Innovative ‘Artificial Mussels’ technology for assessing spatial and temporal distribution of...
-
Upload
golam-kibria -
Category
Documents
-
view
218 -
download
0
Transcript of Innovative ‘Artificial Mussels’ technology for assessing spatial and temporal distribution of...

Environment International 50 (2012) 38–46
Contents lists available at SciVerse ScienceDirect
Environment International
j ourna l homepage: www.e lsev ie r .com/ locate /env int
Innovative ‘Artificial Mussels’ technology for assessing spatial and temporaldistribution of metals in Goulburn–Murray catchments waterways, Victoria,Australia: Effects of climate variability (dry vs. wet years)
Golam Kibria a,⁎, T.C. Lau b, Rudolf Wu c
a Goulburn Murray Rural Water Corporation, Tatura, VIC 3616, Australiab Department of Biology and Chemistry, City University of Hong Kong, Hong Kongc School of Biological Sciences, The University of Hong Kong, Hong Kong
⁎ Corresponding author at: 45 Lowson St, Fawkner, V0438 043 494.
E-mail addresses: [email protected], Kibriah(G. Kibria).
0160-4120/$ – see front matter © 2012 Elsevier Ltd. Allhttp://dx.doi.org/10.1016/j.envint.2012.09.006
a b s t r a c t
a r t i c l e i n f oArticle history:Received 18 May 2012Accepted 16 September 2012Available online 13 October 2012
Keywords:Artificial mussel (AM)Trace metalsHot spotsClimate variability
The “Artificial mussel” (AM), a novel passive sampling technology, was used for the first time in Australia infreshwater to monitor and assess the risk of trace metals (Cd, Cu, Hg, Pb, and Zn). AMs were deployed at 10sites within the Goulburn–MurrayWater catchments, Victoria, Australia during a dry year (2009–2010) and awet year (2010–2011). Our results showed that the AMs accumulated all the five metals. Cd, Pb, Hg weredetected during the wet year but below detection limits during the dry year. At some sites close to orchards,vine yards and farming areas, elevated levels of Cu were clearly evident during the dry year, while elevatedlevels of Znwere found during the wet year; the Cu indicates localized inputs from the agricultural applicationof copper fungicide. The impacts from old mines were significantly less compared ‘hot spots’. Our studydemonstrated that climate variability (dry, wet years) can influence the metal inputs to waterways viadifferent transport pathways. Using the AMs, we were able to identify various ‘hot spots’ of heavy metals,which may pose a potential risk to aquatic ecosystems (sub-lethal effects to fish) and public (via food chainmetal bioaccumulation and biomagnification) in the Goulburn–Murray Water catchments. The State ProtectionPolicy exempted artificial channels and drains from protection of beneficial use (including protection of aquaticecosystems) and majority of sites (‘hot spots’) were located within artificial irrigation channels.
© 2012 Elsevier Ltd. All rights reserved.
1. Introduction
Contamination of aquatic systems through discharges frommining, industrial and agricultural activities may render water notsuitable for irrigation, drinking, and supporting livestock and aqua-culture. Metal pollution may affect ecosystem biodiversity, eliminatesensitive species or reduce species abundance through reproductiveimpairment and increased incidence of diseases. Invertebrates andfish can bioaccumulate metals at concentrations up to thousands tomillion times higher than the ambient environment, thereby posingrisks to predators of humans through biomagnifications (Luoma andRainbow, 2008). As such, monitoring of metals in the environmentis essential to safeguard ecosystem health and public health.
Traditional monitoring of metals in the aquatic environmentinvolves determining and comparing metals in water, sediment andbiota, but each method has its own problems and limitations. Forexample, temporal variations in metal concentrations in water are
IC 3060, Australia. Tel.: +61 3
rights reserved.
typically large, which often require frequent sampling and analysisthat are not cost effective. Metal concentration in sediment, on theother hand, is highly dependent upon the sediment characteristics(e.g. particle size, organic content), which cannot be standardized forsite comparison. Because of these limitations, bio-monitoring has beenused extensively to monitoring metals in the last two decades; thenotable example is the global mussel watch program (Kimbroughet al., 2008). However, the metal concentration in bio-monitors issignificantly affected by physical and biological factors. Importantly,the restricted natural distributions of biomonitoring species often pre-vent direct comparison between different geographical/hydrographicalregimes (Wu et al., 2007).
