JOURNAL OF CHEMISTRY Vol. 257, 10. of May 25. pp. in Rabbit Liver Glycogen … · 2001-08-25 ·...
Transcript of JOURNAL OF CHEMISTRY Vol. 257, 10. of May 25. pp. in Rabbit Liver Glycogen … · 2001-08-25 ·...

THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 257, No. 10. Issue of May 25. pp. 5873-5876, 1982 Printed in U.S.A.
Rabbit Liver Glycogen Synthase Kinases CHARACTERIZATION OF A PROTEIN KINASE (PC,,) ABLE TO PHOSPHORYLATE GLYCOGEN SYNTHASE AND PHOSVITIN*
(Received for publication, August 7, 1981)
Zafeer Ahmad, Anna A. DePaoli-Roach$, and Peter J. Roach From the Department of Biochemistry, Indiana University School of Medicine, Indianapolis, Indiana 46223
A rabbit liver protein kinase (PCO.,), able to phos- phorylate glycogen synthase and phosvitin, has been extensively purified. The enzyme had apparent M, = 170,000-190,000 as judged by gel filtration and was associated with two major polypeptide species, a (Mr = 43,000) and lp (Mr = 25,000). Two other polypep- tides, Mr = 38,000 and M, = 35,000, were also detected. Treatment with trypsin led to an enzyme composed only of polypeptides of M, = 35,000 and M, = 25,000. The @polypeptide underwent autophosphorylation when incubated with M&+ and ATP or GTP. The pro- tein kinase was effective in utilizing both ATP and GTP as the phosphoryl donor (apparent K,,, values 5-11 PM and 9-19 PM, respectively). The enzyme phosphorylated phosvitin, casein, and glycogen synthase but not his- tone or phosphorylase and was inhibited by heparin. Phosphorylation of glycogen synthase proceeded to ap- proximately 0.5 phosphate/subunit with little inacti- vation of the glycogen synthase. The phosphorylation occurred predominantly in a 21,000-dalton CNBr frag- ment of glycogen synthase that had been previously shown to reside toward the COOH terminus of the molecule. The liver PCo., appeared very similar to an analogous enzyme isolated from rabbit muscle (De- Paoli-Roach, A. A., Ahmad, Z., and Roach, P. J. (1981) J. BioL Chem 256,8955-8962). The present work, there- fore, provides a point of contact between the Ca2+ and cyclic nucleotide-independent glycogen synthase ki- nases of rabbit liver and muscle.
Covalent phosphorylation of glycogen synthase (EC 2.4.1.11), the enzyme believed to regulate the rate of glycogen synthesis, plays an important role in the hormonal control of glycogen metabolism (see reviews in Refs. 1-5). The rabbit skeletal muscle enzyme, which has been the most studied, can undergo phosphorylation at multiple sites of its 85,000-dalton subunit through the action of four or five distinct protein kinases (1-5). These protein kinases include cyclic AMP-de- pendent protein kinase (EC 2.7.1.37), phosphorylase kinase (EC 2.7.1.38), and two or three members of a class of cyclic nucleotide and Ca2’-independent protein kinases.’ Although
* This research was supported in part by Grants AM27221 and AM27240 from the National Institutes of Health, The Wayne Newton Research Grant of the American Diabetes Association, and the Grace M. Showalter Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “aduertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
$ Recipient of Fellowship AM06218 from the National Institutes of Health.
Nomenclature has been difficult for this general class of protein kinase. We will continue to use an operational nomenclature (see Ref. 6) for those protein kinases of this class that we separate by phospho-
the enzymology of muscle glycogen synthase kinases is far from complete, even less is known of glycogen synthase ki- nases from liver, especially those in the Ca2+- and cyclic nucleotide-independent category. Schlender and Reimann (7) demonstrated that a large proportion of glycogen synthase kinase in extracts of rat liver was distinct from cyclic AMP- dependent protein kinase. Partial purification of rat liver glycogen synthase kinases of this class has been reported by Jett and Soderling (8) and by Itarte et al. (9). We have now initiated studies aimed at classifying and characterizing the glycogen synthase kinases of rabbit liver, using the same approach as we have been applying to rabbit muscle (6, IO). We report here a clear point of contact between the liver and muscle systems with the purification of a protein kinase, PCo.?, that phosphorylates glycogen synthase and phosvitin and that appears analogous to the PCo.7 isolated from muscle (10).
