In-vitro antimicrobial activity of lichen Ramalina ...
Transcript of In-vitro antimicrobial activity of lichen Ramalina ...

http://jsrr.net 99 ISSN: 2249-7846 (Online)
Science Research Reporter, 6(2):99-108, (Oct - 2016)
© RUT Printer and Publisher
Online, Open Access Available at http://jsrr.net
ISSN: 2249-2321 (Print); ISSN: 2249-7846 (Online)
Research Article
In-vitro antimicrobial activity of lichen Ramalina conduplicans Vain.
collected from Eastern Ghats, India
Anjali Devi B.1, Satish Mohabe
1, Sanjeeva Nayaka
2 and A. Madhusudhana Reddy
1*
1 Lichenology Laboratory, Department of Botany, Yogi Vemana University, Vemanapuram, Kadapa -
516003, Andhra Pradesh, India 2Lichenology Laboratory, CSIR-National Botanical Research Institute, Rana Pratap Marg, Lucknow - 226
001, Uttar Pradesh, India
Article Info
Abstract
Received: 02-08-2016
Revised: 26-09-2016
Accepted: 29-09-2016
The research work investigates the in-vitro antimicrobial efficacy of lichen
Ramalina conduplicans Vain. (Ramalinaceae). The 2-Propanol, methanol,
acetone and petroleum ether extract of the species were tested each against
eleven bacterial and fungal pathogens by using Kirby-Bauer disc diffusion
method with 15 μL extract per disc. It is observed that the extracts are effective
against bacteria in than to the fungi. Among various extracts methanol was
much effective against most of the bacteria and diameter of inhibition zone
ranged from 12.66±0.66 to 16.66±1.6 mm. In case of antifungal activity the
extract formed zone of inhibition against only six pathogens. The R.
conduplicans can be a potential source of bioactive agents against disease
causing microbes of humans and plants.
Keywords:
Antifungal, antibacterial,
Kirby-Bauer disc diffusion
assay, bioprospecting,
lichenized fungi.
INTRODUCTION
The lichens are symbiotic organisms
composed of a fungal partner (mycobiont) and an
algal partner (photobiont), which may be either a
green algae or cyanobacteria (Nash, 1996). The alga
provides nutrients by photosynthesis to the
mycobiont, and the fungus helps in absorption of
water and nutrients from surroundings to the
photobiont. Lichens usually grow on the surface of
rocks, on non-fertile ground, as well as on the barks
of trees and leaves as epiphytic lichens. A variety of
secondary compounds synthesized from lichens are
unique and called as “lichen compounds”. These
secondary metabolites differ in the chemical
structure as well as in their biological activities. The
lichen metabolites are found be effective anti-biotic,
anti-viral, anti-microbial, analgesic, anti-
inflammatory, antipyretic, antioxidant, anticancer,
and antiproliferative antifungal agents (Wei et al.,
2008). Since the times of the Chinese and Egyptians
civilizations lichens are used as food materials and
also in traditional medicine (Palo, 1993; Bernasconi
et al., 2000; Turk et al., 2006; Vinayaka et al., 2009;
Behera et al., 2009; Temina et al., 2010; Nayaka et
al., 2010; Kowalski et al., 2011; Verma et al.,
2012).
The genus Ramalina Ach. is a fruticose
lichens belonging to the family Ramalinaceae, order
Lecanorales, class Ascomycetes. The genus was first
described by Acharius (1810) and comprises
approximately 200 species known from the world in
which 22 species reported from India (Singh &
Sinha, 2010). R. conduplicans occur in diverse
vegetation types and on different substrates such as
rocks, wood, bark, peaty soil etc. The thallus is
attached to the substratum by the basal holdfast. The
thallus may be long, erect to decumbent greenish
grey to yellowish brown, branched, branches
uniformly wide upper side smooth with scarcely
pseudocyphellate, lower side of the thallus is rugose,
with raised, round to oblong, pseudocyphellate
prominent, sorediate, chondroid tissue uneven

http://jsrr.net 100 ISSN: 2249-7846 (Online)
Anjali Devi et al.,
in the thickness, with distinctly cracked hyphal
bundles, medulla solid, apothecia lecanorine,
ascospores straight or curved and thallus contains
usnic acids, salazinic acid and sekikaic acid in three
chemical strains (Awasthi, 2007).
