Lipids
-
Upload
mazz4 -
Category
Technology
-
view
2.750 -
download
5
description
Transcript of Lipids


Lipids are:water-insoluble organic substances but readily soluble in organic solutions e.g.
ether

The term ‘lipid’ defines compounds:
• not on the basis of structural similarity BUT
• in terms of their solubility

‘’;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;
Water is so attracted to other water molecules that anything between them is squeezed out of
the way.

Lipids do not form polymers. Why?
Not made of monomers.
Lipids are:structurally functionally
DIVERSE

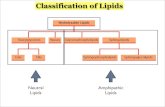
Types of Lipid
1. Triglycerides [Fats & Oils]2. Waxes3. Phospholipids4. Steroids5. Glycolipids6. Lipoproteins7. Terpenes

Types of Lipid
1. Triglycerides [Fats & Oils]
2. Waxes3. Phospholipids4. Steroids5. Glycolipids6. Lipoproteins7. Terpenes

1. Fats & Oils
•the commonest lipids in nature
•the constituents of fats are:-
fatty acids (alkanoic acids)
glycerol (propane 1-2-3 triol)

i) Fatty acids- general formula: R.COOH
- most have an even number of C - most commonly 16-18
C16H32O2
R
- fatty acids may be: saturated or unsaturated

Saturated fatty acid[Single bonds only]
Unsaturated fatty acid[Double bonds]

Stearic acid, C17H35COOH –Saturated fatty acid Oleic acid, C17H33COOH –
Unsaturated fatty acid

The more double bonds present, the more bent the molecule is

What is the difference between a fat and an oil? fats - solid at 20C oils - liquid at 20C

Why are fats solid and oils liquid at room temperature?
Molecule has kinks, keeping the fatty acids apart and as a result, oil is liquid at room
temperature.

A glyceride forms when an –OH in glycerol is replaced by an organic acid
Fatty acid
glycerol

Types of glycerides:Monoglyceride
Diglyceride
Triglyceride

Why are fats and oils classified as triglycerides?

Triglycerides:- are non-polar and float on
water
Polar head - Hydrophilic
Non-polar tail - Hydrophobic


Animal fat solidifies at a low temperature

Why do plants and poikilotherms tend to have a high proportion of unsaturated fatty acids?
To prevent their triglycerides from solidifying.

Fatty acids link to glycerol as a water molecule is lost

Fats & oils are made of one glycerol and three fatty acids

Formation of a triglyceride
formed by esterification / condensation

Fatty acids may be
different in a triglyceride

Functions of Triglycerides1. Store energy in animals +
Yield energy

Comparison of energy yield from:
Triglycerides: 37 kJ g-1
Carbohydrates: 17 kJ g-1
MORE energy from triglycerides:
Due to more hydrogen.

2. Animals store extra fat when hibernating: acts as an insulator.
Subcutaneous fat

2. Fat around organs: protect against bumps keeps them warm
Kidney surrounded by fat

3. Brown adipose tissue (brown fat) - releases heat but no ATP- heat maintains core temperature in:
small mammals in newborn humans, particularly during cold
exposure without shivering


4. In aquatic mammals blubber contributes to buoyancy.

What is present in the hump? Why?
When fat is metabolised, it yields: 1. energy 2. more than 1 g of water for each 1 g of
fat by respiration.
FAT

5. Water is produced when fats are oxidised - this metabolic water is important in desert animals.
A kangaroo rat never drinks. Mention TWO ways
how it can get water.1. Eating2. Metabolism

6. Nerves are covered with a myelin sheath for insulation of the fibre and for fast conduction of impulses.

Test for Triglycerides
Paper becomes translucent.

Chemical Test for Triglycerides
Sudan III

Chemical Test for Triglycerides
Emulsion Test

Types of Lipid
1. Triglycerides [Fats & Oils]
2.Waxes3. Phospholipids4. Steroids5. Glycolipids6. Lipoproteins7. Terpenes

Waxes: not a food source no enzymes to break them down

Structure of Waxes
one fatty acid an alcohol of high molecular weight instead of glycerol

Feathers do not get wet – WHY?
Raindrops on feathers.


Waxes are mainly for e.g.
cutin makes up the waxy cuticle of leaves
fruits produce a waxy coating to
keep from drying out

sebum in mammalian skin

wax in ears traps:dustsandother foreign
particles
do not go deeper into the ear and cause no damage

suberin in Casparian strips,

exoskeleton in insects[contains wax]

waxes build elaborate structures such as beehives

Types of Lipid1. Triglycerides [Fats & Oils]2. Waxes
3.Phospholipids4. Steroids5. Glycolipids6. Lipoproteins7. Terpenes

Phospholipids• lipids with a phosphate group

Phospholipids are made of one phosphate group and 2 fatty acids
Phospholipids are amphipathic – have both polar & nonpolar portions

• constituents of membranes

Two structures formed by self-assembly of phospholipids in aqueous environments

Types of Lipid1. Triglycerides [Fats & Oils]2. Waxes3. Phospholipids
4.Steroids5. Glycolipids6. Lipoproteins7. Terpenes

Steroids are structurally different from all other
lipids
consist of a complex carbon ring structure
Cholesterol is a steroid.

Cholesterol:• is the steroid present in the
largest amount in humans
• a key intermediate in the synthesis of related steroids
• an important constituent of animal plasma membranes
made in the liver


Sex hormones are Steroids

Types of Lipid
1. Triglycerides [Fats & Oils]2. Waxes3. Phospholipids4. Steroids
5.Glycolipids6. Lipoproteins7. Terpenes

Glycolipids• carbohydrate + lipid • components of cell membranes

Types of Lipid1. Triglycerides [Fats & Oils]2. Waxes3. Phospholipids4. Steroids5. Glycolipids
6.Lipoproteins7. Terpenes

Lipoproteins• occur in membranes • are the form in which
lipids are transported in the blood e.g. fatty acids are bound to albumin
protein
triglyceride &
cholesterol
phospholipid
Lipoproteins vary in size and composition

Types of Lipid
1. Triglycerides [Fats & Oils]2. Waxes3. Phospholipids4. Steroids5. Glycolipids6. Lipoproteins
7.Terpenes

Terpenes may be:volatile : attract pollinators
less volatile:strongly bitter-tasting to prevent plants being eaten by herbivores

Examples of terpenes:
light-absorbing pigments in animals & plants
carotenoidsrubber

β carotene:- traps light in plants and in humans- is broken down into two vitamin A molecules
from which we make rhodopsin

Carotenoids also act as antioxidants:
trap free radicals and inhibit oxidation
Free radicals: highly unstable
molecules that attack
healthy cells through a
damaging chain reaction of oxidation.

Antioxidants: prevent cancer improve the
immune system

Question: [MAY, 2007]
Suggest explanations for the following statement.Fats are used as storage molecules rather than as an immediate energy source. (2)Fats are ideal for storing energy as they release large quantities of energy. This means that small quantities of stored fat carried by the organism provide sufficient energy without having to carry a heavy load. Fat molecules are large and would take a long time to release energy, making them unsuitable for immediate sources of energy.

Question: [SEP, 2007]
Use your knowledge of biology to explain the following. (5 marks)
Most animals use fats, rather than starch, as energy-storage molecules.

No life as we know it can exist without lipids. Comment on the importance of lipids to life.
[1994]
Give an account of the important roles played by lipids in living organisms. [SEP, 1998]
Give an overview of the importance of lipids for living organisms. [SEP, 2009]
Essay Titles