Icbt08i10p1039
Click here to load reader
-
Upload
anuraghearthack56 -
Category
Health & Medicine
-
view
57 -
download
0
Transcript of Icbt08i10p1039

Indian Journal of Pediatrics, Volume 75—October, 2008 1039
Correspondence and Reprint requests : Vijayalakshmi Bhatia,Department of Endocrinology SGPGIMS, Lucknow-226 014,India. Phone: +91-522-2668700x2380, Fax: +91-522-2668017
[Received August 19, 2008; Accepted August 19, 2008]
Symposium on Steroid Therapy
Corticosteroid Physiology and Principles of Therapy
Priyanka Gupta and Vijayalakshmi Bhatia
Department of Endocrinology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow,India
ABSTRACT
The adrenal cortex secretes glucocorticoids (GC), mineralocorticoids (MC) and androgens. GC maintain homeostasis, MCregulate fluid and electrolyte balance and adrenal androgens contribute to development of secondary sexual characteristics.Pharmacologic GC therapy is frequently indicated in the pediatric age group. Besides having many important side effects,prolonged high dose systemic GC therapy has a suppressive effect on endogenous steroid production. Therefore, GCtherapy should be withdrawn gradually and stopped based on assessment of hypothalamo-pituitary-adrenal (HPA) axisrecovery. Patients with HPA axis suppression require physiological replacement of GC along with enhancement of dosesduring periods of stress. Due to its immunosuppressive effects, issues about safety and efficacy of live virus vaccines inpatients receiving systemic high dose GC therapy must be borne in mind. [Indian J Pediatr 2008; 75 (10) : 1039-1044]E-mail: [email protected]
Key words: Corticosteroid; Hypothalamo-pituitary-adrenal axis; Replacement dose; Stress dose
Corticosteroids (CS) are an important class of naturallyoccurring and synthetic steroid hormones that affectvirtually every aspect of human physiology. They are acommon part of our prescriptions, sometimes inphysiological doses and sometimes for pharmacologicaltherapy. CS therapy affects endogenous CS productionand has a suppressive effect on hypothalamo-pituitary-adrenal (HPA) axis. This chapter deals with principlesof endogenous steroidogenesis and CS therapyincluding actions of CS, agents used in CS therapy,dosing and withdrawal regimes, stress dosing andimmunisation related issues.
The adrenal cortex and HPA axis
The adrenal cortex consists of three zones. The zonaglomerulosa, located immediately beneath the capsule,synthesizes aldosterone, the most potentmineralocorticoid (MC) in humans. The zona fasciculata(middle zone) produces cortisol (hydrocortisone), theprinciple circulating glucocorticoid (GC). Adrenalandrogens are secreted by both zona fasciculata and zonareticularis (innermost zone).
GC secretion is regulated by adrenocorticotrophichormone (ACTH), produced in the anterior pituitaryand released in secretory bursts throughout the dayand night. ACTH production is in turn driven by
corticotrophin releasing hormone (CRH) from thehypothalamus. Pulses of ACTH occur every 30-120minutes. Varying amplitude of ACTH pulses leads tothe normal diurnal rhythm of cortisol production.Plasma cortisol is highest in the early morning, low inthe afternoon and evening, and lowest 1 or 2 hours aftersleep begins. Cortisol has a negative feedback on ACTHand CRH production. Thus when GC production isimpaired as in Addison disease, ACTH is elevated.Similarly, excess GC (either endogenous or exogenous)suppresses ACTH.
In contrast to the ACTH driven GC pathway, MCsynthesis is regulated mainly by the renin-angiotensinsystem and by potassium levels in blood, with ACTHhaving only a short term effect. This is the reason whypatients with primary adrenal insufficiency requireboth GC and MC for treatment, whereashypopituitarism patients with ACTH deficiency requireonly GC and no MC replacement.
The mechanism of regulation of adrenal androgensis not completely understood. Adrenarche, the onset ofadrenal secretion of dehydroepiandrosterone andandrostenedione, is a maturational process andusually sets in prior to the onset of puberty.
