How are molecules depicted? Ch. 9, sections 3 & 4.
-
Upload
kory-doyle -
Category
Documents
-
view
224 -
download
0
description
Transcript of How are molecules depicted? Ch. 9, sections 3 & 4.

How are molecules How are molecules depicted?depicted?
Ch. 9, sections 3 & 4Ch. 9, sections 3 & 4

Lewis electron-dot Lewis electron-dot structuresstructures
1. Electrons in the outermost energy 1. Electrons in the outermost energy level of an atom are involved in level of an atom are involved in bonding, both ionic and covalent. bonding, both ionic and covalent.
2. These outer-level electrons are 2. These outer-level electrons are called called VALENCE ELECTRONSVALENCE ELECTRONS..
3. A 3. A LEWIS STRUCTURELEWIS STRUCTURE represents represents the valence electrons in a molecule.the valence electrons in a molecule.

The diatomic hydrogen molecule (H2) is the simplest model of a covalent bond, and is represented in Lewis structures as:
The shared pair of electrons provides each hydrogen atom with two electrons in its valence shell (the 1s) orbital.
In a sense, it has the electron configuration of the noble gas helium

When two chlorine atoms covalently bond to form Cl2, the following sharing of electrons occurs:
Each chlorine atom shared the Each chlorine atom shared the bonding pair of electrons and bonding pair of electrons and achieves the electron configuration of achieves the electron configuration of the noble gas argon. the noble gas argon.

In Lewis structures the bonding pair of electrons is usually displayed as a line, and the unshared electrons as dots:

The shared electrons are not located in a fixed position between the nuclei. In the case of the H2 compound, the electron density is concentrated between the two nuclei:
The two atoms are bound into the H2 molecule mainly due to the
attraction of the positively charged nuclei for the negatively charged electron cloud located between
them

How do we represent more-How do we represent more-complicated molecules with Lewis complicated molecules with Lewis
structures, such as CHstructures, such as CH33I ?I ?
1. 1. Determine the total number of Determine the total number of valence electrons in the compound.valence electrons in the compound.
1 C atom with 4 electrons = 1 x 4 = 41 C atom with 4 electrons = 1 x 4 = 4 3 H atoms with 1 electron = 3 x 1 = 33 H atoms with 1 electron = 3 x 1 = 3 1 I atom with 7 electrons = 1 x 7 = 7 1 I atom with 7 electrons = 1 x 7 = 7 Total # valence electrons =14Total # valence electrons =14

2. Determine which element will 2. Determine which element will be the central atom. be the central atom.
The central atom is the element The central atom is the element that that
*has the lowest electronegativity *has the lowest electronegativity or or
*is the only single element or*is the only single element or *Hydrogen and the halides are *Hydrogen and the halides are
usually NOT the central atom.usually NOT the central atom.

In the compound, CHIn the compound, CH33I, carbon has I, carbon has the lowest electronegativity so it the lowest electronegativity so it will be in the middle. will be in the middle.
HH H C I H C I H H

3. Place two electrons in each 3. Place two electrons in each bond. A BOND is the space bond. A BOND is the space between the central atom and between the central atom and the other atoms around it. the other atoms around it. (Electrons are found in pairs and (Electrons are found in pairs and by doing this, you are pairing by doing this, you are pairing them up.)them up.)
Look on the chalk board as I draw Look on the chalk board as I draw this for you. (I couldn’t figure out this for you. (I couldn’t figure out how to draw it on this how to draw it on this powerpoint!!!!!)powerpoint!!!!!)

4. Complete the octets of the 4. Complete the octets of the atomsatoms attached to the central attached to the central atomatom by adding electrons in by adding electrons in pairs.pairs.
See the chalk board again! (You See the chalk board again! (You should be writing these examples should be writing these examples down as I write them on the white down as I write them on the white board.)board.)

5. Place any remaining 5. Place any remaining electrons on the central atom in electrons on the central atom in pairs. pairs.
REMEMBER! The total electrons in REMEMBER! The total electrons in the Lewis structure MUST equal the Lewis structure MUST equal the number of electrons in step the number of electrons in step #1!!!!!!!!!#1!!!!!!!!!
See the chalk board again!See the chalk board again!

6. Now, replace each electron pair 6. Now, replace each electron pair attaching the central atom to the attaching the central atom to the outer atoms with a line. Each line outer atoms with a line. Each line represents where the electrons are represents where the electrons are shared.shared.
For example: For example: H : O : HH : O : H
would be written as would be written as
H - O - HH - O - H

Draw Lewis Structures for Draw Lewis Structures for the following compounds:the following compounds: 1. NF1. NF33
2. SiCl2. SiCl44 3. ClF3. ClF 4. CCl4. CCl22FF22 5. HOCl5. HOCl

7. If the central atom does not have an 7. If the central atom does not have an octet after you have equaled out the dots octet after you have equaled out the dots and # of valence electrons, form double and # of valence electrons, form double or triple bonds to give the central atom 8 or triple bonds to give the central atom 8 electrons.electrons.
Multiple bonds The sharing of a pair of electrons represents
a single covalent bond, usually just referred to as a single bond
In many molecules atoms attain complete octets by sharing more than one pair of electrons between them.
Two electron pairs share a double bond Three electron pairs share a triple bond

Example of a TRIPLE BOND is below.Example of a TRIPLE BOND is below.
Because each nitrogen contains 5 valence electrons, they need to share 3 pairs to each achieve a valence octet.