Until recently there were no reliable time integrated techniques toassess metal concentrations in water which could be used to assessthe risk or conformity to water quality guidelines. However, Wuet al. (2007) have now developed the ‘Artificial Mussel’ (AM), andthis novel device has been used to monitor heavy metals in water inEurope, South Africa and China. This new device is a cost effective mon-itoring tool which provides a time-integrated concentration of metals inthe aquatic environment during the deployment period. Both laboratoryand field studies showed that the AMs confers significant advantagescompared with traditional monitoring techniques using mussels,

39G. Kibria et al. / Environment International 50 (2012) 38–46
water and sediment (Degger et al., 2011; Gonzalez-Rey et al., 2011;Leung et al., 2008; Wu et al., 2007).
Goulburn–Murray Water (G-MW) is the Australia's largest ruralwater authority, covering 68,000 square kilometres in northernVictoria. The primary goal of G-MW is the maintenance of water qual-ity for the customers and various objectives including maintenance ofbiodiversity, irrigation, drinking and recreation. As part of the Global
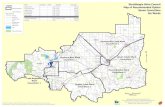
Fig. 1. Showing the location of Australia (top left), Victoria (top right) and the location of G-in North Victoria, Australia. [Sites are: 1—Buffalo at Myrtleford (Ref‐Murray River); 2—Howq(intensive orchards); 5—Mooroopna (orchards and tomatoes); 6—Tatura (mixed farming);farming) and 10—Kangaroo lake at Mystic park (intensive vine)].
Artificial Mussel Watch program being run in ten countries includingAustralia (Kibria et al., 2010a), the main objectives of this study were:
• To use the ‘Artificial Mussel’ for monitoring and assessing thespatial and temporal changes of metals (Cd, Cu, Hg, Pb, Zn) inGoulburn–Murray catchments waterways
• To identify the ‘hot spots’ in the catchment
MWAMmonitoring sites (bottom) during 2009–2010 (dry) and 2010–2011 (wet) yearua at Mansfield (Ref‐Goulburn River); 3—Cobram (intensive orchards); 4—Shepparton7—Rochester (vine and tomatoes); 8—West Boort (intensive olive); 9—Kerang (mixed

40 G. Kibria et al. / Environment International 50 (2012) 38–46
• To assess the ecological and public health risks of metal contamina-tion within the catchments.
2. Material and methods
Levels of heavy metals at ten potential risk sites (Fig. 1) in thecatchment was monitored for a dry year (2009–2010) and a wetyear (2010–2011) to assess the climate variability (dry vs. wetyears) impacts on trace metals inputs, transport and bioavailability.The study was conducted during the irrigation season (15 August–15May of each year; Note in 2009–2010, irrigation season started inSeptember due to insufficient supply of water due to drought).
Table 1Description of tracemetalsmonitoring sites in Goulburn–MurrayWater (G-MW) catchmentsd/s=downstream.
