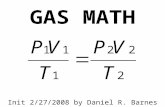Init: 4/20/2009 by Daniel Raymond Barnes
description
Transcript of Init: 4/20/2009 by Daniel Raymond Barnes

Init: 4/20/2009 by Daniel Raymond Barnes

. . . Explain how reversible reactions result in equilibrium.

Please open your textbooks to page 549.
2SO2(g) + O2(g) ↔ 2SO3(g)
How would you translate this into English?
“Sulfur dioxide gas reacts reversibly with oxygen gas to form sulfur trioxide gas.”
You could just as truthfully say, “Sulfur trioxide decomposes reversibly into sulfur dioxide and oxygen gas.”

Please open your textbooks to page 549.
2SO2(g) + O2(g) ↔ 2SO3(g)
How would you translate this into English?
“Sulfur dioxide gas reacts reversibly with oxygen gas to form sulfur trioxide gas.”
You could just as truthfully say, “Sulfur trioxide decomposes reversibly into sulfur dioxide and oxygen gas.”

2SO2(g) + O2(g) ↔ 2SO3(g)
Let’s say you have an airtight, rigid cannister of pure sulfur trioxide gas.
According to the above equation, sulfur trioxide turns into . . . sulfur dioxide gas and oxygen gas.

2SO2(g) + O2(g) ↔ 2SO3(g)
Let’s say you have an airtight, rigid cannister of pure sulfur trioxide gas.
According to the above equation, sulfur trioxide turns into . . . sulfur dioxide gas and oxygen gas.
The amount of SO3 starts out high . . . . . . But then immediately begins to fall
because of SO3 turning into SO2 & O2.
Time
SO
3 C
once
ntra
tion

2SO2(g) + O2(g) ↔ 2SO3(g)
At first, the rate of SO3 turning into
SO2 & O2 is very high because, at first,
the concentration of SO3 is very high.
Time
SO
3 C
once
ntra
tion
Notice the slope of the line. It’s a pretty steep, downward slope.
As the reaction proceeds, there is less and
less SO3 left, so the reaction rate slows
down. Notice the slope of the line.
Not quite as steep, is it?

2SO2(g) + O2(g) ↔ 2SO3(g)
As more and more SO3 turns into
SO2 & O2, the rate of SO3 disappearing
slows down . . .
Time
SO
3 C
once
ntra
tion
. . . and eventually reaches zero.
When the amount of SO3 stops changing,
the graph becomes horizontal.

2SO2(g) + O2(g) ↔ 2SO3(g)
The reason the SO3 graph goes horizontal
is because at some point, there is so
much SO2 & O2 and so little SO3 that the
rate at which SO2 & O2 turn back into SO3
is just as fast as the rate at which SO3
turns into SO2 & O2.
Time
SO
3 C
once
ntra
tion

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
SO
3 C
once
ntra
tion
When opposite processes occur at the same rate, their effects cancel each other out, and the amounts of things remain constant.
When opposite processes occur at the same rate in a system, the system is said to be in a state of “equlibrium”.

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
SO
3 C
once
ntra
tion
Let’s re-run the animation, but this time also graph the amount of sulfur dioxide gas and oxygen gas.

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
Con
cent
ratio
n
At first, there are 10 moles of SO3 . . .Zero moles of SO2, and . . .
Zero moles of O2.
During the first hour, four SO3’s turn
into . . . . . . four SO2’s and two O2’s

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
Con
cent
ratio
n
At first, there are 10 moles of SO3 . . .Zero moles of SO2, and . . .
Zero moles of O2.
During the first hour, four SO3’s turn
into . . . . . . four SO2’s and two O2’s
During the next two hours, two SO3’s
turn into . . . . . . two SO2’s and one O2

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
Con
cent
ratio
n
Another two moles of SO3 turn into two
moles of SO2 one mole of O2, but it takes
a much longer time.

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
Con
cent
ratio
n
Another two moles of SO3 graph turn into
two moles of SO2 one mole of O2, but it
takes a much longer time.At that point, the system has reached equilibrium and the amounts of the chemicals stop changing.

2SO2(g) + O2(g) ↔ 2SO3(g)
Time
Con
cent
ratio
n
A WORD OF CAUTION:
At equilibrium, the forward reaction rate equals the reverse reaction rate.
BUT
The amounts of the chemicals do NOT equal each other.
They’ve just stopped changing.

