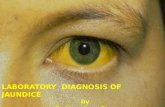
Laboratory diagnosis of salmonella
-
Upload
drmalathi13 -
Category
Health & Medicine
-
view
188 -
download
2
Transcript of Laboratory diagnosis of salmonella
Laboratory diagnosis of Salmonella
By,Dr.M.MalathiPostgraduate
Department of MicrobiologyChengalpattu Medical College
Introduction
The genus Salmonella consists of bacilli that parasitise the intestines of a large number of vertebrate species and infect human beings, leading to enteric fever, gastroenteritis, septicemia with or without focal suppuration and the carrier state
Clinical Manifestations
• Typhoidal salmonella – Enteric fever• Non typhoidal salmonella – Gastroenteritis
• Bacteremia• Osteomyelitis• Localised infections• Carriers
Laboratory diagnosis of Enteric fever
• Typhoid fever + Paratyphoid fever
• Typhoid fever – S.Typhi• Paratyphoid fever – S.Paratyphi A, B, and C
• Confirmed case of typhoid fever is defined(WHO), as a patient with fever (> 38°C) that has lasted for at least three days, with a laboratory confirmed positive culture of S.Typhi.
• Probable case of typhoid fever is a patient with fever (> 38°C) that has lasted for > 3 days, with a positive serodiagnosis or antigen detection test but without S.Typhi isolation.
• Chronic carrier is determined as excretion of S.Typhi in stools or urine for longer than one year after the onset of acute typhoid fever.
Specimen collection
BloodSerumUrineFecesBoneMarrowBile
PusCSFSputumGall bladderLiverSpleenMesentric lymph nodes
Ideal specimen
First week Blood (culture)
Second week Serum (Antibodies)
Third week Stool
Fourth week Urine
Chance of isolation
Specimens First week Third week
Blood 50 to 80% 30%
Feces 40 to 50% 80%
Urine - 25%
Blood culture
• Volume of blood : 10 to 15 ml from adults and adolescents , 2 to 4 ml in children
• Ratio of blood to bile broth: 1:10• Or add saponin to BHI broth with 0.05% SPS• Inoculate the blood immediately• Transport immediately, never store under 15degC• Incubate as soon as possible
• When blood culture bottles are not available, direct plating of blood buffy coat from 5 to 10ml sterile heparinised blood onto columbia agar plates containing 0.05% saponin is recommended(Wain, J et al)
• Check for turbidity and evidence of growth after 1,2,3 and 7 days
• Bottles showing signs of growth – Do culture on solid media
• Subculturing done in Mac Conkey agar and Blood agar
• On day 7, all the bottles subcultured before being discarded as negative
• Casteneda`s method• Blood agar – Non hemolytic, 2 to 3mm,
smooth white colonies• Mac Conkey agar – non lactose fermenting
colonies• Confirmed by biochemical reactions and slide
agglutination test with high titre sera
Slide agglutination test - Serotyping
• Prepare a milky suspension of overnight slope culture with saline
• Place a drop in clean glass slide• Check autoagglutination• Add diagnostic sera in the following order for
serotyping
1. Salmonella polyvalent O (Groups A-G)2. Salmonella polyvalent H phases 1 and 2
serum and polyvalent H phase 2 serum3. Individual Salmonella O group sera O2 – O13 4. Single factor H sera
Unusual Serotype ?Send to National Salmonella Reference Centre
Central Research Institute, Kasauli
Rapid detection tests from culture• MUCAP test – 4 methylumbelliferyl caprylate
test – rapid identification of Salmonella strains directly from agar plates
• The substrate combines with Salmonella C8 esterase – releases the umbelliferone – strongly fluoresent at 365 nm
• Apply a drop the reagent directly over the suspected colonies on agar plat and observe under a wood`s lamp within 5 minutes
• 100% sensitivity and specificity
• OBIS Salmonella test – Oxoid Biochemical Identification System – rapid colorimetric spot test
• For the determination of PYRase and NPA activity
• Sample from the colony on an agar plate and applied to the PYR and NPA test areas on the card
• Drop of buffer solution added to both test areas, after 5 minutes, one drop PYR reagent added in PYR test area, NPA reagent in NPA area
InterpretationPYRase negative
NPA negativeSalmonella
PYRase positiveNPA negative
Citrobacter
NPA positivePYRase negative
Proteus, Morganella and Providencia
Clot culture
• Allow the blood to clot and serum pipetted off and used for widal test
• Clot is broken up with sterile glass rod and added to bottle of bile broth