Ramalina conduplicans is an edible lichen
species in Central and South Eastern Asian
countries. It is used to prepare a traditional cold dish
served at marriage banquets in Yunnan province of
south western China, and in a stir-fried pork dish
(Wang et al. 2001). R. conduplicans is used in the
preparation of traditional food by the Limbu and Rai
communities of East Nepal (Bhattarai et al., 1999).
Vinayaka et al. (2009) reported the proximate
composition of R. conduplicans collected at Bhadra
Wildlife Sanctuary, Karnataka which has high
protein (5.95%) and carbohydrates (79.08%). Upreti
et al., (2005) reported that the tribals of Himachal
Pradesh use R. conduplicans as food materials and
are being sold in the markets for commercial
purposes. The fruticose lichen R. conduplicans
exhibits antioxidant, insecticidal antihelmentic (Luo
et al., 2010; Vinayaka et al., 2009; Kumar et al.,
2010a). India exhibits a rich diversity of lichens,
found in all phytogeographical regions of the
country. A total of 20,000 species of lichens are
known in the world, more than 2,450 species
(Awasthi, 2007) of lichens are reported from Indian
Subcontinent and India alone has 2,300 species
(Singh and Sinha, 2010) and more than 500 species
were reported from South India but a few data on
the biological screening of lichens are available.
Recently, Kambar et al., (2014) studied the
antimicrobial activity of R. conduplicans against six
bacterial and five fungal pathogens with ethyl
acetate, petroleum ether and ethanol extracts in
which petroleum ether extracts showed effective
results against the bacterial pathogens while all the
three solvents showed effective results against
fungal pathogen. Therefore in search of a new
molecules of natural origin for the production of
biosafety products lichens can play a dynamic role
and the present study on In-vitro antimicrobial
efficacy R. conduplicans against the bacterial and
fungi pathogens was initiated with this aim.
MATERIAL AND METHODS
Collection and identification
The thalli of R. conduplicans (Fig. 1A) were
collected from Mangifera indica orchards near
Talakona Jungle Thrills, Chittoor District of Andhra
Pradesh at an altitude of N 14°28.698’ E
078°42.749’ alt. 539 m. The specimen identification
was carried out by following the standard
procedures given by Nayaka (2014). The literature
of Awasthi, (2007) and Mohabe, (2016) was referred
for taxonomic characters and Orange et al., (2001)
was followed for chemical analysis. The voucher
specimens (ADB & SM 3734, 3760, 3772) were
deposited at the Lichen Herbarium, Department of
Botany, Yogi Vemana University (YVUH), Kadapa,
Andhra Pradesh, India.
Extraction of bioactive compounds The freshly collected lichen material was
cleaned, shade dried and powdered mechanically.
About 10g of the powdered samples was wrapped in
Whatman No.1 filter paper and placed inside the
extractor tube of Soxhlet apparatus. The extraction
was carried out in 250 ml of solvents of different
polarity such as 2-Propanol, petroleum ether,
acetone, methanol and water (Soxhlet, 1879;
Harwood and Moody, 1989) for 48 h at room
temperature at the specific boiling temperature of
the solvents (2-propanol: 82.3ºC, Petroleum ether:
60ºC, acetone: 56ºC and methanol: 65ºC). The
lichen extracts obtained were filtered and
concentrated to dryness in vacuum under reduced
pressure at 40°C using a Heidolph rota-vapour. Few
drops of the extract were used to identify the major
secondary compounds for Thin Layer
Chromatography (TLC). Extracts were preserved in
a Deep Freezer at -80°C until they are used for
further assays.