Resonance StructuresResonance Structures http://wine1.sb.fsu.edu/chm1045/nhttp://wine1.sb.fsu.edu/chm1045/n
otes/Bonding/Resonan/Bond07.htmotes/Bonding/Resonan/Bond07.htm

Draw Lewis Structures for Draw Lewis Structures for the following compounds:the following compounds:
6. O6. O2 2 (multiple bonds)(multiple bonds) 7. CS7. CS2 2 (multiple bonds)(multiple bonds) 8. HCN 8. HCN (multiple bonds)(multiple bonds) 9. SO9. SO2 2 (resonance)(resonance) 10. O10. O3 3 (resonance)(resonance)

Exceptions to the Octet RuleExceptions to the Octet Rule
1.1. Molecules with an odd # of Molecules with an odd # of valence electronsvalence electronsEX: NOEX: NO22
ClOClO22
NONO2. Compounds with fewer than 8 2. Compounds with fewer than 8
valence electrons present (this is valence electrons present (this is very rare)very rare)EX: BHEX: BH33

3. Compounds in which the central 3. Compounds in which the central atom has more than 8 valence atom has more than 8 valence electrons -- called an electrons -- called an EXPANDED EXPANDED OCTETOCTET ; ; occurs in energy levels occurs in energy levels of elements in period 3 and upof elements in period 3 and up
**Extra lone pairs are added to the **Extra lone pairs are added to the central atom OR more than 4 central atom OR more than 4 bonding atoms are present.bonding atoms are present.
EX: IFEX: IF44
PClPCl55

VSEPRVSEPR Model : Model : VValence alence SShell hell EElectron lectron PPair air RRepulsion epulsion model model Based on an arrangement that Based on an arrangement that
minimizes the repulsion of shared and minimizes the repulsion of shared and unshared pairs of electrons around unshared pairs of electrons around the central atomthe central atom
READREAD pages 259 – 261. pages 259 – 261. Look at Table 9-3 as you read.Look at Table 9-3 as you read. Complete #49 – 53 on pg. 262.Complete #49 – 53 on pg. 262.

Electronegativity and Electronegativity and PolarityPolarity Electronegativity Electronegativity indicates the indicates the
relative ability of an atom to relative ability of an atom to attract electrons in a chemical attract electrons in a chemical bond.bond.
It generally increases as the It generally increases as the atomic number increases atomic number increases ACROSS ACROSS A PERIOD A PERIOD and generally decreasesand generally decreases as you go as you go DOWN A GROUP.DOWN A GROUP.

Polar or Polar or Nonpolar???????????Nonpolar??????????? Identical atoms, like NIdentical atoms, like N22, have an , have an
electronegativity difference of zero, and electronegativity difference of zero, and the electrons in the bond are equally the electrons in the bond are equally shared between the two atoms. This is shared between the two atoms. This is a a NONPOLAR COVALENT BOND, or a PURE COVALENT BOND.
A covalent bond between atoms of A covalent bond between atoms of different elements does not have equal different elements does not have equal sharing of the electron pair, due to the sharing of the electron pair, due to the difference in electronegativity.difference in electronegativity.

Unequal sharing results in a Unequal sharing results in a POLAR COVALENT BOND. The shared electrons are . The shared electrons are pulled toward one of the atoms and spend pulled toward one of the atoms and spend more time around that atom than the other more time around that atom than the other atom. Partial charges occur at the ends of atom. Partial charges occur at the ends of the bond. This bond is often referred to as the bond. This bond is often referred to as a a dipole (two poles).(two poles).
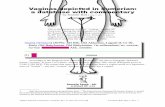
To determine if the bond is To determine if the bond is polar or or nonpolar, you must look at the , you must look at the shapeshape of the of the molecule. Draw the molecular structure, molecule. Draw the molecular structure, using what you know from Table 9-3.using what you know from Table 9-3.
SYMMETRIC MOLECULES ARE USUALLY SYMMETRIC MOLECULES ARE USUALLY NONPOLARNONPOLAR AND AND ASYMMETRIC ARE POLARASYMMETRIC ARE POLAR AS LONG AS THE BOND TYPE IS POLAR.AS LONG AS THE BOND TYPE IS POLAR.

Do #60 – 63 on page 266. Do #60 – 63 on page 266.