Site number and description Active region Prime targ
01—Buffalo–Murray River system@Yarrabulla creek, Lake Buffalo; GPS:55-429519E-5879188NElevation—285 m
Myrtleford Reference-River
02—Howqua–Goulburn River system@ Howqua River @Glen-esk-Woods point &Howqua River RoadGPS: 55-429700E-5879100NElevation—318 m
Mansfield Reference-River
03—Cobram–Murray River System@ irrigation channel (6/1 off take of no 1 main);Murray Valley HWYGPS: 55-379788E-6021820 NElevation-114 m
Cobram East, MurrayValley
Intensive o
04—Shepparton–Goulburn River system@Channel 12; Barmah-Shepparton Rd; GPS:55-356255E-5977834NElevation-125 m
Shepparton North,Shepparton
Intensive O
05—Mooroopna–Goulburn River system@channel15/6/4,Ebbots regulator; MidlandHWY-Cemetery RdGPS: 55-351062E-5975117NElevation—116 m
Mooroopna, CentralGoulburn
Channel o
06—Tatura–Goulburn River system@ channel 3/5; Murchison–Tatura Rd;GPS: 55-340656E-5961583NElevation- 121 m
Tatura, CentralGoulburn
Intensive oand town
07—Rochester–Goulburn River system@ channel1 Waranga Western Channel; WinterRd–Freeman RdGPS: 55-303-6831E-5972339NElevation—110 m
Rochester,Rochester-Campaspe
Town supp
08—West Boort–Goulburn River system@ channel 5 Waranga Western Channel;Boort-Charlton Rd–Hummel–Grandview RdGPS: 54-734789E-5997485NElevation—103 m
Boort, Pyramid- Boort Intensive o(Olive) ansupply
09—Kerang–Murray River system@channel 14/2; Murray Valley HWY–Collins RdGPS: 54-763963E-6039331NElevation—80 m
Kerang, Torrumbarry Town supp
10—Kangaroo Lake–Murray River system@channel 7; Murray Valley HWY–Mystic park RdGPS: 54-750197E-6061606NElevation—75 m
Kerang, Torrumbarry Ramsar lak
New G-MW operational water districts (2010): Murray Valley=Murray North-East Operatioperations; Rochester–Campaspe=Campaspe operations; Pyramid-Boort=Loddon operati
2.1. Sampling sites
A brief description of each of the ten sampling sites is given inTable 1 (see also Fig. 1):
2.2. Field deployment and retrieval of artificial mussel
2.2.1. What is an artificial mussel (AM)The ‘Artificial Mussel (AM)’ passive sampler is a device that collects
or accumulates metals through a diffusion barrier onto a sorbentmedium. The device (AM) consists of non permeable Perspex tubing(60 mm×25 mm) in which 200 mg Chelex-100® resin (50–100
, Victoria, Australia during 2009–2010 (dry) and 2010–2011 (wet) year. u/s=upstream;
ets Comments
Murray u/s: national forest; Old mines during 1800 s- at BucklandValley upstream of the site.d/s: water supply to Murray Valley and Torrumbarry irrigation districtsfor irrigation, stock & domestic, aquaculture and town supply
Goulburn u/s: national forest; Old mines during 1800 s were at SheepYard flat, Little Nell, Mountain Chief and Great Rand upstream of the sited/s: water supply to Shepparton, Central Goulburn,Rochester-Campaspe and Pyramid Hill-Boort irrigationdistricts for irrigation, stock & domestic, aquaculture andtown supply
rchards u/s: intensive orchardsd/s: pasture, horticulture, crops, stock & domestic supply
rchards u/s: intensive orchardsd/s: pasture, horticulture, crops, stock & domestic supply
utfall u/s: intensive orchards and tomatoes;d/s: outfall to Goulburn River
rchardssupply
u/s: pasture and crop;d/s: town supply off take point.
ly u/s: vine and tomatoes;d/s: town supply off take point and stock & domestic supply.
rchardsd town
u/s: intensive olives;d/s: town supply off take point and stock & domestic supply.
ly u/s: pastured/s: raw town supply
e u/s: intensive vines and Ramsar Lake;d/s: water supply to Swan Hill.
ons; Shepparton=Goulburn-Broken Operations; Central Goulburn=Central Goulburnons; Torrumbarry=Central Murray Operations.

Fig. 4. Deployment of AMs in irrigation channels (Rochester) (photo by Golam Kibria).
Fig. 2. A schematic diagram showing the design of Artificial Mussel chemical structureof chelex-100 is shown in the inset (Wu et al., 2007).
41G. Kibria et al. / Environment International 50 (2012) 38–46
mesh from Bio-Rad) is suspended in 8 mL seawater/freshwater insidethe tubing (see Figs. 2 and 3). Both ends of the tubing are further cappedby a layer of polyacrylamide gel (thickness: 1 cm), to protect the gelfrom possible mechanical damages (Figs. 2 and 3). Water diffusesthrough the polyacrylamide gel into the chelax-100 (metal bindingagent) from which the complexed metals can later be extracted (seeFig. 2). After several weeks, the chelating agent is sampled to determineitsmetal content. Laboratory studies have found that uptake ofmetals isaffected only slightly by salinity and temperature (Wu et al., 2007).