EXPERIMENTAL PROCEDURES
Enzyme Assays-The assay of protein phosphorylation was based on the method of Huang and Robinson (11) using the conditions described previously (IO). Rabbit skeletal muscle glycogen synthase was present a t 0.2 mg/ml, liver phosphorylase (the gift of Dr. D. J. Graves, Iowa State University) at 0.4 mg/ml, and other protein substrates at 2.0 mg/ml, and [y3’]ATP was 0.1 m~ (600-900 cpm/ pmol) uniess noted otherwise. When used, [Y-~*P]GTP had a specific activity of 800 cpm/pmol. For studies of purified PC0.7, the assay contained 1-2 pg/ml of protein kinase. Glycogen synthase was assayed by the method of Thomas et al. (12). The per cent Z activity is the ratio of activity measured in the absence of glucose-6-P to that measured in the presence of 7.2 m~ glucose-6-P multiplied by 100.
Glycogen synthase was purified from rabbit skeletal muscle as described previously (13, 14).
Protein Determination-Protein concentration was determined by the Coomassie blue-binding assay (kit obtained from Bio-Rad) (15) using bovine serum albumin as a standard.
Gel Electrophoresis-Polyacrylamide gel electrophoresis in the presence of SDS’ followed the method of Laemmli (16) as described before (IO). Some gels were stained using the silver nitrate stain as described by Merril et al. (17). Quantitation of Coomassie blue-stained protein bands in dried gels was performed by scanning at 565 nm in a Beckman DU8 spectrophotometer. Chemicals for the silver stain were purchased from Fisher Scientific Co. CNBr cleavage of glycogen synthase was performed as previously described (IO), and the resulting fragments were separated using the SDS/urea system of Swank and Munkres (18).
Buffers-Homogenization buffer contained 4 m~ EDTA, 250 mM sucrose, 1 m~ (NH4)2S04, 0.5 m~ phenylmethylsulfonyl fluoride, 0.1 m~ N-or-p-tosyl-L-lysine chloromethyl ketone HCl, 250 pg/liter of leupeptin, and 25 mM mercaptoethanol, pH 7.0. Buffer A consisted of 50 mM Tris, 2 mM EDTA, 2 m~ ethylene glycol bisw-aminoethyl
cehdose chromatography, e.g. PC0.7, the fraction eluting at 0.7 M KC1 from phosphocellulose. We use the terms phosvitin kinase and gly- cogen synthase kinase generically to describe any protein kinase that phosphorylates the named substrate. ’ The abbreviations used are: SDS, sodium dodecyl sulfate.
5873
by guest on October 7, 2020
http://ww
w.jbc.org/
Dow
nloaded from

5874 Phosphorylation of Glycogen Synthase and Phosvitin
ether)-N,N,N’,N’-tetraacetic acid, 0.5 m~ phenylmethylsulfonyl flu- oride, 0.1 mM N-a-p-tosyl-L-lysine chloromethyl ketone HCl, 250 pg/ liter of leupeptin, and 25 mM mercaptoethanol, pH 7.5.
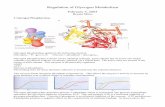
Enzyme Purification-Fresh rabbit liver (approximately 180 g) was homogenized in 3 volumes of homogenization buffer in a Waring blender. The homogenate was centrifuged at 13,000 X g for 40 min and the resulting supernatant filtered through glass wool. The pH of the supernatant was brought to 5.9-6 by adding 3.5 N acetic acid before centrifugation at 13,000 X g for 40 min. The supernatant was taken to 60% saturation with ammonium sulfate and centrifuged at 13,000 X g for 30 min. The precipitate was dissolved in Buffer A plus 0.1 M KC1 and dialyzed against three 2-liter volumes of the same buffer for a total of 6 h. The dialyzed material was absorbed in batch for 4 h, with gentle stirring, to phosphocellulose (Whatman P-11) which had been washed as described by Kish and Kleinsmith (19) and equilibrated with Buffer A plus 0.1 M KC1. The phosphocellulose was then washed batchwise with 300 ml of Buffer A plus 0.2 M KC1 and Buffer A plus 0.4 M KC1 successively. The phosphocellulose was packed to form a column (2.2 X I1 cm) and was washed with an additional 225 ml of Buffer A plus 0.4 M KCl. A gradient (200 ml) formed of equal volumes of Buffer A plus 0.4 M KC1 and Buffer A plus 1.4 M KC1 was then applied. PC0.7 was eluted at approximately 0.7 M KC1 and was identified as a peak of phosvitin and glycogen synthase kinase activity. The active fractions were pooled and chro- matographed on a phosvitin-Sepharose 4B column as previously described (IO). The active fractions (7 ml) from the phosvitin-Seph- arose 4B column were applied to a column (2.6 X 88 cm) of Bio-Gel A-1.5m (Bio-Rad) equilibrated with Buffer A plus 0.4 M KC1 and eluted with the same buffer (Fig. 1). Active fractions from the gel frltration were pooled, absorbed to DE52 (Whatman), and eluted as previously described (10). The PC0.7 appeared as a peak of glycogen synthase and phosvitin kinase activities (Fig. 2). All steps were performed at 4 “C and the whole procedure took 5 days.