• Add streptokinase (alternative)• Higher rate of isolation than blood cultures
(bactericidal action of the serum is obviated)
Serum
• 1 to 3 ml of blood inoculated into a tube without anticoagulant
• Second sample should be collected during convalescent phase
• Used for serological assays
Widal test
• Aim: Measurement of H and O agglutinins for typhoid and paratyphoid
• Principle : Tube Agglutination• Requirements:• Serum, Tubes, Antigen, Incubator, Waterbath• Tubes : Dreyer`s tube and Felix tube
Antigens
• O antigen of S.Typhi• H antigen of S.Typhi• H antigen of S.Paratyphi A and B
• Strain used to prepare : S.Typhi 901, O and H
• Procedure:1. Serial dilutions of equal volumes of
serum and antigen mixed.2. Put controls3. Incubated overnight at 37degC4. Read the results5. No agglutination in controls6. For O antigen – disc like pattern7. For H antigen – loose, cotton wooly clumps
• Highest dilution – TITRE• Moderate sensitivity and specificity• 30% of culture proven cases found to be Widal
negative ( WHO – TFguide)
• Slide Widal test – undiluted patient serum and antigens
CAUTION• Stage of disease• Prior antibiotics• Prior immunisation• Anamnestic response• Antigen preparation – free from fimbriae• False positive – typhus, acute falciparum
malaria, chronic liver disease, rheumatoid arthritis, nephrotic syndrome
• False negative – antibiotics, severe hypoproteinemia
Interpretation
• Always rising titre by testing paired sera – fourfold rise in the titre needed
• Single report with caution• Baseline titre in endemic areas
O - > 1:100H - > 1:200
IDL Tubex ® test
• Simple, Rapid• Slide latex agglutination test• O 9 antigen – Highly specific for S.Typhi, used
here , immunodominant epitope• Only for Typhoid fever, does not give positivity
for S.Paratyphi• Detects IgM antibodies
• Test Pack:1. Sets of V shaped tubes – six samples per set –
tested simultaneously2. Reagent A, magnetic particles coated with
S.Typhi LPS3. Reagent B, Blue coloured latex particles
coated with a monoclonal antibody specific for the O9 antigen
• Test serum ( one drop ) + Reagent A (one drop) – 1 minute – mix
• Then add two drops of Reagent B• Keep the tubes in magent embedded stand,
and slid it several times• Read the results immediately• Based on the colour of the reaction –
compared with the chart – Titre value noted• Stored sera has a better result in tubex than
widal
Typhidot ® test
• Simple, speed, economical• Sensitivity is 85.9%, Specificity 96.7%• To detect specific IgM and IgG antibodies to
S.Typhi• Typhidot - M ® - To detect IgM alone• Replaces the widal when used in conjunction
with the culture (Gold Standard)• High negative predictive value – useful in high
endemic areas
IgM dipstick test
• Detects IgM antibodies in serum and whole blood
• Materials:1. Dipstick2. Lyophilised non enzymatic detection reagent3. Liquid to reconsitute the detection reagent4. Liquid to wet the test strip of dipstick
• Wet the test strip in a mixture of serum and detection reagent ( 1:50)
• Incubate for 3 hours at RT• Rinse the test strip with water• Allow it to dry• Compare the color with reference strip• Grade it as 1+, 2+, 3+ and 4+• Sensitivity – 65% to 77%• Specificity – 95% to 100%
Enterocheck - WB
• Immunochromatographic test in cassette from • 30 minutes test• Sensitivity – 79.3%• Specificity – 90.2%
Coagglutination test
• Demonstration of circulating antigen• Done in the blood and in urine• Done in early phase of the disease• S.aureus (Cowan I Strain) which contains
protein A is stabilised with formaldehyde and coated with S.Typhi antibody
• 1% above suspension + patient serum – in a slide – visible agglutination (2 min) – positive
Urine
• Irregular and infrequent shedding of bacilli• Positive only in second and third weeks• 25% cases +• Clean voided urine samples are inoculated
into enrichment and selective media
Feces
• Collected in a container • Spoonful amount• Transport immediately• 6ml of buffered glycerol saline transport
mediumAlternate specimen:• Rectal swabs• Fecel swabs
• Shed throughout the course of the disease and also in convalescence
• Valuable in patients on antibiotics ( drug does not eliminate the bacilli from the gut)
• Fecal samples plated directly on MacConkeyDCA / XLD
Wilson Blair Media• Enrichment also done in selenite or
tetrathionate broth , incubated for 6 to 8 hours and subcultured.