2.2.2. How does AM accumulate trace metals?Chelex-100 is ametal complexing resin that can adsorb heavymetals
by forming stable complexes with them. The main function of the per-meable gel is to control the rate of diffusion of water to the resin andhence control the rate of metal uptake. The rate of diffusion across thegel can be tuned by varying the thickness and the pore size of the gel.The pore size can be tuned by varying the ratio of themonomer acrylam-ide and the crosslinker N,N′-methylene-bis-acrylamide. Moreover, thepolyacrylamide gel is very robust and not bio-degradable.
2.2.3. Deployment and retrieval of AMAMs were placed in an rock filled autoclaved/plastic basket
(17.8×16.8×15.6 cm; mesh 10 mm; Interpath Services, Heidelberg,Melbourne) with one AM per basket (3 replicates at each site)(note: rock were placed to enhance sinking of the basket into
Fig. 3. ‘Artificial mussels’ device used in heavy metals monitoring (photo by GolamKibria).
water). The plastic basket was then submerged in rivers, creeks andchannels sites (1 m below surface water, Fig. 4). Nylon rope andpulleys were attached to star pickets to facilitate deployment andretrieval of autoclave baskets and operated from the shore. Eachbatch of AM deployed in rivers, creeks and channels were retrievedat the end of a four week (28 day) exposure period and sent by courierto City University of Hong Kong for analysis. The following procedureswere followed after retrieval of AMs: the fouling organisms attachedon the surface of AMs were washed down and AMs were briefly rinsedwith the site water. Each AM was then wrapped within a wet sponge/cotton pad with identification tags included inside each whirl packbag /sterile bag (Solar-Cult cellulose Swab-SBDCS-100-1, ArrowScientificPty Ltd, NSW) before shipment to Hong Kong for analysis.
2.3. Analytical techniques and quality control
The AMs were analyzed by the Chemistry Lab, City University ofHong. In the laboratory, the contents of each AM were emptied ontoa sintered glass filter, washed twice with nitric acid (6 M, analyticalgrade; 2×12.5 mL), and the elutriant made up to 25 mL volumewith deionized water. The concentrations of metals were determinedby either inductively coupled plasma atomic emission spectrometry(ICP-AES, Perkin Elmer Optima 2100DV) or inductively coupledplasma mass spectrometry (ICP-MS, Perkin Elmer Sciex Elan DRCPlus, for metal concentrations below 20 μg/L) (Wu et al., 2007). Theinstruments were calibrated by using standard solutions.
The CityU used an in-house analytical manual and a quality manualthat were written according to the guidelines of HOKLAS (Hong KongLaboratory of Accreditation Scheme). The instruments ICP-AES andICP-MS were calibrated using standard reference material (SRM 3171a,batch 4929080) obtained from the National Institute of Standards andTechnology (USA). SRM 317a is a multielement solution prepared gravi-metrically with a nitric acid concentration (V/V) of 5%.
2.4. Water quality
Data on rainfall was obtained from the Victorian data warehouseand averaged for the year http://www.vicwaterdata.net/vicwaterdata/data_warehouse_content.aspx?option=2 and data on water tempera-ture andwater hardnessweremeasured at all the 10 sites and averagedfor the year to elucidate the relationships between metal inputs, trans-port and bioavailability with water quality and climate variability (dryvs. wet year). The yearly average for dry (2009–2010) and wet yearare given below:
2009-2010
2010-2011Rainfall
708±2.84 mm 1080±22.45 mm Water temperature 20.8±2.18 °C 19.17o±1.11 °C Water hardness 88.42 mg/L CaCO3 73.93±6.66 mg/L CaCO3
Table 2Average (±S.EM) metal concentration (μg/g dry weight) in the AMs at sites within the Murray River catchment during dry (2009–2010) and wet year (2010–2011) (– indicate notdetected or below the limit of detection). Limit of detection (LoD): Cd=0.01 μg/g; Cu=1.0 μg/g; Pb=0.02 μg/g; Hg=0.01 μg/g; Zn=1.0 μg/g.