RESULTS
Purification of P C O , ~ - T ~ ~ enzyme was purified by the procedure described under “Experimental Procedures” and which was similar to the method used for the isolation of the muscle enzyme (6, 10). One significant difference in the liver preparation was the more complex pattern of glycogen syn- thase kinase activity that eluted from the phosphocellulose column with the KC1 gradient (not shown) compared with the analogous step in the muscle purification. The Pc0.7 was selected as the major peak of phosvitin and glycogen synthase kinase activity eluting around 0.7 M KC1. Another difference from the muscle preparation was the presence of a lower molecular weight phosvitin kinase (Mr = -30,000) separated by gel fitration (Fig. 1). This material could represent a protein kinase activity not present in muscle or could reflect a less stringent pooling of the Pc0.7 fraction eluted from the
I
0.0
30 40 50 60 70 80 9
FRACTION NUMBER
FIG. 1. Gel filtration. The pooled fractions of PC0.7 from the phosvitin-Sepharose column were applied to a column (2.6 X 88 m) of Bio-Gel A-1.5 m eluted at 24 ml/h with fractions of 4.7 ml collected. In this run, only phosvitin kinase activity was monitored (solid circles), as described under “Experimental Procedures.”
7.5
5.0
2.5
0.0
0.4
0.3
“
0.2 ’ ZI GL Y
0.1
0.0
5 1 0 15 20
FRACTION NUMBER
FIG. 2. DEAE-cellulose chromatography. The elution of a col- umn of DE52 (Whatman) with a gradient of KC1 concentration (dashed line) is shown. Activity toward glycogen synthase (open circles, lower scale) and phosvitin (solid circles, higher scale) was determined as described under “Experimental Procedures.”
phospho~ellulose.~ The elution profile of the final DEAE- cellulose step (Fig. 2) indicated coincident peaks of glycogen synthase kinase and phosvitin kinase activity eluting at around 0.17 M KC1. The peak fractions from such a column were used for the characterization presented below. Fifty pg of Pc0.7 could be purified from 180 g of liver with 9% yield and a final specific activity (when freshly prepared) of 650 nmol/ min/mg using phosvitin as substrate compared with a value of 602 nmol/min/mg for the muscle enzyme (10).
Polypeptide Composition-Analysis of peak fractions from DEAE-cellulose chromatography (cfi Fig. 2) by polyacryl- amide gel electrophoresis in the presence of SDS indicated the presence of polypeptides a (Mr = 43,000), a’ (Mr = 38,000), a” (Mr = 35,000), and P (Mr = 25,000) (Fig. 3). From spectro- photometric scanning after staining with Coomassie blue, a typical a/a’/a“/j3 ratio would be 1:1.05:0.52:1.9. Enzyme activ- ity that eluted from DEAE-cellulose strictly correlated with the presence of the polypeptides described above (not shown). Our best muscle Pc0.7 preparations contained similar polypep- tides, but with a greater proportion of a, less a’, and very little or no a” (Fig. 3, see Ref. 10). Enzyme from rabbit reticulocytes could be obtained almost devoid of a’ and a” (20). However, we have previously reported (10) how apparent degradation of the a-polypeptide can occur during purification of muscle Pc0.7, leading to a decreased proportion of a and increased amounts of lower molecular weight species. Some prepara- tions of Pc0.7 from muscle have displayed a polypeptide pat- tern, upon gel electrophoresis, with increased proportions of a’ and a”. Treatment of liver Pc0.7 with 5 p g / d of trypsin led to the loss of a and a’ and the accumulation of a polypeptide of similar electrophoretic mobility to a” (Fig. 3). Similar behavior has been observed with muscle PC0.7.4
In the course of this work, the sensitive silver stain (17) was applied to polyacrylamide gels and this led to the observation that the P-polypeptide of PC0.7 stained abnormally well com- pared with its staining by Coomassie blue (compare a and P intensities of Coomassie blue- and silver-stained tracks in Fig. 3).