Microorganisms and media
The microorganisms used in the present
study were procured form National Collection of
Industrial Microorganisms (NCIM), Pune. Eleven
bacterial pathogens viz. Bacillus cereus, B. subtilis,
Corynebacterium rubrum, Staphylococcus aureus
Streptococcus pyogenes (Gram +ve) Enterobacter
cloacae, Escherichia coli, Klebsiella pneumonia,
Pseudomonas aeruginosa, Salmonella abony, S.
typhimurium (Gram –ve) were selected and
maintained on Muller Hington Agar (MHA) media
at 37°C. Among all the bacterial pathogens a single
pathogen E. cloacae is plant pathogen rest of all
human pathogens. Similarly, eleven fungal
pathogens viz. Aspergillus niger, Colletotrichum
falcatum, Fusarium solani, Rhizoctonia bataticola,
Trichoderma lignorum, Yeast sp. (plant pathogens)
Fusarium moniliformae, P. notatum Mucor sp.
(human pathogens) Aspergillus flavus and Fusarium
oxysporum (plant and human pathogens) were
selected and maintained on Potato Dextrose Agar
(PDA) at 27°C.

http://jsrr.net 101 ISSN: 2249-7846 (Online)
Science Research Reporter, 6(2):99-108, (Oct - 2016)
Screening of antimicrobial activity The antimicrobial efficacy of crude lichen
extracts was studied by Kirby-Bauer disc diffusion
method using selected bacterial and fungal micro-
organisms (Bauer et al., 1966). Bacterial cultures
(1.5 × 108
CFU/ml) were seeded onto the Muller
Hinton Agar plates with the help of L – spreader
while the fungal mat of 2–3 days old cultures were
seeded onto PDA plates. On the seeded plates the
sterile filter paper discs of 5 mm soaked with 15 μL
of lichen extract concentration were placed. The
inoculated plates with bacteria were incubated at
37°C for 24 hrs while fungal plates were incubated
at 27°C for 2–3 days. Amphicillin (30μg/ml) and
Nystanin (10μg/ml) were taken as a standard
positive control for bacteria and fungus respectively,
while solvent alone used as negative control. All the
experiments were carried out in triplicates. The
diameters of the zone of inhibitions were measured
in millimeter (including size of paper disc) and the
mean and standard error was calculated.
RESULTS
Among the different solvents 2-propanol
extract showed maximum zone of inhibitions of
7.33±0.33 mm against B. cereus, E. cloacae, S.
abony and S. typhimurium while it showed
minimum inhibition zone of 2.33±2.33 mm was
against C. rubrum. However,2-propanol did not
have effect against the E. coli. Petroleum ether
extract exhibited maximum and minimum zone of
12.66±0.33 mm and 7.33±3.17mm against P.
aeruginosa and C. rubrum respectively. Methanolic
extract showed highest and lowest inhibition zones
of 16.66±1.6 mm and 12.33±1.45 mm against S.
typhimurium and B. subtilis respectively. Acetone
extract presented a highest inhibition zone of
11.66±0.33 mm against S. typhimurium and lowest
inhibition zone of 7.66±0.66 mm against S. abony.
The standard Amphicillin showed an inhibition zone
of 18.0±0.57 mm and E. cloacae and lowest
inhibition zone of 13.33±0.33 mm was observed
against P. aeruginosa (Plate-1). In case of
antifungal activity the extracts showed zone of
inhibition against only six fungal pathogens only.
The 2-propanol extract showed maximum and
minimum inhibition zones of 10.0±0.57 and
8.66±4.66 mm against C. falcatum and and T.
lignorum respectively. Petroleum ether extract
exhibited a highest zone of inhibition of 19.0±3.0
mm against F. moniliformae and lowest inhibition
zone of 9.33±9.33 mm against T. lignorum. The
methanolic extracts formed maximum and minimum
inhibition zone of17.33±4.09 mm and.0±0.57 mm
against F. moniliformae and C. falcatum
respectively.
DISCUSSION The present investigation evaluated antimicrobial
efficacy of R. conduplicans against diverse human
and plant fungal pathogens in different solvents viz.
