane.
a. The lecithin head dissolves in water but the tails do not.
b, In an aqueous medium, the non-polar tails repel water and can congregate together, the polar heads interact with water through hydrogen bonding.
c, Lecithin molecules arrange in double layer.
d, Hydrophilic heads are linked with hydrogen bonds with water molecules where the hydrophobic tails link together with each other with weak Van der Waals.
e, This is the basic arrangement of the cell membrane. Deposit of the protein molecules at double layer contributes to cell membrane.

d, as shown on fig. 2 above, Phospholipids are derived from one molecule of glycerol bonded with two molecules of fatty acids.
e, One molecule of phosphoric acid forming phosphotidil acid through ester bonds.
f, Choline is an alcohol complex bonded to the phosphotidil acid forming lecithin.
g, Distribution of phospholipid- it appears at the plasma membrane and organelle membrane.
h, widely distributed at nerves, heart and kidney.

Physical properties:1. They are amphipathic, containing hydophobic and hydrophilic groups.
When phospolipids are added to water, they self-assemble into aggregates that shield their hydrophobic portions from water.
One kind of cluster is a micelle, a phospholipid droplet with the phosphate heads on the outside, in contact with water.
The hydrocarbon tails are restricted to the water- free Interior of the micelle. (fig. 3)
fig. 3

at the surface of a cell, phospholipid are arranged in a bilayer, or double layer look at fig b.
The hydrophilic head of the molecules are on the outside of the bilayer, in contact with the aqueous solutions inside and outside the cell.
The hydrophobic tails point toward the interior of the membrane, away from the water. The phospholipid bilayer forms a boundary between the cell and its external environment. In fact, phospholipid are major components of cell membranes.
Hydrophilic head
Hydrophobic tail

Function: essential as the component of cell membrane.
involved in fat transportation in our body.
Lipids normally are transported in our body as phospholipid in the blood system.
The hydrophilic head dissolves in water and can be transported in our blood stream.

Waxes -
• Waxes are found in many plants and animals.
• Coatings of carnauba wax on fruits and the leaves and stems of plants help to prevent loss of water and damage from pests.
• Waxes on the skin, fur, and feathers of animals and birds provide a waterproof coating.
• A wax is an ester of a saturated fatty acid and a long-chain alcohol, each containing from 14 to 30 carbon atoms.
• The formulas of some common waxes are given in table below.
• Beeswax obtained from honeycombs and carnauba wax obtained from palm trees are used to give a protective coating to furniture, cars and floors.
• Jojoba wax is used in making candles and cosmetics such as lipstick.
• Lanolin, a mixture of waxes obtained from wool, is used in hand and facial lotions to aid retention of water, which softens the skin.
Fatty acid Long-chain alcohol
Ester bond

Some typical waxes

STEROIDS
• Are lipids that are made of four fused hydrocarbon rings as shown below.
• Different steroids vary in the functional groups attached to this ensemble of rings.
• One steroid, cholesterol is a common component of animal cell membranes and is also the precursor from which other steroids are synthesised.
• Many hormones, including vertebrate sex hormones, are steroids produced from cholesterol.
Fig. of cholesterol, a steroid.

• Generally, • Steroid has the similar basic skeleton which is 17 carbon atoms that is
arranged in one 5-C atom ring and 3 6-C atom ring.
• Steroids exist in both plants and animals.• Steroids are classified as lipid because they are insoluble in water.• Example of steroids are cholesterol, bile acids, corticosteroids,
estrogens and progesterone, Testosterone, calciferol, D3 and Ecdysone.

AMINO ACIDS• Proteins are composed of molecular
building blocks called amino acids.
• Amino acids contain- 2 functional group,
I, amino group(-NH2) ii, carboxylic acid group (-COOH).
• General structure of an amino acid.
• Although, there are many amino acids, only 20 different amino acids are present in the proteins in human.
• The unique characteristics of the 20 a.a are due to a side chain R, which can be an – alkyl, hydroxyl, thiol, amino, sulphide, aromatic or heterocyclic group.
• There are 4 different group of a.a: I, Non-polar a.a
ii, polar a.aiii, acidic a.aiv, basic a.a
Also known as the electrically charged a.a