Endogenous steroidogenesis
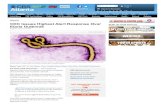
The substrate for steroid production is cholesterol. It ismobilised from the outer to the inner mitochondrialmembrane (by the steroidogenic acute regulatory (StAR)protein), where it is converted to pregnenolone (Figure1). ACTH regulation of StAR protein is the rate limitingstep in adrenal steroidogenesis.

P. Gupta and V. Bhatia
1040 Indian Journal of Pediatrics, Volume 75—October, 2008
Structure and mechanism of action
Glucocorticoid (GC) activity is determined by ahydroxyl group at carbon-11 of the steroid molecule.Cortisone and prednisone are 11-keto compounds,lacking GC activity. They are converted in the liver tocortisol and prednisolone respectively, thecorresponding 11-β hydroxyl compounds. All GCpreparations marketed for topical or local use (likeintra-articular) are 11-β hydroxyl compounds,obviating the need for biotransformation.1
The actions of all CS are mediated by interaction ofhormone with CS receptor, which regulates genetranscription. CS continue to act inside the cell evenafter their disappearance from the circulation, as theevents initiated and the products of these events (suchas specific proteins) may be present even afterdisappearance of CS from the circulation.
Pharmacodynamics
Systemically used GC are classified as short acting,intermediate acting and long acting (Table 1) based ontheir duration of ACTH suppression. They also differ intheir relative GC versus MC potency (Table 2) (1, 2).However one must remember that even those CS whichhave low MC activity (eg. hydrocortisone) may have MCeffects when used in high doses. The relative potency ofCS differ due to their affinity for the receptor. However,observed potency is determined by both intrinsicbiologic potency and duration of action. There is littlecorrelation between circulating half life (t1/2) and itspotency. Similarly little correlation exist between t1/2and its duration of action (1).
Actions of corticosteroids
1. Glucocorticoids
a. Carbohydrate metabolism: GC increasegluconeogenesis and conserve glucose for use byessential tissues like brain and red blood cells, atthe expense of less essential tissues like muscle,during the times of stress or starvation.
b. Protein metabolism: Overall effect is catabolic so
Fig. 1. Endogenous Steroidogenesis
TABLE 1. Classification of Glucocorticoids Based on Durationof Action
Short acting Intermediate acting Long acting(bioligical half (biological half (biological halflife 8-12 hr) life 12-36 hr) life 36-72 hr
Cortisol (hydrocortisone Triamcinolone BetamethasoneCortisone DexamethasonePrednisolone, PrednisoneMethylprednisolone
TABLE 2. Relative Potency of Commonly Used Corticosteroids.
Preparation Potency relative to hydrocortisone
Glucocorticoid* Mineralocorticoid Growthinhibitory
Hydrocortisone 1 1 1Cortisone 0.8 0.8 0.8Prednisolone 4 0.8 5Prednisone 4 0.8 5Methylprednisolone 5 0.5 7.5Dexamethasone 25 0 80Fludrocortisone 10 125 -
*Anti-inflammatory potency

Corticosteroid Physiology and Principles of Therapy
Indian Journal of Pediatrics, Volume 75—October, 2008 1041
that there is negative nitrogen balance with musclewasting, osteoporosis, growth slowing, skinatrophy, increased capillary fragility, bruisingand striae. Healing of wounds is delayed.
c. Fat deposition: It is increased on shoulders, faceand abdomen.
d. Maintainance of blood pressure: GC enhances thevascular reactivity to other vasoactive substancessuch as nor-epinephrine and angiotensin-II.
e. Anti-vitamin D action: They decrease calciumabsorption from the gut and increase urinarycalcium excretion, thus are useful in treatment ofhypercalcemia in sarcoidosis and vitamin Dintoxication.
f. Fluid and electrolyte balance: GC exert their effecton tubular function and glomerular filtration rate.They play a permissive role in renal free waterexcretion.
g. Renal excretion of urate is increased.
h. Anti-inflammatory and immunosuppressiveeffects: GC decrease recruitment and function ofinflammatory cells and vascular permeability atthe site of inflammation. They also inhibitprostaglandin and leucotriene synthesis byinhibiting the release of arachidonic acid from thephospholipids. By these mechanisms, GC protectthe organism from the damage caused by its owndefense reactions and the products of thesereactions during stress. Consequently, the use ofGC as anti-inflammatory and immuno-suppressive agents represent the application ofphysiological effects to the treatment of diseases.