2-propanol, petroleum ether, acetone and methanol
extract. R. conduplicans consists of a yellowish
cortical pigment and occurs in two enantiomeric
forms differing in the direction of the methyl group
located in the stereo genic centre at the 9b position.
It is noticeable that inhibitory effects of the species
against the microbes exhibited in very low
concentration (15 μL) especially against bacteria.
The lichens and their extracts containing usnic acid
have been used for several purposes such as
medicinal, perfumery, cosmetic etc. As a pure
substance, usnic acid has been formulated in the
preparations of toothpaste, mouthwash, creams,
sunscreen lotions and deodorants products. Kilpiö
(1952) studied that the Topical formulations of an
usnic acid salt (“Usno”) have been successfully used
clinically against bacterial infections. Cocchietto et
al., (2002) reported that the usnic acid has effective
result against Tinea pedis and furthermore reports on
usnic acid showed that the acid has been used
topically as an adjuvant against papilloma virus
infection. Usnic acid is being used in antifeedants by
Durazo et al., (2004). It exhibits several important
biological and pharmacological properties (Yilmaz
et al., 2004; Ribeiro-Costa et al., 2004; Behera et
al., 2009). Sati et al., (2011) used Parmotrema
nilgherrense extracts of Chloroform, Ethanol and
Methanol and recorded maximum zone of inhibition
against Bacillus subtilis and Escherichia coli by
using 200μL of the extract while in the present
study, R. conduplicans extracts inhibited the same
bacterial pathogens with a very less quantity (15 μL)
of the bioactive compound. Pavithra et al., (2013)
enumerated that the usnic acid compound from U.
pictoides exhibited a noticeable zone of inhibition
against the Gram +ve bacteria i.e. S. aureus than the
Gram ˗ve bacteria P. aeruginosa. Whereas in the
present study with R. conduplicans the bioactive
compound exhibited highest zone of inhibition
against the P. aeruginosa with the petroleum ether
extract and S. aureus showed less zone of inhibition
with the petroleum ether extract. The studies of
Kambar et al., (2014) assessed antifungal effect of
solvent extracts of R. conduplicans against five

http://jsrr.net 102 ISSN: 2249-7846 (Online)
Anjali Devi et al.,
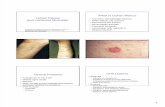
Plate–1.Antibacterial efficacy of R. conduplicans A. Habit of the thallus, B. Bacillus cereus, C. B. subtilis,
D. Corynebacterium rubrum E.Enterobacter cloacae, F. Escherichia coli, G. Klebsiella pneumonia, H.
Pseudomonas. aeruginosa, I. Salmonella abony, J. S. typhimurium, K. Staphylococcus aureus,L.
Streptococcus pyogenes; Abbreviations: Am=Amphicillin (control), Extract used: 2P=2-Propanol,
Ac=Acetone, M=Methanol, PE=Petroleum ether, W=Water; Scale bars: B–L = 10 mm.

http://jsrr.net 103 ISSN: 2249-7846 (Online)
Science Research Reporter, 6(2):99-108, (Oct - 2016)
Table-1: Antibacterial efficacy of R. conduplicans Vain.