Pale non lactose non sucrose fermenting colonies – DCLS
Red, black centred colonies – XLD
• Rule out proteus by urease test• Check for purity by subculturing in nutrient
agar• Do biochemical reactions and sugars• Do serotyping by slide agglutination test
InterpretationProvisional report – given on third or fourth day and
inform the clinician
Secondary confirmation test panel:1. Citrate agar slope2. Lysine decarboxylase medium with control3. Salicin peptone water4. ONPG5. Mac Conkey secondary purity plate6. Nutrient agar slope7. Sensitivity agar plate
If the secondary tests – confirm – pure culture of Salmonella seed it on to two Dorset egg slopes and send one to a Salmonella Reference Laboratory for final serotyping.
Send a confirmation report to clinician
Antimicrobial susceptibility testing
Drugs :1. Amoxycillin 2. Co-amoxiclav3. Cefuroxime4. Cotrimoxazole5. Ciprofloxacin6. Chloramphenicol
• Most of the strains are sensitive• Resistant – depends on serotype, phage type
and country of origin• 1990 – 20% strains resistant to
Chlorampenicol isolated in UK• 90% of strains are resistant to ampicillin and
trimethoprim• In Multidrug resistant areas – Ciprofloxacin is
the drug of choice
• A multidrug resistant strain of S.Typhimurium definitive type 104 that is resistant to five antibiotics emerged around 1990s
• 50% of the S.Typhimurium isolates were resistant to one or more drugs and 28% had a five drug resistance pattern
• 1998 – S.Newport – emerged as a major MDR pathogen
Diagnosis of Carriers
• High incidence due to carrier state• Contamination of food by food handlers
(Carriers)• Convalescent carriers • Temporary carriers• Chronic carriers ( 2 to 5%)• Bacilli persists in the Gallbladder and kidney• Intermittent shedding
• Repeated sampling – Bile or Faeces, urine for culture (Confirmatory)
• Demonstration of antibodies to Vi antigens (Screening test)
• IgG is the primary indicator of carriers• IgA and secretory IgA are seen. In Vaccinated –
No secretory IgA• Hence High IgA content indicate typhoid
carrier state
Public health ?
• In cities – tracing of carriers – Sewer Swab technique
• Sewage – Filtration through Millipore membrane – culture in Wilson and blair media
• Food safety – Carriers in hotels – Eg: Typhoid mary
Salmonella and Eggs
• According to the Centers for Disease Control, 1 in 10,000 eggs contain Salmonella.
• Experts say that chickens carry the bacteria in their own bodies, and pass Salmonella along to the yolk and white while the egg is forming in the ovaries.
• Chickens can also pass bacteria to the eggshell—and through the shell pores into the inner egg—when the egg is laid.
• The eggs are then submerged in all-natural water bath, where computer-controlled temperature zones monitor & heats the eggs in their shells to the exact temperature needed to destroy all bacteria, without cooking the egg.
• After pasteurization, the eggs are sealed with an FDA-approved, food-grade wax coating to prevent contamination and preserve product freshness.
• After pasteurization, the eggs are dried, cooled, and then stamped as P , which identifies them as pasteurized .
Typing methods
Bacteriophage typing :• Depends on Vi antigen• Used in epidemiological surveillance• National Salmonella Phage Typing Centre –
Lady Hardinge Medical College• S.Typhi phage types A and E1 – common in
India• S.Paratyphi A , types 1 and 2
Summary
• Culture – Gold standard – Late results – AST• Widal – Duration – Endemicity – paired sera• Slide tests – Discrepancies between labs
Ideal diagnostic test rapid, specific, sensitiveTYPHIDOT EIA
In this study ,• 66% blood culture +• 66% Widal test +• 74% Typhidot +
Typhidot is found to have high sensitivity and good specificity , alternate to blood culture
References• Mackie & McCartney – Practical Medical Microbiology –
14th Edition• Konemann – Colour atlas of diagnostic microbiology – 6th
edition• Harrisons – principle of internal medicine – 18th edition• District laboratory practive in tropical countries – 2nd
edition – Monica cheesbrough• Wain J et al, Specimens and culture media for the
laboratory diagnosis of typhoid fever• WHO – Salmonella surveillance report• Nitte journal of health science• Malaysian journal of medical sciences