2. Mineralocorticoids
MC primarily act on the distal tubules and collectingducts of kidneys, beside their actions on gut, salivaryand sweat glands, where they stimulate reabsorption ofsodium and excretion of potassium and hydrogen ions,thus maintaining electrolyte balance. Hyperaldos-
teronism causes positive sodium balance withconsequent expansion of extracellular volume, normal/slight increase in plasma sodium concentration,hypokalemia and alkalosis. In contrast, MC deficiencyleads to sodium wasting, contraction of extracellularvolume, hyponatremia, hyperkalemia and acidosis.
3. Adrenal androgens
Physiologic development of pubic and axillary hair andodour during normal puberty is regulated by adrenalandrogens. Increased adrenal androgen productionresults in virilisation in girls and peripheral precociouspuberty in boys.
Physiological replacement of CS
Prolonged GC therapy suppresses the HPA axis.During the period which the axis takes to recover (whichmay be as long as 12-18 months), the adrenal gland will notbe able to make GC in sufficient amounts needed for dailyphysiology. Furthermore, it will not be able to increase GCproduction to the levels required during stress, the requiredrelease being anywhere from three times (for moderate stress)to ten times (for severe stress) the daily production rate. Thisadjustment has to be done through exogenous GCreplacement and patient education, until the patient hasbeen documented to have HPA axis recovery.
Glucocorticoids
The physiological secretory rate of cortisol in the intactsystem is approximately 6 mg/m2/day (3, 4, 5). Theusual maintainance GC dose is adjusted above thisestimated secretory rate as the bioavailability of cortisolis reduced by gastric acids and first pass metabolism inliver. Thus 8-10 mg/m2/day of oral hydrocortisone(HC) is a reasonable initial starting dose, thoughpatients with primary adrenal insufficiency mayrequire slightly higher doses of 10-12 mg/m2/day (6, 7,8, 9). Later on, the dose may be individualised to avertsigns and symptoms of adrenal insufficiency on theone hand while avoiding growth retardation andcushingoid features on the other.
TABLE 3. Guideline for Glucocorticoid Withdrawal After Prolonged Therapy
<2 weeks No need to taper; can stop abruptly
2-4 weeks Taper over 1-2 weeks
>4 weeks Decrease* 8 am < 3 Continue physiological Reassess HPA axis 3dose slowly cortisol μg/dL replacement montly
stepwise > 20 Stop treatmentover 1-2 or μg/dL
more months 3-20 Do ACTH Normal Stop treatmentto physiologic μg/dL stimulation Abnormal Continue Reasses
dose# test physiologic HPA axisreplacement 3 monthly
*Increase the dose, if the underlying disease flares up#Physiological dose: 10 mg/m2 per day of hydrocortisone or 2.5 mg/m2 per day prednisolone HPA: hypothalamopituitaryadrenal

P. Gupta and V. Bhatia
1042 Indian Journal of Pediatrics, Volume 75—October, 2008
HC is preferred in infancy and childhood because of itsrelatively low growth suppressing effects. Long lastingGC (prednisolone and dexamethasone) may be anoption at/or near the completion of linear growth.However, cost may be an important concern for ourpatients. An equivalent dose of tablet hydrocortisone(Hisone®) is approximately 30 times costlier thanprednisolone (Wysolone®). A hydrocortisone dose of 10mg/m2/day corresponds to 2.5 mg/m2/day ofprednisolone and 0.25 mg/m2/day of dexamethasone(Table 2). HC is given in 3 divided doses at 6-8 am, 2pm and 8 pm, prednisolone is given in two divideddoses at 6-8 am and 4 pm, whereas dexamethasone isgiven in once daily dose at 6-8 am (6, 8, 10). Theseschedules are designed to mimic the normal diurnalrhythm of cortisol production, wherein the serumcortisol approaches very low levels by midnight.