S.No. Bacterial
pathogens
Amphicillin Diameter of zone of inhibition
2pol Methanol Acetone PE
1 B. cereus 13.66±0.33 7.33±0.33 12.66±0.66 10.0±0.0 11.0±0.57
2 B. subtilis 14.66±0.33 6.66±0.33 12.33±1.45 10.0±0.33 10.0±0.66
3 C. rubrum 13.33±2.02 2.33±2.33 15.66±1.45 11.0±1.0 7.33±3.17
4 E. cloacae 18.0±0.57 7.33±0.33 13.33±4.37 8.33±0.33 8.66±0.33
5 E. coli 13.66±0.88 0.0±0.0 13.0±1.15 9.66±0.66 11.33±1.45
6 K. pneumonia 13.66±1.20 7.0±0.0 13.33±0.6 9.33±0.33 12.33±0.66
7 P. aeruginosa 13.33±0.33 4.66±2.33 16.0±1.15 9.66±0.66 12.66±0.33
8 S. abony 14.33±1.33 7.33±0.33 12.66±4.66 7.66±0.66 8.0±0.57
9 S. typhimurium 14.66±0.66 7.33±0.33 16.66±1.6 11.66±0.33 11.66±0.33
10 Sta. aureus 13.33±1.76 4.66±2.33 14.66±1.45 11.0±0.57 9.33±0.33
11 Str. pyogenes 15.33±1.33 2.66±2.66 15.66±2.02 9.33±0.33 11.33±1.76
(*values are in mean ± standard error)
collected from different sources namely
anthracnose of chilli, foot rot of finger millet and
mouldy grains of sorghum. Whereas, the present
work with different solvents of R. conduplicans
resulted in the activity against six fungal pathogens
in which most of them are plant and human disease
causing fungal microbes. Tiwari et al., (2014)
reported that the acetone, methanol and chloroform
extracts of Flavoparmelia caperata exhibited
effective results against Aspergillus flavus, A. niger,
Fusarium solani and Fusarium oxysporum. The
bioactive compounds of R. conduplicans have not at
all having any activity against the same fungal
pathogens.
Fig.1. Graph showing zone of inhibitions of R. conduplicans extract in different solvents against bacterial
pathogens; 2Pol = 2-Propanol, PE= Petroleum ether

http://jsrr.net 104 ISSN: 2249-7846 (Online)
Anjali Devi et al.,
Acetone extract revealed highest and lowest inhibition zones of 13.66±1.85 mm and 8.66±0.88 mm against
F. moniliformae and R. bataticola respectively. The standard Nystanin showed effective results against P.
chrysogenum with an inhibition zone of 31.0±2.08 mm and lowest activity against T. lignorum with an
inhibition zone of 7.33±0.33 mm (Plate-2). Bacterial and fungal pathogens taken for the study has no
activity with the water extracts. The mean and standard values of R. conduplicans were shown in (Table-1
& 2)
Plate-2. Antifungal efficacy of R. conduplicans A. Fusarium oxysporum, B. F. solani, C. Mucor sp., D.
Penicillium chrysogenum, E P. notatum, F. Rhizoctonia bataticola, G. Trichoderma lignorum, H.
Yeast sp.; Abbreviations: NS=Nystanin (control), Extract used: 2P=2-Propanol, Ac=Acetone,
M=Methanol, Pe=Petroleum ether, W=Water; Scale bars: A–I = 10 mm.

http://jsrr.net 105 ISSN: 2249-7846 (Online)
Science Research Reporter, 6(2):99-108, (Oct - 2016)
Table-2: Antifungal efficacy of R. conduplicans Vain.
S.No Fungal pathogens Diameter of zone of inhibition
Nystanin 2pol Methanol Acetone PE
1 A. flavus 25.66±0.66 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0
2 A. niger 26.33±0.88 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0
3 C. falcatum 24.33±1.33 10.0±0.57 8.0±0.57 11.66±1.76 0.0 ±0.0
4 F. moniliformae 2.33±2.33 0.0 ±0.0 17.33±4.09 13.66±1.85 19.0±3.05
5 F. solani 21.0±3.51 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0
6 Mucor sp. 14.0±1.52 0.0 ±0.0 0.0 ±0.0 10.0±1.15 0.0 ±0.0
7 P. chrysogenum 31.0±2.08 10.0±0.88 11.0±1.52 9.0±1.15 9.66±1.20
8 P. notatum 29.0±1.05 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0 0.0 ±0.0
9 R. bataticola 17.0±1.15 0.0 ±0.0 10.0±1.73 8.66±0.88 0.0 ±0.0
10 T. lignorum 7.33±0.33 8.66±4.66 6.0±6.0 0.0±0.0 9.33±9.33
11 Yeast sp. 11.33±2.40 0.0 ±0.0 12.33±3.38 9.33±1.85 10.0±2.51
(*values are in mean ± standard error)
Fig. 2 Graph showing zone of inhibitions of R. conduplicans extract in different solvents against fungal
pathogens. 2Pol = 2-Propanol, PE= Petroleum ether

http://jsrr.net 106 ISSN: 2249-7846 (Online)
Anjali Devi et al.,
The usnic acid, a low molecular weight
dibenzofurans derivative formed by the fungal
partner, is one of the important lichen substances
found in plenty of genera especially in some
Alectoria, Cladonia, Evernia, Ramalina, Usnea and
Lecanora. In this connection the major bioactive
compound like usnic and salazinic acid occurred in
R. conduplicans exhibited effective results against
eleven bacterial pathogens and seven fungal
pathogens with 2-propanol, petroleum ether, acetone
and methanolic extracts.