Molecular Weight-Calibration of the gel fitration column
In the purification of muscle Pc0.7 (6), a small peak of phosvitin and glycogen synthase activity, PCo.6, flanked the PCo.7. With liver, enzyme activity possibly corresponding to Pc0.6 was much more prominent and so might have contaminated the liver PC,,, fraction to a greater extent than with muscle.
4A. A. DePaoli-Roach, 2. Ahmad, and P. J. Roach, unpublished data.
by guest on October 7, 2020
http://ww
w.jbc.org/
Dow
nloaded from

Phosphorylation of Glycogen Synthase and Phosvitin 5875
used in the purification (Fig. 1) led to an apparent molecular weight of 170,000-190,000 for Pc0.7.
Nucleotide Specificity-Liver PC0.7 was effective in utilizing both ATP and GTP as phosphoryl donors. The kinetic param- eters obtained by varying ATP or GTP concentrations, with either phosvitin or glycogen synthase as substrate, are sum- marized in Table I. The apparent K,,, values for both ATP (5-11 p ~ ) and GTP (9-19 p ~ ) were close to the corresponding values obtained with muscle PCo.7 (13 p~ and 20-35 p ~ , respectively).
Phosphorylation of the P-Polypeptide-Incubation of mus- cle PC0.7 with ATP and Mg2‘ led to phosphorylation, predom- inantly in the P-polypeptide (10). The same phenomenon was found for liver Pc0.7 (Fig. 3) and we show additionally that autophosphorylation could occur when the enzyme was incu- bated with GTP (Fig. 3).
Protein Substrate Specificity-Liver PCo.7, using standard conditions, phosphorylated glycogen synthase (0.2 mg/ml), phosvitin (2 mg/ml), and mixed casein (2 mg/ml) at specific activities of 13.2, 356, and 336 nmol/min/mg, respectively. The protein kinase was ineffective in phosphorylating histone (with or without cyclic AMP) or phosphorylase (from rabbit liver or muscle). Up to the maximum glycogen synthase concentration that could be used (1.6 mg/ml, 19 p~ in terms of subunits), Pc0.7 activity was not saturated, defining an apparent K, in excess of 9.5 pM (see Ref. 10). PCo.7 activity
43 35
25 T
0- TI
1 2 3 4 5 6 7 8 9 10 FIG. 3. Polyacrylamide gel electrophoresis of PCO.,. Gel elec-
trophoresis, in the presence of SDS, was run as described under “Experimental Procedures.” The tulo digit numbers at the side are molecular weights, divided by 1000, of the indicated polypeptides. In A, the gel (10% acrylamide) was stained by the silver nitrate proce- dure. Truck 1 contained 0.75 pg of muscle PcO.7, truck 2,0.375 pg of muscle and 0.125 pg of liver Pc0.7, and trucks 3 and 4 contained approximately 0.25 pg of peak tubes of liver Pc0.7 from DEAE- cellulose column (see Fig. 2). The spurious bands in the region of X are artifacts of the staining procedure. B shows a portion of an autoradiogram of a gel comparable to that in A. Liver PC0.? was incubated with [y-”PIATP (truck 5) or [y-32P]GTP (truck 6) in a standard phosphorylation reaction (see “Experimental Procedures”) but lacking protein substrates. Approximately 0.1 pg of the Pc0.7 was run and the autophosphorylation of the P-polypeptide is seen. In C is shown portion of a gel (10% acrylamide) stained with Coomassie blue. In truck 7 was 1.12 pg of muscle PC0.7 and in truck 8 0.75 pg of liver Pc0.7. The 25,000-dalton P-polypeptide is scarcely visible in this gel. D shows portions of a gel (12% acrylamide) stained by the silver nitrate procedure. Liver Pc0.7 was incubated in the absence (truck 9) or presence (truck 10) of 5 pg/ml of trypsin for 15 min at 30 “C before addition of an excess of soybean trypsin inhibitor. 0.5 pg of Pc0.7 was applied to each track. The trypsin inhibitor and trypsin are indicated by TI and T, respectively.