One must monitor blood pressure, weight andheight beside other clinical and laboratory variables,during treatment with CS.
Mineralocorticoids
Fludrocortisone is the synthetic MC used in all patientswith primary adrenal insufficiency and classiccongenital adrenal hyperplasia (CAH). Dosagerequirements in early infancy range from 0.05-0.3 mg/day and remain the same or decrease to 0.05-0.2 mg/day with age (6, 8, 9, 11). Infants need simultaneoussodium chloride supplementation at 1-3 gm/day (17-51mEq Na+/day), distributed in several feedings, as milkalone does not contain adequate amounts of sodium. Asexplained above, MC replacement is not required inchildren with secondary adrenal insufficiency. By thesame analogy, the child recovering from prolongedsteroid therapy, who has ACTH deficiency, does notneed MC replacement.
Stress dosing
With stress, cortisol secretion increases. Consequently,all patients with primary or secondary adrenalinsufficiency and CAH must be educated about theneed for increasing their GC dose during stress to avoidan adrenal crisis, which can be fatal. They shouldalways carry medical identification and informationconcerning therapy for stress. Care givers should havean emergency supply of I/M HC.
There is controversy about the definition of “stress”and the need to increase GC doses. Mild stresses likeimmunisation, uncomplicated viral illness and upperrespiratory tract infections may not require a stress dosesteroid regimen, if the patient otherwise feels well. GCdoses should be increased during fever ≥ 38oC,vomiting, diarrhea, decreased oral intake, lethargy,surgery, trauma, dental work and large burns (6, 8, 9). Ithas been reported that for short term high intensityexercise in adolescents with CAH, an additional
morning dose of HC did not alter blood glucose, lactate,free fatty acids, or epinephrine levels compared withplacebo. Thus exercise, although a physical stressor,does not require increased dosing (12). In theirconsensus statement on CAH, the Lawson WilkinsPediatric Endocrine Society and European Society forPediatric Endocrinology did not recommend increasingthe GC dose during psychological and emotional stress(3).
For stress dosing, HC is the preferred agent due to itsMC activity (6). For patients able to take orally, it shouldbe 2-3 times the maintainance dose (6, 8, 9). Traumapatients or those unable to take oral steroids requireparenteral (I/M or I/V) HC. At home, this can beinitiated by I/M hydrocortisone sodium succinate in adose of 50 mg/m2. This will provide coverage for 6-8hours. The more severe stresses such as major surgeryand sepsis should be treated aggressively with doses upto 100 mg/m2/day divided every 6 hourly intravenously(6). Blood glucose should be monitored, and I/V sodiumand glucose replacements given as needed. In thepostoperative period, dose of hydrocortisone may betapered on a daily basis depending on the patient’scondition and the level of stress, most of the patientsreaching physiological dosage by 7th postoperative day.
HPA axis suppression and glucocorticoid withdrawal
Systemic treatment courses as brief as 2 weeks result only intransient suppression of endogenous cortisol production.Topical/local GC therapy has a lower chance of HPAaxis suppression. However, long term use of high dosesof potent inhaled GC like fluticasone may lead toimpaired adrenal functions (13). In a study of childrenbeing treated for leukemia, a 4 week course of GCresulted in suppression of HPA axis for up to 8-10 weeksafter discontinuation (14, 15). The time course of recoverycorrelated with the total duration and total previous doseof GC. However, HPA axis suppression may persist foras long as 12 months after withdrawal of treatment. Therecovery may be more rapid in children than adults (1).
During recovery, hypothalamo-pituitary functionrecovers before adrenocortical function. Patients withmild suppression of the HPA axis (i.e. normal basalplasma and urine CS but impaired response to ACTH)resumes normal HPA function more rapidly than dothose with severe depression of HPA axis (i.e. low basaland impaired response to ACTH).
