Ionic and Covalent Bonds Science 10 Ms. Lowrie. Using Lewis Diagrams to Show: Covalent Bonding...
-
Upload
merry-phoebe-wood -
Category
Documents
-
view
218 -
download
0
Transcript of Ionic and Covalent Bonds Science 10 Ms. Lowrie. Using Lewis Diagrams to Show: Covalent Bonding...

Ionic and Covalent Bonds
Science 10
Ms. Lowrie

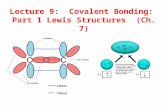
Using Lewis Diagrams to Show:
Covalent Bonding Steps:
1. Draw a Lewis diagram for both elements
2. Determine how to fill valance shell of both by sharing electrons
3. Redraw Lewis diagrams together
4. Circle ‘shared’ electrons

Covalent Examples
a) Hydrogen (H) and Chlorine (Cl)
b) Water = Hydrogen (H) and Oxygen (O)

More Covalent Examples
c) Carbon (C) and Hydrogen (H)
d) Fluorine (F) and Fluorine (F)
e) Phosphorus (P) and Chlorine (Cl)
f) Carbon (C) and Chlorine (Cl)

Practice: Draw Covalent Bonds
1. Hydrogen and Hydrogen
2. Ammonia = Hydrogen and Nitrogen
3. Chlorine and Chlorine
4. Nitrogen and Fluorine
5. Oxygen and Chlorine
6. Hydrogen and Fluorine
7. Iodine and Iodine
8. Carbon and Fluorine

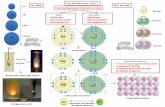
Using Lewis Diagrams to Show:
Ionic Bonding Steps:
1. Draw a Lewis diagram for each element
2. Use arrows to show how the electrons move
Example (a): Potassium (K) and chlorine (Cl)

More Ionic Examples
b) Sodium (Na) and oxygen (O)
c) Calcium (Ca) and chlorine (Cl)
d) Barium (Ba) and fluorine (F)
e) Aluminum (Al) and Oxygen (O)

Practice: Draw Ionic Bonds
1. Potassium and Phosphorus
2. Sodium and Fluorine
3. Aluminum and Chlorine
4. Potassium and Fluorine
5. Lithium and Oxygen
6. Calcium and Phosphorous
7. Sodium and Nitrogen
8. Aluminum and Bromine

Writing Chemical Formulas
Crossover Method Shortcut to writing
ionic compound formulas Steps:
1. Determine ions (write cation first)
2. “Crossover” - Write ion # as subscript (bottom #) for other element
Notes: Do not write +1 Write -1 as - If charges are equal (+2 & -2) = 1 each

Crossover Method – Example
a) Barium and Chlorine
BaCl2

More Crossover Examples
b) Beryllium and Phosphorous

More Crossover Examples
c) Potassium (K) and Phosphorous (P)
d) Sodium (Na) and Fluorine (F)
e) Aluminum (Al) and Oxygen (O)
f) Potassium (K) and Fluorine (F)

Practice: Crossover Method
1. Lithium and Oxygen
2. Calcium and Phosphorous
3. Calcium and Chlorine
4. Aluminum and nitrogen
5. Beryllium and Oxygen
6. Beryllium and Nitrogen
7. Potassium and Sulphur
8. Sodium and Oxygen

Practice Worksheets
1. “Ionic vs. Covalent Bonds” Last 2 questions
2. “Write formulas for the following:” Using Crossover Method

Polyatomic Ions
Ions made up of 2+ ions
“poly” = many

Polyatomic Ion Formulas
Notes on formula writing: Use brackets to keep polyatomic ion
“together” Write cation first

Example
a) Write formula for:
NH41+ and S2-
1. Draw:
2. Use Crossover Method:

Examples & Practice
b) Calcium & Nitrate
c) Sodium & Hydroxide
d) Sodium & Sulfate
e) Calcium & Phosphate
f) Lithium & Hydrogen carbonate

Practice Worksheets
1. “Polyatomic Ion Practice”
2. “Writing Formulas” No naming yet!

Naming Ionic Compounds
Steps:1. Write cation first
2. Write anion last with suffix (ending) “ide”
Potassium Chloride
Note: Do not include ‘numbers’!

Naming Ionic Compounds: Examples
a) Ba and Cl
b) Sodium and Fluorine
c) Be and P
d) Ca2+ and Cl-
e) Al3+ and N3-
f) Na2O
g) K3P

Naming Ionic Compounds: Practice
1. Be2+ and O2-
2. Be2+ and N3-
3. K+ and S2-
4. Al2O3
5. KF
6. Li2O
7. Ca3P2
Polyatomic Ions: Same “ordering” rule! Don’t change to “ide”
1. Ca & NO3
2. Na & OH
3. Na & SO4
4. Ca & PO4
5. Li & HCO3

Naming Ionic Compounds: Practice
1. “Writing Formulas” worksheet With names now!
Complete the following textbook questions: #9, 10 (on page 113) #2 (pg 115) #4 (on pg 117)

Naming Covalent Compounds
Steps:1. Add numerical prefix (beginning) to both
elements Ignore ‘mono’ for 1st
element
2. Add “ide” suffix (ending) to second element
Note: When prefix ending in O or A
is added to oxide, drop last vowel of prefix

Naming Covalent Compounds: Examples
a) N2S4 dinitrogen tetrasulfide
b) NI3 nitrogen triiodide
c) XeF6 xenon hexafluoride
d) CCl4 carbon tetrachloride
e) P2O5 diphosphorus pentoxide
f) SO3 sulfur trioxide
1 mono
2 di
3 tri
4 tetra
5 penta
6 hexa
7 hepta
8 octa
9 nona
10 deca
* Second element in ‘ide’ from
* Drop –a & -o before ‘oxide’

Naming Covalent Compounds: Practice “Nomenclature – Covalent Compounds”
worksheet
Complete the following textbook questions: #9, 10 (on page 123)