Upstream (site 1) Downstream sites (3, 9, 10)
Metals Year Reference MurrayRiver-site 1 (Buffalo)
Irrigation channelsite-3 Cobram
Irrigation channelsite-9 Kerang
Irrigation channel site-10Kangaroo lake
Cadmium (Cd) 2009–2010 – – – –
2010–2011 0.015±0.0 0.012±0.0 0.010±0.0 0.013±0.0Copper (Cu) 2009–2010 15.83±1.4 16.28±2.4 20.98±1.4 18.12±2.0
2010–2011 12.78±1.4 12.33±0.5 11.26±0.8 10.97±0.8Lead (Pb) 2009–2010 – – – –
2010–2011 0.393±0.0 0.467±0.8 0.773±0.1 0.936±0.9Mercury (Hg) 2009–2010 – – 0.5±0.1 –
2010–2011 0.017±0.0 0.0.15±0.0 0.015±0.0 0.011±0.0Zinc 2009–2010 27.02±4.9 27.85±5.1 29.16±3.5 23.7±3.1
2010–2011 74.76±13.1 68.07±10.2 88.29±9.1 79.21±8.5
42 G. Kibria et al. / Environment International 50 (2012) 38–46
3. Results
The AM accumulated all the five targeted metals (Cd, Cu, Hg, Pband Zn). Elevated levels of Cu were detected during the dry year(2009–2010), while elevated levels of Zn were detected during thewet year (2010–2011). Cd, Pb and Hg were generally below detectionlimits (detection limit of Cdb0.01 μg/g; Pb b0.020 μg/g; Hg b0.01 μg/g)during the dry year but detected during the wet year (Tables 2, 3).
3.1. Copper
Significant spatial and temporal difference in Cuwas found. Elevatedcopper levels in the AM were found during dry year (2009–2010) atfour sites: site 4 (Shepparton): an area of intensive orchards (Dec-Jan;Fig. 5); site 8 (West Boort): an area of intensive olive culture (Jan–Feb; Fig. 5.2); site 9 (Kerang): mixed farming (Dec-Jan; Fig. 5.3); andsite 10 (Kangaroo Lake): vineyards (Dec–Jan; Fig. 5.4). Cu concentra-tions were highest during December–February in 2009–2010, whilethe lowest concentrations of Cuwere foundduring September–October.The copper concentrations during the dry year (2009–2010) were sig-nificantly higher than the wet year (2010–2011) (Fig. 5 to 5.4).
3.2. Zinc
Spatial and temporal variations in Zn concentrations were found.Four irrigation channel sites had elevated zinc levels during wetyear (2010–2011), these were: site 6 (Tatura): mixed farming (Jan–Feb; Fig. 6); site 7 (Rochester): vineyards & tomatoes (Dec-Feb;Fig. 6.2); site 8 (West Boort): intensive olive culture (Jan–Feb;Fig. 6.3); site 9 (Kerang): mixed farming (Jan–Feb; Fig. 6.4). DuringJanuary-February (2010–2011), the highest zinc concentrations inAMs were observed, whereas the lowest concentrations were found
Table 3Average (±S.EM)metal concentration (μg/g dryweight) inAMs deployed at siteswithin theGodetected or below the limit of detection. Limit of detection (LoD): Cd=0.01 μg/g; Cu=1.0 μg/
Upstream (site 2) Downstream sites (4, 5, 6
Metals Year Reference GoulburnRiver-site 2 (Howqua)
Irrigation channelsite-4 (Shepparton)
Irrigsite
Cadmium (Cd) 2009–2010 – – –
2010–2011 0.014±0.0 0.011±0.01 0.01Copper (Cu) 2009–2010 15.59±1.1 19.87±1.5 16.9
2010–2011 11.02±0.6 11.88±1.0 12.0Lead (Pb) 2009–2010 – 2.1±0.0 2
2010–2011 0.247±0.1 0.387±0.0 0.31Mercury (Hg) 2009–2010 – – –
2010–2011 0.011±0.0 0.013±0.0 0.01Zinc 2009–2010 22.8±1.5 32.95±4.2 32.4
2010–2011 69.93±13 90.78±10.2 71.3
during September–October periods. The zinc concentrations duringthe wet year (2010–2011) were significantly higher compared tothe dry year (2009–2010) (Fig. 6 to 6.4).