What does this mean?

is the opposite of

Statues are static.
They don’t do anything.
They don’t move. They don’t talk.

Living things are dynamic.

Systems of living things are dynamic, too.
Let’s look at an example of a system of living things in
“dynamic equilibrium”

Imagine a town where there are 100 weddings every year . . .
. . . and 100 divorces every year.
If the number of weddings equals the number of divorces, then the two processes cancel each other out and the number of married couples remains constant.
This town is in a state of “dynamic equilibrium” with regard to marriage.
Stuff is happening.The system is not static.

Consider the reversible decomposition of carbonic acid into carbon dioxide and water.
This happens in your blood, and also in soda, beer, champagne, and other “carbonated” beverages.
carbonicacid
carbondioxide
water

There’s stuff going on here, isn’t there? It’s not static. It’s “dynamic”.
These opposite processes cancel each other out, so the amounts of carbonic acid, carbon dioxide, and water remain constant.
Carbonic acid is forming in one place, and decomposing in another.

[virtual school project has a good equlibrium videoIncludes hole filling/hole digging example, etc.]

If the force of gravity is equal and opposite to the force created by a vehicle’s engine, the vehicle will be in a state of equlibrium and will not accelerate upward or downward. Vertical velocity will be constant, maybe even zero, which would cause the vehicle to hover in place, motionless.


. . . Predict the outcome of disturbing a system that is in equilibrium

LeChatelier’s Principle is a chemical shock absorber that helps make life possible.
It helps prevent extremes by opposing change.
Henry Louis Le Châtelier (October 8th 1850 -
September 17th 1936)
LeChatelier’s principle, according to Wikipedia [snicker]:
“If a chemical system at equilibrium experiences a change in concentration, temperature or total pressure, the equilibrium will shift in order to minimize that change.”
http://www.howjsay.com/index.php?word=le+Chatelier

If we say that disturbing an equlibrium “shifts the equilibrium to the left”, this means that the system responds to the disturbance by increasing the amount of reactants and decreasing the amount of products.
If we say that disturbing an equlibrium “shifts the equilibrium to the right”, this means that the system responds to the disturbance by increasing the amount of products and decreasing the amount of reactants.
A + B C + D
A + B C + D

http://phet.colorado.edu/en/simulations/category/chemistry/general
Download & run the “reactions & rates” simulation.

Mr. Barnes’LeChatelier’s Principle Worksheet
¿Porque?
Porque es bonito



I hate this question. I’m deactivating it. It’s a CST reject.




NO2(g) N2O4(g) + heat

Nitrogen dioxide-rich smog in Sao Paolo, Brazil






How many gas molecules on each side?
No gas on the left Two gas molecules on the right
System tries to raise the pressure back up again by . . . . . . increasing the number of gas molecules by . . . . . . shifting the equilibrium to the right.


http://www.youtube.com/watch?v=c4BmmcuXMu8
Here’s a link for a YouTube video about the Haber process. It’s one of the most important industrial processes in the world.It’s a great example of how understanding how pressure, temperature, concentration, and catalysts affect reaction rates can help people make useful stuff.This process, by some estimates, creates one third of the food eaten by the human race. That means it, alone, increases human population on the planet 50%, if I’ve interpreted it right. Without it, one out of every three people couldn’t exist.It may smell awful, but, Indirectly, ammonia feeds a LOT of people.
Fritz Haber1868-1934
3H2 + N2 2NH3

All of my PowerPoint presentations are eternal works-in-progress, but this one is
espcially incomplete. Sorry.

Definition of equilibrium opposite processes occur at equal rates effects cancel amounts problably NOT equal; rates are equal dynamic, not static; effects cancel, but plenty of activity balance, stability; amounts of chemicals remain constantNon-chemical examples marriage/divorce income/expenses mortality/natality gravity/lift immigration/emmigration
LeChatelier’s Principle: definitionExample problems Concentration changes Temperature changes Pressure changes Non-effect (!) of catalysts – same eq pos, just reached fasterApplications in industry

Fe3+ + SCN- FeSCN2+ (Light Yellow) (Deep Red)
NEAT TRICK: To get the double headed arrow, , type a capital “D”, and then change its font to Wingdings3.
Potassium thiocyanate, KSCN, also dissolves easily in water.KSCN(cr) K+(aq) + SCN-(aq) (217 g / 100 mL @ 20oC)
Iron (III) chloride, FeCl3, dissolves easily in water.FeCl3(cr) Fe3+(aq) + 3Cl-(aq) (92 g / 100 mL @ 20oC)
If you dissolve both FeCl3 and KSCN in water, the Fe3+ from the FeCl3 and the SCN- from the KSCN get together to form FeSCN2+ ions.