TABLE I Kinetic parameters for ATP and GTP
Using the standard reaction conditions, except that ATP was varied in the range 2.5-300 PM and GTP was varied in the range 3.0-380 p ~ , the activity of liver Pc0.7 toward glycogen synthase and phosvitin was measured. In all cases, the dependence of reaction rate on nucleotide concentration was close to hyperbolic.
Protein substrate ATP varied GTP varied
K, V,. K, V,.
mg Glycogen synthase 5 33 9 16 Phosvitin 11 333 19 192
C M nmol/min/
m(F PM nmol/min/
was totally inhibited by 5 p g / d of heparin. The phosphoryla- tion of glycogen synthase proceeded to no more than 0.4-0.5 phosphate/subunit, with insignificant inactivation of glycogen synthase as judged by per cent I activity. Phosphorylation occurred in a 21,000-dalton fragment of glycogen synthase (see Ref. 10). All of the above properties coincide with those found for skeletal muscle PCo.7 (10, 20).
DISCUSSION
Compared with muscle, relatively little is known of the glycogen synthase kinase complement of liver, especially of the cyclic nucleotide and Ca“-independent enzymes. The main point of this paper is to suggest that one enzyme of this class, PCo.?, is present in both rabbit skeletal muscle and liver. Liver and muscle Pc0.7, purified extensively by almost iden- tical procedures, displayed the following properties in com- mon: specific activity of freshly purified enzyme (600-650 nmol/min/mg with phosvitin), molecular weight by gel fiitra- tion (170,000-190,000), ability to utilize GTP and ATP with apparent K,,, values in a similar range, phosphorylation of phosvitin, casein, and glycogen synthase but not phosphoryl- ase or histone, specificity for a 21,000-dalton CNBr fragment of glycogen synthase, phosphorylation of glycogen synthase to 0.4-0.5 phosphate/subunit, inhibition by heparin, the presence of polypeptide species of similar molecular weights in the purified enzyme (see below), and the autophosphorylation of one of these, the P-polypeptide. Purification through the same procedure, which involves four chromatographic steps, implies further similarities of properties. On the basis of these fairly extensive criteria, we propose that liver and muscle PCo.7 are very similar enzymes.
There is, however, a question concerning polypeptide com- position since liver contained greater proportions of the species a’ and a” compared with muscle PCo.7. We note that such species have been observed in greater proportions in some muscle PCo.7 preparations. Treatment of liver (or mus- cle) Pc0.7 with trypsin led to the disappearance of a and a’ and an accumulation of a“. Given our previous experience with muscle PCo.7 from which we suggested that the a-poly- peptide was susceptible to proteolysis (lo), one hypothesis is that during purification from liver, noted for its protease content, the Pc0.7 has been degraded from a putative a& structure analogous to that of the muscle (10) and reticulocyte (20) enzymes. Similar arguments have been advanced in other studies of PCo.7-like enzymes (21-23). However, definitive proof of this structure would require us to obtain liver PCo.7 lacking a’ and a”, something we have not achieved.
Rabbit liver PCo.7 shares several properties with the “casein kinase 2” partially purified by Itarte et al. (9) from rat liver. Casein kinase 2 had M, = 190,000 (by gel filtration) and phosphorylated glycogen synthase and phosvitin and eluted from phosphocellulose at high KC1 concentration. The casein kinase 2, however, phosphorylated glycogen synthase to a
by guest on October 7, 2020
http://ww
w.jbc.org/
Dow
nloaded from

5876 Phosphorylation of Glycogen Synthase and Phosvitin
level of almost two phosphates per subunit with significant inactivation of glycogen synthase, in contrast to the data presented here. The reason for the apparent discrepancy is not understood, but it is fair to point out that the casein kinase 2 was only partially purified.
In conclusion, we have reported the characterization of a protein kinase, PCO.~, from rabbit liver that is analogous to a glycogen synthase and phosvitin kinase previously isolated from muscle, and that appears similar to enzymes studied, in a variety of contexts other than glycogen metabolism, in a number of tissues (20-30). The work represents a fist , positive cross-correlation in our comparative studies of the glycogen synthase kinases of rabbit liver and skeletal muscle.
Acknowledgments-We wish to thank Sandya Ananth for techni- cal assistance and Peggy Smith for typing the manuscript.
1.
2. 3. 4.
5. 6.
7.
8.
9.