4. Discussion
Elevated levels of Cu was found during the dry year (2009–2010)(Fig. 7); while elevated levels of Zn were found during the wet year(Fig. 8), showing climate variability (dry vs. wet year) affects out-flows from the catchment, thereby affecting the inputs, transportand availability of individual metals. Furthermore, during the dryyear (2009–2010), the water temperature and water hardness values(see Section 2.4), were found higher compared to the wet year.However, it is unknown whether any of the water quality factors con-tributed in higher accumulation of copper in AMs during the dry yearand zinc in the wet year. For instance, copper fungicides are typicallyinsoluble in water and it may be mainly transported to the waters bywind during dry years. On the other hand zinc salts (in fertilizer) areusually more water soluble and therefore more zinc may be washedto the waters during wet year. However, there may be other reasonsthat are not due to rainfall.
Elevated Cu was found during Dec-Feb periods of the dry year(2009–2010) at some sites (‘hot spots’). These sites were close to inten-sive orchards, vineyards or mixed farming, suggesting that localizedcopper inputs as copper dust from these areas, possibly from the useof fungicide containing copper hydroxide or copper sulphate (seeFig. 5 to 5.4 for sites 4, 8, 9, and 10). Similarly, the sites where elevatedZnwas found during thewet year (2010–2011) (‘hot spots’) were closeto mixed farming or vine and tomatoes. This may be due to localizedzinc inputs in these areas, possibly from usage of agriculture fertilizerssuch as phosphate fertilizer or windblown dust containing Zn. Roseand Kibria (2007) also found elevated trace metals concentrations
ulburnRiver catchment during dry (2009–2010) vs. wet year (2010–2011).− indicate notg; Pb=0.02 μg/g; Hg=0.01 μg/g. Zn=1.0 μg/g.
, 7, 8)
ation channel-5 (Mooroopna)
Irrigation channelsite-6 (Tatura)
Irrigation channelsite-7 (Rochester)
Irrigation channelsite-8 (West Boort)
– – –
0±0.0 0.010±0.0 0.012±0.0 0.012±0.00±2.9 16.54±2.2 15.51±1.4 19.57±1.17±0.7 11.52±0.3 12.74±0.7 10.63±0.6.0±0.1 – – –
7±0.0 0.442±0.4 0.620±0.1 0.615±0.7– 1.5±1.1 –
3±0.0 0.014±0.0 0.009±0.0 0.011±0.00±2.6 28.10±4.4 21.45±5.1 37.33±4.65±13.6 79.30±9 80.66±14 79.66±8.6

Fig. 5. 1. Shepparton (site 4). Four week average copper concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deployment period for AM. Months without abar indicate concentrations level was below the detection limits of 1.00 μg/g. 2. West Boort (site 8). Four week average copper concentrations in artificial mussels (μg/g dry weight);mean (±S.EM) for each deployment period for AM. Months without a bar indicate concentrations level was below the detection limits of 1.00 μg/g. 3. Kerang (site 9). Four weekaverage copper concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deployment period for AM. Months without a bar indicate concentrations level wasbelow the detection limits of 1.00 μg/g. 4. Kangaroo lake (site 10). Four week average copper concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deploy-ment period for AM. Months without a bar indicate concentrations level was below the detection limits of 1.00 μg/g.
43G. Kibria et al. / Environment International 50 (2012) 38–46
(copper) atWest Boort (site 8), Kerang (site 9), Kangaroo Lake (site 10)during 2004–2006 pesticide monitoring and concluded that copperlevels at those sites may have been originated from use of copper fungi-cide in those areas. In general, Cu and Zn are potential sources fromfertilizers, fungicides, copper dust, farmland manures, and pesticides(Marcotullio, 2007). The impacts from old mines (sites 1 and 2) weresignificantly less compared to sites close to orchards, vineyards ormixed farming (see Figs. 7 and 8).
