Ions and Ionic bonding Chemistry. What’s in an atom? (review and new Info) Atoms are made up of 3...
-
Upload
matilda-hardy -
Category
Documents
-
view
216 -
download
2
Transcript of Ions and Ionic bonding Chemistry. What’s in an atom? (review and new Info) Atoms are made up of 3...

Ions and Ionic bondingChemistry

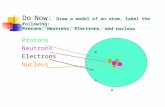
What’s in an atom? (review and new Info)• Atoms are made up of 3 particles:1.Protons-Located in the nucleus (center of atom)
2.Neutrons-Located in the nucleus (center of atom)
3.Electrons-Located in cloud or shells around the nucleus-Outermost shell of an atom is called the valence shell.-The number of electrons in the valence shell tells us how the atom will bond (with other atoms).

Atom drawings…• Blue are electrons.• Red are protons.• White are neutrons.• This is a drawing of…• Carbon (C).
• The closest “electroncloud” can only have2 electrons. EveryOther cloud can haveUP TO 8 electrons in it.

The Octet Rule
• The octet rule is…• -Atoms are most stable (content, happy, not
reactive) when their valence (outermost) shells have 8 electrons.• The only elements that have eight electrons in
their valence shell naturally are the noble gases.

•STOP for Atomic Structure Activity

Noble Gases are on far right

The 8 main groups of the periodic table12 3 4 5 6 7 8

Sodium and Chlorine example: Let’s make a deal!• Sodium (Na) had 1 electron in its valence shell and
chlorine (Cl) had 7 electrons in its valence shell.• They both need 8 electrons in their valence shells to be
stable.• It was easier for Na to give up 1 electron than try to gain
7 electrons.• It was easier for Cl to gain 1 electron than try to lose 7
electrons.• So, sodium gave up its 1 electron. The shell below the
valence shell already has an octet (8 electrons). Therefore, sodium is now stable.• Cl gained 1 electron, so it now has 8 electrons in its
valence shell, which makes it stable.

12 3 4 5 6 7
8Sodium (Group 1) and Chlorine (Group 7)

Forming the bond
• Sodium loses 1 electron so it has one less electron than Neon (Ne). Protons have a positive (+) charge so the Na will have an overall +1 charge because it lost 1 negatively charged electron.• This Sodium atom will now be a cation. (Positively
charged ion). Na+
• Chlorine gains one electron so it has one more electron than Argon (Ar). Electrons have a negative (-) charge so the Cl atom will have an overall -1 charge because it gained 1 negatively charged electron.• This Chlorine atom will now be an anion (Negatively-
charged ion). Cl-

Sodium and Chlorine example: Let’s make a deal!• (Let’s draw out what happened when Na and Cl formed
an ionic bond…)• Na: 1 valence electron Cl: 7 valence electrons• is shown by 1 dot are shown by 7 dots

Sodium and Chlorine example: Let’s make a deal!
• (Let’s draw out what happened when Na and Cl formed an ionic bond…)• When they form an ionic bond and exchange electrons,
Na loses 1 electron and Cl gains that 1 electron. Now both have 8 in their valence shells (Na had 8 electrons in its next lowest shell)

Which elements like to gain or lose electrons?•Metals (Groups 1-3) have less than 4
electrons in their valence shells. They would rather lose electrons to form cations.• Nonmetals (Groups 5-7) have more than 4
electrons in their valence shells. They would rather gain electrons to form anions.

12 3 4 5 6 7
8Groups 1-3 want to lose Electrons

12 3 4 5 6 7
8Groups 5-7 want to Gain Electrons

12 3 4 5 6 7
8Group 8 is happy how it is (already has a full 8 (Octet))

12 3 4 5 6 7
8
Group 4 can go either way (Gain or lose) depending on the atom…

More examples
• K and F• -K (Potassium) is in group 1 so it has 1 valence
electron.• -F (Flourine) is in group 7 so it has 7 valence electrons.• -Which atom will…• -lose electrons? _______ How many?________• -gain electrons? _______ How many?_______• Which atom becomes a(n)…• -Cation? ___________• -Anion? ____________
KF
11
K+
F-

More examples
• Ca and S• -Ca (Calcium) is in group 2 so it has 2 valence
electron.• -S (Sulfur) is in group 6 so it has 6 valence electrons.• -Which atom will…• -lose electrons? _______ How many?
________• -gain electrons? _______ How many?_______• Which atom becomes a(n)…• -Cation? ___________• -Anion? ____________
CaS
22
Ca2+
S2-

Your turn – Turn this page in!Your name here:________________• Al and N• -Al (Aluminum) is in group _____ so it has _____
valence electrons.• -N (Nitrogen) is in group ____ so it has ______
valence electrons.• -Which atom will…• -lose electrons? _______ How many?________• -gain electrons? _______ How many?_______• Which atom becomes a(n)…• -Cation? ___________• -Anion? ____________