While withdrawing GC therapy, one has to balancethe risk of adverse effects of prolonged steroid use withthe risk of flare up of the underlying condition forwhich GC therapy was being given. There is also apotential risk of adrenal insufficiency with rapidwithdrawal. If the treatment duration was less than 2weeks, GC can be stopped abruptly. In children gettingtreatment for 2-4 weeks, the dose of GC may be

Corticosteroid Physiology and Principles of Therapy
Indian Journal of Pediatrics, Volume 75—October, 2008 1043
decreased over 1 or 2 weeks. Step-wise tapering ismandatory in patients on prolonged GC therapy. GCdose is slowly decreased to physiological dose overabout 1 to 2 or more months, and then discontinuedafter assessment of adrenal functions has shownrecovery (Table 3). Tests for recovery of adrenalfunctions may be performed approximately every 3months once the GC has been tapered to physiologicaldoses. Use of shorter acting preparations, single ratherthan multiple daily doses and alternate day therapyfavour early recovery of the HPA axis. More detailsabout GC withdrawal are described in theaccompanying article on steroid toxicity.
Assessment of the HPA axis
Before testing for recovery, the replacement GC iswithdrawn, 24 hours before the test in the case of HC(i.e. the dose of the previous morning may be the lastone taken) and 48 hours before with prednisolone. Thisis done as these medicines will cross react in a cortisolassay. Dexamethasone does not cross react in thecortisol assay and thus can be used during the periodoff prednisolone, if the patient resides in a remote areafar away from medical facilities and is expected not tohave recovered. Unless 8 am cortisol is clearly low (< 3-5 µg/dL) or clearly normal (>20 µg/dL), an ACTHstimulation test is indicated. This test can be performedat any time of the day, not necessarily at 8 am. Serumcortisol ≥20 µg/dL 60 minutes after an I/M injection of250 µg of synthetic ACTH (Synacthen®, Cortrosyn®) isregarded as normal and reflects normal response tostress. Other tests used, such as the low dose (1 µg)ACTH test and insulin hypoglycaemia test are notdescribed here in detail as they are not the practical testsfor paediatricians to use and are beyond the scope ofthis review.
Immunisation related issues
As recommended by the Indian Academy of Pediatrics,children receiving CS in the dose of 2 mg/kg/day formore than 14 days should not receive live-virusvaccines until they are off-steroids for at least 1 month.However physiological low dose, topical or inhaledtherapy are not contraindications (16).
Similarly, the ACIP (Advisory Committee onImmunisation Practices, of the American Academy ofPediatrics) writes that steroid therapy usually does notcontraindicate administration of live-virus vaccineswhen such therapy is (a) short term (<2 weeks); (b) lowto moderate dose; (c) long term, alternate day treatmentwith short acting preparations; (d) maintenancephysiological doses or (e) administered topically orlocally. A dose of 2 mg/kg/day or a total dose of 20mg/day of prednisolone is considered sufficientlyimmunosuppressive to raise concern about safety ofimmunisation with live-virus vaccines. It may also
reduce the immune response to vaccines. Physiciansshould wait for at least 3 months after discontinuationof therapy for administration of live-virus vaccine topatients who have received high dose systemic steroidsfor greater than or equal to 2 weeks (17).
CONCLUSION
Prolonged glucocorticoid therapy affects endogenousCS production and has a suppressive effect onhypothalamo-pituitary-adrenal (HPA) axis. If thetreatment duration was less than 2 weeks, GC can bestopped abruptly. If given for > 4 weeks, GC should betapered to physiological dose over about 1 to 2 or moremonths, and then discontinued after assessment ofadrenal functions has shown recovery. Until the HPAaxis recovers and endogenous production normalises,GC replacement in physiological dose of 8-10 mg/m2/day of oral hydrocortisone is required. Enhanced doses(from 2 – 3 to 10 times the daily replacement) arerequired during stress such as fever, infection, surgeryand trauma. HPA axis recovery is tested by 8 amcortisol and ACTH stimulated cortisol.
REFERENCES
1. Axelrod L. Glucocorticoid therapy. In DeGroot LJ, JemesonJL, de Kretser D, Grossman AB, Marshall JC, Melmed S et aleds. Endocrinology, 5th ed. Philadelphia: Elsevier Saunders;2006; 2329-2342.