4.1. Implications for biota and humans
Copper and zinc accumulated in AMs may represent the bio-available or free toxic fractions. Cu and Zn are regarded as essentialelements for aquatic biota, however, it is unknown whether elevatedcopper and zinc observed in the ‘hot spots’ in the present study mayexert any sub-lethal effects to fish and other aquatic biota in thearea, and this require further investigations. In general, the toxicityof Cu and Zn generally decreases as hardness increases (ANZECCand ARMCANZ, 2000) and in our present study the hardness valuewas quite high, ranging between 73 and 88 mg/L. Similar to aquaticbiota, both zinc and copper are also regarded as essential elementsfor humans and is not carcinogenic (drinking water guideline valuesfor copper and Zinc are 2 mg/L andb3 mg/L respectively) (WHO, 1996).Nevertheless, higher concentrations may still pose a risk to human
consumers via food chain bio-accumulation and bio-magnification(Chapman, 1997).
In order to convert AM chelex resin concentrations into time-weighted average water concentrations for the period of AM deploy-ment, calibration (or uptake) factors for each metal are required forcomparison with the ANZECC and ARMCANZ (2000) water qualityguideline values for the protection of aquatic ecosystems in Australia.These are not currently available. A preliminary calibration andconcentration factor experiment with AM was conducted at DPIQueenscliff Centre (Kibria et al., 2010b), but results were inconclu-sive, perhaps due to high levels of Cu (540 μg/l) found in the tapwater used, and thus they cannot be used to produce quantitativeaverage water metal concentration.
5. Conclusion
Metals accumulated in AM provide an indication of the levels oflabile-metal species (the most toxic fractions consists of free ion) inthe water over the deployment period, which has been shown to bedifferent between dry and wet years (Figs. 7 and 8). This studysuggests that climate variability (e.g. dry and wet years) may have asignificant impact on metal (Cu and Zn) input, transport levels ofmetals in aquatic systems. Using the AMs, we were able to identifyvarious ‘hot spots’ in the water catchments due to localized inputs

Fig. 6. 1. Tatura (site 6). Four week average zinc concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deployment period for AM. Months without a barindicate concentrations level was below the detection limits of 1.00 μg/g. 2. Rochester (site 7). Four week average zinc concentrations in artificial mussels (μg/g dry weight); mean(±S.EM) for each deployment period for AM. Months without a bar indicate concentrations level were below the detection limits of 1.00 μg/g. 3. West Boort (site 8). Four weekaverage zinc concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deployment period for AM. Months without a bar indicate concentrations level werebelow the detection limits of 1.00 μg/g. 4. Kerang (site 9). Four week average zinc concentrations in artificial mussels (μg/g dry weight); mean (±S.EM) for each deployment periodfor AM. Months without a bar indicate concentrations level were below the detection limits of 1.00 μg/g.
44 G. Kibria et al. / Environment International 50 (2012) 38–46
from usage of copper fungicide. Since these ‘hot spots’ are in closeproximity of orchards, vine yards and farms, the potential publichealth concerns should not be overlooked. Furthermore, chemicalsdetected in this study are results of customer's usage of chemicals(fungal pesticides or fertilizers) for which G-MW does not have anycontrol. The State Protection Policy (Waters of Victoria of SEPPWoV; EPA, 2003) exempted artificial channels and drains fromprotection of beneficial use (including protection of aquatic ecosys-tems) and majority of the sites were located within artificial irrigationchannels.
Acknowledgments
The work described in the paper was supported by a grant from theUniversity Grants Committee of the Hong Kong Special AdministrativeRegion, China (AoE/P-04/04). Gavin Rose and Graeme Allinson(Department of Primary Industries, Victoria, Australia) took part inour initial AM program in Australia (pilot trial, calibration and concen-tration factor experiments with AM) and alsomade valuable commentson the draft manuscript. We are also grateful to two anonymousreviewers for their helpful comments and suggestions. G-MW fieldstaffs at Buffalo, Eildon, Cobram, Shepparton, Tatura, Rochester, Boort,and Kerang assisted the first author (GolamKibria) during field deploy-ment and retrieval process (2009–2011).