CONCLUSION
Lichens have been documented as potent
antimicrobial agents since ancient times and many
studies conducted all over the world showed the
potential of lichen extracts and purified metabolites
to inhibit a wide range of bacterial and fungal
pathogens (Yilmaz et al., 2004; Turk et al., 2006;
Canarasan et al., 2006, Candan et al., 2006;
Vinayaka et al., 2009; Kekuda et al., 2011; Kambar
et al., 2014; Prabhu & Sudha 2015). Hence the
present study has created an enthusiasm to workout
with different solvents for R. conduplicans against
diverse human and plant disease causing bacterial
and fungal pathogens. The present species used for
microbial assay has screened out as potential lichen
especially from Sheshachalm Biosphere Reserve of
Andhra Pradesh in Eastern Ghats of India. The study
also includes three different solvents viz. 2-
propanol, methanol and acetone whereas the use of
these solvents with R. conduplicans were not yet
reported from the study area (Anjali et al., 2014,
2015a, b). Now it became clear that the bioactive
compound present in R. conduplicans attributed the
best results against the bacterial pathogens instead of
fungal pathogens with the standard controls. The
study encourages to explore the novel antimicrobial
bio-molecules within lichen biodiversity. The
availability of R. conduplicans in Eastern Ghats of
India may also yield potential antimicrobial
compound in some other organic solvent systems. It
is very useful for the pharmacological industries for
further development of novel drug discovery.
ACKNOWLEDGMENTS
The authors are thankful to the Department
of Science and Technology (INSPIRE) and Director
and Dr. D.K. Upreti, Chief Scientist, CSIR-National
Botanical Research Institute, Lucknow for their kind
permission to utilize the infrastructure facilities of
Lichenology Laboratory for the identification of
lichens. The authors are also thankful to the Forest
Official of Andhra Pradesh for their help.
REFERENCES
Acharius E, 1810. Lichenographia Universalis.
Göttingae. 122: 598.
Anjali DB, Mohabe S, Reddy MA, Nayaka S,
Ponmurugan P and Ayyappadasan G, 2014. Antimicrobial Activity of Lichen Roccella
montagnei Bél. obtained from Horsley hills, Andhra
Pradesh, India. In: Madhusudhana Rao J. (Eds).
Bioactives from Natural Products, Special Issue
(pp13–20), Proceedings of Andhra Pradesh
Academy of Sciences.
Anjali DB, Mohabe S, Reddy AM and Nayaka S,
2015a. Efficacy of a potential lichen Parmotrema
andinum (Müll. Arg.) Hale against pathogenic
microorganisms. Journal on New Biological
Reports, 2(4): 149–156.
Anjali DB, Mohabe S, Reddy MA and Nayaka S,
2015b. Antimicrobial activities of 2-Propanol crude
extract from lichen Parmotrema tinctorum (Despr.
ex. Nyl.) Hale, collected from Eastern Ghats, India.
Curr. Res. Environ. Appl. Mycol. J. Fungal Biol., 5
(3): 160–168.
Awasthi DD, 2007. A compendium of the
Macrolichens from India, Nepal and Sri Lanka.
Bishen Singh Mahendra Pal Singh, Dehra Dun.
Behera BC, Verma N, Sonone A and Makhija U,
2009. Optimization of culture conditions for lichen
Usnea ghattensis G. Awasthi to increase biomass
and antioxidant metabolite production. Food
Technol. Biotech., 47(1): 7–12.