REFERENCES
Roach, P. J., and Larner, J. (1977) Mol. Cell. Biochem. 15,
Soderling, T. R. (1979) Mol. Cell. Endocrinol. 16, 157-179 Cohen, P. (1981) Adu. Cyclic Nucleotide Res. 14, 345-359 Preiss, J., and Walsh, D. A. (1981) in Biology of Carbohydrates
(Ginsburg, V., ed) Vol. 1, pp. 199-314, Academic Press, New York
179-200
Roach, P. J. (1981) Curr. Topics Cell. Regul. 20,45-I05 DePaoli-Roach, A. A., Roach, P. J., and Larner, J. (1979) J. Biol.
Schlender, K. K.. and Reimann. E. M. (1977) J. Biol. Chem. 252, Chem. 254,12062-12068
2384-2389
10.
11.
12.
13.
14.
15. 16. 17.
18.
19.
20.
21.
22. 23. 24. 25.
26.
27. 28.
334-397
Chem. 256,8955-8962
593-599
Biochem. 25,486-499
Chem. 250,8943-8950
251, 1913-1919
DePaoli-Roach, A. A., Ahmad, Z., and Roach, P. J. (1981) J. Biol.
Huang, K.-P., and Robinson, J. C. (1976) Anal. Biochem. 72,
Thomas, J. A., Schlender, K. K., and Larner, J . (1968) Anal.
Takeda, Y., Brewer, H. B., Jr., and Larner, J . (1975) J. Biol.
Roach, P. J., Takeda, Y., and Larner, J. (1976) J. Biol. Chem.
Bradford, M. (1976) Anal. Biochem. 72,248-259 Laemmli, U. K. (1970) Nature (Lond.) 227,680-685 Merril, C. R., Goldman, D., Sedman, S. A., and Ebert, M. H.
Swank, R. T., and Munkres, K. D. (1971) Anal. Biochem. 39,
Kish, V. M., and Kleinsmith, L. J. (1975) Methods Enzymol. 40,
DePaoli-Roach, A. A., Roach, P. J., Pham, K., Kramer, G., and
Hathaway, G. M., Lubben, T. H., and Traugh, J. A. (1980) J. Biol.
Hathaway, G. M., and Traugh, J. A. (1981) Fed. Proc. 40, 1607 Dahmus, M. E. (1981) J. Biol. Chem. 256,3319-3325 Dahmus, M. E., and Natzle, J. (1977) Biochemistry 16,1901-1908 Thornburg, W., and Lindell, T. J. (1977) J. Biol. Chem. 252,
Cochet, C., Job, D., Pirollet, F., and Chambaz, E. M. (1981)
Walinder, 0. (1973) Biochim. Biophys. Acta 293,140-149 Pinna, L. A., Meggio, F., and Dediukina, M. M. (1981) Biochem.
(1981) Science 211,1437-1438
462-477
198-208
Hardesty, B. (1981) J. Biol. Chem. 256,8871-8874
Chem. 255,8038-8041
6660-6665
Biochim. Biophys. Acta 658,191-201
BioDhvs. Res. Commun. 100.449-454 Jett, M. F., and Soderling, T. R. (1979) J. Biol. Chem. 254, 29. V&r:Phasi, C., and Kumon; A. (1981) J. Biol. Chem. 256,
Itarte, E., Mor, M. A., Salavert, A., Pena, J . M., Bertomeu, J. F., 30. Rose, K. M., Bell, L. E., Siefkin, D. A., and Jacob, S. T. (1981) J. 6739-6745 7409-7415
and Guinovart, J. J . (1981) Biochim. Biophys. Acta 658, Biol. Chem. 256,7468-7477
by guest on October 7, 2020
http://ww
w.jbc.org/
Dow
nloaded from

Z Ahmad, A A DePaoli-Roach and P J Roach(PC0.7) able to phosphorylate glycogen synthase and phosvitin.
Rabbit liver glycogen synthase kinases. Characterization of a protein kinase
1982, 257:5873-5876.J. Biol. Chem.
http://www.jbc.org/content/257/10/5873Access the most updated version of this article at
Alerts:
When a correction for this article is posted•
When this article is cited•
to choose from all of JBC's e-mail alertsClick here
http://www.jbc.org/content/257/10/5873.full.html#ref-list-1
This article cites 0 references, 0 of which can be accessed free at
by guest on October 7, 2020
http://ww
w.jbc.org/
Dow
nloaded from