References
Australian and New Zealand Environment and Conservation Council (ANZECC),Agriculture and Resource Management Council of Australia and New Zealand(ARMCANZ). Aust N Z Guidel FreshMarWater Qual 2000;1. The Guidelines (Chapters1–7).
Chapman PM. Is bioaccumulation useful for predicting impacts? Mar Pollut Bull1997;34(95):282–3.
Degger N, Wepener V, Richardson BJ, Wu RS. Application of artificial mussels (AMs)under South African marine conditions: a validation study. Mar Pollut Bull2011;63(5–12):108–18.
EPA. State environment protection policy (waters of Victoria): policy impact assessment.Southbank, EPA, Victoria: EPA Publication no 905; 2003 [91 pp.].
Gonzalez-Rey M, Lau TC, Gomes T, Maria VL, Bebianno MJ, Wu R. Comparison of metalaccumulation between 'ArtificialMussel' andnaturalmussels (Mytilus galloprovincialis)in marine environments. Mar Pollut Bull 2011;63(5–12):149–53.
Kibria G, Rose G, Lau TC, Lung YK, ChanAKY,WuR.Monitoring heavymetals in Goulburn–Murray waterways using passive sampling with artificial mussels (AM) — pilotstudy (trial of AM technology). Report prepared under a research collaborationagreement between Goulburn Murray Rural Water Corporation, Tatura, Australia.Werribee, Victoria, Australia: the City University of Hong Kong, the University ofHong Kong, and the Department of Primary Industries; 2010a [29 pp. G-MWDM#2806033].
Kibria G, Allinson G, Allinson M, Lau TC, Wu R. Calibration and Concentration FactorsExperiments with Artificial Mussels at DPI Queenscliff Centre, Melbourne, Australia.Report prepared under a research collaboration agreement between GoulburnMurray Rural Water Corporation, Tatura, the Department of Primary Industries,Werribee, Victoria Australia. the City University of Hong Kong, and the University ofHong Kong, and, Australia; 2010b [30 pp.].
Kimbrough KL, JohnsonWE, Lauenstein GG, Christensen JD, Apeti DA. An assessment oftwo decades of contaminant monitoring in the Nation's Coastal Zone. Silver Spring,MD. NOAA Technical Memorandum NOS NCCOS, 74; 2008 [105 pp.].

Fig. 7. Yearly average of copper concentrations in artificial mussels (μg/g dry weight; mean (±S.EM)) for each monitoring site during 2009–2010 (dry year) and 2010–2011 (wetyear). Note Ref=Reference site.
45G. Kibria et al. / Environment International 50 (2012) 38–46
Leung KM, Furness RW, Svavarsson J, Lau TC, Wu RS. Field validation, in Scotland andIceland, of the artificial mussel for monitoring trace metals in temperate seas.Mar Pollut Bull 2008;57(6–12):790–800.
Luoma SN, Rainbow P. Metal contamination in aquatic environments: science andlateral management. Cambridge University Press; 2008 [588 pp. ISBN0521860571].
Marcotullio PJ. Urban water-related environmental transitions in Southeast Asia.Sustainability Sci 2007;2:27–54.
Rose G, Kibria G. Pesticide and heavy metal residues in Goulburn Murray IrrigationWater 2004–2006. Australas J Ecotoxicol 2007;13:65–79.
WHO. Guidelines for drinking water quality. Vol. 2 Health criteria and other supportinginformation, (pp. 940–949) and Addendum to Vol. 2. 1998 (pp. 281–283). 2ndedition. Geneva: World Health Organisation; 1996. [http://www.who.int/water_sanitation_health/GDWQ/summary_tables/sumtab.htm. http://www.fao.org/DOCREP/005/Y4263E/y4263e0g.htm].
Wu RSS, Lau TC, Fung WKM, Ko PH, Leung KMY. An artificial mussel for monitoringheavy metals in a marine environments. Environ Pollut 2007;145:104–10.

Fig. 8. Yearly average of zinc concentrations in artificial mussels (μg/g dry weight; mean (±S.EM)) for each monitoring site during 2009–2010 (dry year) and 2010–2011 (wetyear). Note Ref=Reference site.
46 G. Kibria et al. / Environment International 50 (2012) 38–46