Bernasconi ES, De Vito IE, Martinez LD and
Raba J, 2000. Heavy metals determination by ICP-
AES coupled with ultrasonic nebulization using the
lichen Usnea densirostra (Tayl.) as biomonitor
pollution in San Luis, Argentina. Ars
Pharmaceutical, 41(3): 249-257.
Bhattarai T, Subba D and Subba R, 1999. Nutritional value of some edible lichens of east
Nepal. Journal of Applied Botany and Food Quality
– Angewandte Botanik, 73:11–18.
Canarasan D, Kahya D, Yurdakulol E and
Atakol O, 2006. Identification and quantitation of
Usnic acid from the lichen Usnea species of
Anatolia and antimicrobial activity. Z. Naturforsch.,
61c: 773–776.
Candan M, Yilmaz M, Tay T, Kivanc M and
Turk H, 2006. Antimicrobial activity of extracts of
the lichen Xanthoparmelia pokornyi and its
Gyrophoric and Stenosporic acid constituents. Z.
Naturforsch., 61c: 319-323.

http://jsrr.net 107 ISSN: 2249-7846 (Online)
Science Research Reporter, 6(2):99-108, (Oct - 2016)
Harwood LM and Moody CJ, 1989. Experimental
organic chemistry: Principles and Practice
(Illustrated Edn.), Blackwell Scientific Publications,
122–125.
Kambar Y, Vivek MN, Manasa M, Kekuda TRP
and Ramesh Kumar KA, 2014. Proximate and
elemental analysis of Ramalina conduplicans Vain.
(Ramalinaceae) and Parmotrema tinctorum (Nyl.)
Hale (Parmeliaceae). J. Chem. Pharm. Res., 6(6):
2668–2674.
Kekuda TRP, Vinayaka KS, Swathi D, Suchitha
Y, Venugopal TM and Mallikarjun N, 2011.
Mineral composition, total phenol content and
antioxidant activity of a macrolichen Everniastrum
cirrhatum (Fr.) Hale (Parmeliaceae). E- J. Chem.,
8(4): 1886-1894.
Kowalski M, Hausner G and Piercey-Normore
MD, 2011. Bioactivity of secondary metabolites and
thallus extracts from lichen fungi. Mycoscience, 52:
413–418.
Kumar PSV, Kekuda PTR, Vinayaka, KS,
Swathi D and Chinmaya A, 2010. Insecticidal
efficacy of Ramalina hossei H. Magn and G.
Awasthi and Ramalina conduplicans Vain-
Macrolichens from Bhadra wildlife sanctuary,
Karnataka. Biomedicine, 30(1): 100–102.
Lawrey JD, 1986. Biological role of lichens
substances. The Bryologist, 89: 111–122.
Luo H, Wei X, Yamamoto Y, Liu Y, Wang L,
Jung JS, Koh YJ and Hur J, 2010. Antioxidant
activities of edible lichen Ramalina conduplicans
and its free radical-scavenging constituents.
Mycoscience, 51(5): 391–395.
Mohabe S., Anjali D. B., Reddy A.M., Nayaka S.
and Chandramati P. S. (2015c). An appraisal of
lichen biota in Chittoor district of Andhra Pradesh,
India. In (Ed.) Biodiversity in India, T. Pulaiah &
Sandhya Rani (8): 247–296.
Nash TH, 1996. Lichen Biology. 1st Edn.
Cambridge University Press, Cambridge.
Nayaka S, Upreti DK and Khare R, 2010. Medicinal lichens of India. In PC Trivedi (Ed.),
Drugs from Plant. Avishkhar Publishers,
Distributors, Jaipur, India.
Orange A, James PW and White FJ, 2001. Microchemical methods for the identification of
lichens. British Lichen Society, U.K.
Palo TR, 1993. Usnic acid, a secondary metabolite
of lichens and its effect on in vitro digestibility in
reindeer. Rangifer, 13(1): 39–43.