2. Schimmer BP, Parker KL. Adrenocorticotropic hormone;Adrenocortical steroids and their synthetic analogs;inhibitors of the synthesis and actions of Adrenocorticalhormones. In Brunton LL, Silazo J, Parker KL, eds. Goodmanand Gilman’s The Pharmacological basis of Therapeutics, 11th ed.USA; The McGraw-Hill Companies, 2006; 1587-1612.
3. Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, RogolAD. Estimation of daily cortisol production and clearancerates in normal pubertal males by deconvolution analysis. JClin Endocrinol Metab 1993; 76: 1505-1510.
4. Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, BoothJD, Winterer JC et al. Daily cortisol production rate in mandetermined by stable isotope dilution/mass spectrometry.J Clin Endocrinol Metab 1991; 72: 39-45.
5. Linder BL, Esteban NV, Yergey AL, Winterer JC, LoriauxDL, Cassorla F. Cortisol production rate in childhood andadolescence. J Pediatr 1990; 117 : 892-896.
6. Shulman DI, Palmert MR, Kemp SF; Lawson Wilkins Drugand Therapeutics Committee. Adrenal insufficiency: still acause of morbidity and death in childhood. Pediatrics 2007;119 : e484-494. Epub 2007 Jan 22.
7. Lukert BP. Editorial: glucocorticoid replacement—howmuch is enough? J Clin Endocrinol Metab 2006; 91 : 793-794.
8. Clayton PE, Miller WL, Oberfield SE, Ritzén EM, SippellWG, Speiser PW; ESPE/ LWPES CAH Working Group.Consensus statement on 21-hydroxylase deficiency from theEuropean Society for Paediatric Endocrinology and theLawson Wilkins Pediatric Endocrine Society. Horm Res 2002;58 : 188-195.

P. Gupta and V. Bhatia
1044 Indian Journal of Pediatrics, Volume 75—October, 2008
9. Merke DP, Bornstein SR. Congenital adrenal hyperplasia.Lancet 2005; 18-24; 365(9477): 2125-2136.
10. Maguire AM, Ambler GR, Moore B, McLean M, Falleti MG,Cowell CT. Prolonged hypocortisolemia in hydrocortisonereplacement regimens in adrenocorticotrophic hormonedeficiency. Pediatrics 2007; 120 : 164-171.
11. American Academy of Technical report: congenital adrenalhyperplasia. Section on Endocrinology and Committee onGenetics. Pediatrics 2000; 106: 1511-1518.
12. Weise M, Drinkard B, Mehlinger SL, Holzer SM, EisenhoferG, Charmandari E et al. Stress dose of hydrocortisone is notbeneficial in patients with classic congenital adrenalhyperplasia undergoing short-term, high-intensity exercise.J Clin Endocrinol Metab 2004; 89 : 3679-3684.
13. Paton J, Jardine E, McNeill E, Beaton S, Galloway P, YoungD et al. Adrenal responses to low dose synthetic ACTH(Synacthen) in children receiving high dose inhaledfluticasone. Arch Dis Child 2006; 91 : 808-813. Epub 2006
Mar 23.14. Einaudi S, Bertorello N, Masera N, Farinasso L, Barisone E,
Rizzari C et al. Adrenal axis function after high-dose steroidtherapy for childhood acute lymphoblastic leukemia.Pediatr Blood Cancer 2008; 50 : 537-541.
15. Felner EI, Thompson MT, Ratliff AF, White PC, DicksonBA. Time course of recovery of adrenal function in childrentreated for leukemia. J Pediatr 2000; 137 : 21-24.
16. Shah RC, Shah NK, Kukreja S. Immunization in SpecialCircumstances. In Shah RC, Shah NK, Kukreja S eds. IAPGuide Book on Immunization, 4 th ed. Mumbai: IndianAcademy of Pediatrics; 2007; 52-58.
17. Centers for Disease Control and Prevention. Recommen-dations of the Advisory Committee on ImmunizationPractices (ACIP): Use of vaccines and immune globulins inpersons with altered immunocompetence. MMWR 1993; 42(No.RR-4): {inclusive page numbers}.