SCN-
OH H
OH H
OH H
OH H
OH H
OH H
OH H
OH H
Fe3+
FeSCN2+
When clear/yellow (?) Fe3+ and clear SCN- ions get together they form FeSCN2+, which is red

Fe3+ + SCN- FeSCN2+ (Light Yellow) (Deep Red)
SCN-Fe3+ FeSCN2++
Fe3+ and SCN- can get together to form FeSCN2+,
but this reaction is reversible,
so FeSCN2+ can fall apart to re-form Fe3+ and SCN- again.

SCN-Fe3+ FeSCN2+

SCN-Fe3+ FeSCN2+

SCN-Fe3+ FeSCN2+
I could do that all day. Couldn’t you?
Okay, Humans get bored, but molecules don’t.

SCN-Fe3+
FeSCN2+SCN-
Fe3+
FeSCN2+Fe3+
SCN-
Fe3+
SCN-
Imagine a beaker with Fe3+, SCN-, and FeSCN2+ ions wandering around in it. Imagine that it’s been sitting around long enough to reach equilibrium.
Fe3+ Fe3+

SCN-Fe3+
SCN-
Fe3+
Fe3+
SCN-
Fe3+
SCN-
If it’s at equilibrium, then for every FeSCN2+ ion that forms, there is an FeSCN2+ ion that breaks up.
Fe3+ Fe3+FeSCN2+
Fe3+SCN-FeSCN2+
FeSCN2+
Fe3+SCN-FeSCN2+
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2

FeSCN2+Fe3+
Fe3+
SCN-
Fe3+
SCN-
Even though there’s plenty of activity going on, the amount of each kind of ion remains constant.
Fe3+
FeSCN2+
SCN-Fe3+
Fe3+
SCN-
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2

FeSCN2+Fe3+
Fe3+
SCN-
Fe3+
SCN-
Are the amounts of the ions equal to each other? No, but they did remain constant over time. The amounts did not change.
Fe3+
FeSCN2+
SCN-Fe3+
Fe3+
SCN-
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2


FeSCN2+Fe3+
Fe3+
SCN-
Fe3+
SCN-
Let’s see what happens if we add some extra Fe3+ ions. We could do this by adding FeNO3 to the solution.
Fe3+
FeSCN2+
SCN-Fe3+
Fe3+
SCN-
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2 NO3-
NO3- NO3
-
NO3-
NO3-
Fe3+
Fe3+
Fe3+
Fe3+
Fe3+

FeSCN2+Fe3+
Fe3+
SCN-
Fe3+
SCN-
At first, this only changes the amount of Fe3+ ions in the solution.
Fe3+
FeSCN2+
SCN-Fe3+
Fe3+
SCN-
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2 NO3-
NO3- NO3
-
NO3-
NO3-
Fe3+
Fe3+
Fe3+
Fe3+
Fe3+
11

FeSCN2+Fe3+
Fe3+
SCN-
Fe3+
SCN-
At first, this only changes the amount of Fe3+ ions in the solution.
Fe3+
FeSCN2+
SCN-Fe3+
Fe3+
SCN-
# Fe3+
ions =
# SCN-
ions =
# FeSCN2+
ions =
6
4
2 NO3-
NO3- NO3
-
NO3-
NO3-
Fe3+
Fe3+
Fe3+
Fe3+
Fe3+
11

Silver thiocyanate, AgSCN, is insoluble in water, so adding AgNO3 to a solution containing the above equilibrium system ions will change the color. Which way and why?
Fe3+ + SCN- FeSCN2+ (Light Yellow) (Deep Red)
Ag+(aq) + SCN-(aq) AgSCN(cr) (white solid “precipitate”)
Silver nitrate, AgNO3, like most nitrates, dissolves easily in water. AgNO3(cr) Ag+(aq) + NO3
-(aq) (216 g / 100 mL @ 20oC)
The nitrate ion, NO3-(aq) is famous for almost always remaining
aqueous in water. In water, it almost never combines with anything else, so it doesn’t affect the equilibrium. However, the Ag+ ion DOES often combine with other ions to form other species.