Pavithra GM, Vinayaka KS, Rakesh KN, Syed J,
Dileep N, Prashith Kekuda TR, Saba S,
Abhishiktha and S Naik, 2013. Antimicrobial and
antioxidant activities of a macrolichen Usnea
pictoides G. Awasthi (Parmeliaceae). Journal of
Applied Pharmaceutical Science, 3 (08); 154–160.
Prabhu SS and Sudha SS, 2015. Evaluation of the
antibacterial properties of some Lichen species
against human pathogens. Int. J. Adv. Res. Biol. Sci.
2(4): 177–181.
Preeti S Babiah, Upreti DK and John SA, 2014. Fungicidal Efficacy of a Foliose Lichen
Flavoparmelia caperata (L.) Hale against
Phytopathogenic Fungi. Int. J. Curr. Res. Biosci.
Plant Biol., 1(5): 38–44.
Ribeiro-Costa RM, Alves AJ, Santos NP,
Nascimento SC, Goncalves ECP, Silva NH,
Honda NK, and Santos-Magalhaes NS, 2004. In
vitro and in vivo properties of usnic acid
encapsulated into PLGA-microspheres. J.
Microencapsulation, 21(4): 371–384.
Sati SC and Joshi S, 2011. Antibacterial Activity of
the Himalayan Lichen Parmotrema nilgherrense
Extracts. Br. Microbiol. Res. J., 1(2): 26–32.
Shukla P, Upreti DK and Tiwari P, 2014. Assessment of dye yielding potential of Indian
lichens. Ind. J. Pl. Sc., 3(1): 57–63.
Soxhlet F, 1879. Die Gewichtsanalytische
Bestimmung des Milchfettes. Dingler’s
polytechnisches J., 232: 461–465.
Temina M, Levitsky DO and Dembitsky VM,
2010. Chemical constituents of the epiphytic and
lithophilic lichens of the genus Collema. Rec. Nat.
Prod., 4(1): 79–86.
Turk H, Yilmaz M, Tay T, Turk AO and Kivanc
M, 2006. Antimicrobial activity of extracts of
chemical races of the lichen Pseudevernia
furfuracea and their Physodic Acid,
Chloroatranorin, Atranorin, and Olivetoric acid
constituents. Z. Naturforsch., 61c: 499-507.
Upreti DK, Divakar PK and Nayaka S, 2005. Commercial and ethnic use of lichens in India.
Econ. Bot., 59: 269–273.
Verma N, Behera BC and Sharma BO, 2012.
Glucosidase inhibitory and radical scavenging
properties of lichen metabolites Salazinic Acid,
Sekikaic Acid and Usnic Acid. Hacettepe J. Biol. &
Chem., 40(1): 7–21.
Vinayaka KS, Kumar PSV, Kekuda PTR,
Krishnamurthy YL, Mallikarjun N and Swathi
D, 2009. Proximate composition, antioxidant,
anthelmintic and insecticidal activity of a
macrolichen Ramalina conduplicans Vain.
(Ramalinaceae). Europ. J. Appl. Sci., 1(3): 40–46.

http://jsrr.net 108 ISSN: 2249-7846 (Online)
Anjali Devi et al.,
Wei X, Jeon H, Han KS, Koh YJ and Hur J,
2008. Antifungal activity of lichen forming fungi
against Colletotrichum acutatum on hot pepper.
Plant Pathol. J., 24(2): 202–206.
Yilmaz M, Turk AO, Tay T and Kivanc M, 2004.
The antimicrobial activity of extracts of the lichen
Cladonia foliacea and its (-)-Usnic acid, Atranorin,
and Fumar protocetraric acid constituents. Z.
Naturforsch., 59c: 249–254.
How to Cite this Article:
Anjali Devi B, Satish Mohabe, Sanjeeva Nayaka and A. Madhusudhana Reddy, 2016. In-vitro
antimicrobial activity of lichen Ramalina conduplicans Vain. collected from Eastern Ghats, India. Science
Research Reporter, 6(2):99-108.