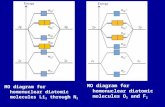
Homonuclear & Heteronuclear bonds Homonuclear bondsHetronuclear bonds Ethane (C 2 H 6 ) Hydrazine (N...
-
Upload
noah-short -
Category
Documents
-
view
220 -
download
2
Transcript of Homonuclear & Heteronuclear bonds Homonuclear bondsHetronuclear bonds Ethane (C 2 H 6 ) Hydrazine (N...
Homonuclear & Heteronuclear bonds
Homonuclear bonds Hetronuclear bonds
Ethane (C2H6)Hydrazine (N2H4)
Hydrogen peroxide (H2O2)
Salt crystals are repeating patterns of positive+ cations and negative- anions held together by electrostatic attraction.
IONIC COMPOUNDS
COVALENT COMPOUNDS
Biological molecules are covalently bound (remember, only covalent bonds can form molecules)
Most consist of the non-metals Carbon, Hydrogen Oxygen,, and Nitrogen. (CHON)
electronegativityThe ELECTRONEGATIVITY of an element helps us understand the difference between ionic and covalent bonding
• Electronegativity is the measure of the ability of an atom in a bond to attract electrons.
• With only a few exceptions, electronegativity values increase as you move from left to right in any period of the periodic table.
• Within any group, electronegativity values decrease as you go down the group.
Every element has an electronegativity value
Francium has the lowest electronegativity 0.7 Fluorine has the highest 4.0
Elements with a HIGH electronegativity have a STRONG pull on electrons.
Elements with a LOW electronegativity have a WEAK pull on electrons.
difference in electronegativity
When two atoms bond their DIFFERENCE in electronegativity determines the bond type.
A large difference in electronegativity means one atom will win the “tug of war” and take the electrons completely. This is an ionic bond.
When the electronegativity of two bonding atoms is very similar, neither
atom wins the “tug of war” and the electrons are shared equally.
This produces a covalent bond
IONIC COVALENT
Transfer electrons Share electrons
Between an atom of high electronegativity and an atom of low electronegativity
Between two atoms of equal or very close electronegativities
NaCl N2
If the electronegativity difference between two bonded atoms is very high the bond is ionic.
If the electronegativity difference is very low the bond is covalent.
What if the difference in electronegativity between the two bonded atoms is in-between?
A POLAR COVALENT BOND occurs when two atoms share electrons unequally.
The atom with a high electronegativity value holds the bonding electrons more often, but it doesn’t remove the electrons completely.
Are the bonds polar covalent, non-polar covalent, or ionic?
1) H-C 2) K-Cl 3) O-F 4) Cl-Cl
5) C-N 6) S-O 7) B-S
Bond polarity and 3D shape determine if a molecule is polar
Bond polarity --- When a bond has a partial negative charge on one atom and a partial positive charge on the other atom.
Molecule shape--- the arrangement of atoms in three dimensions (3-D)
A polar molecule has polar bonds and asymmetry
Polar bonds Non-polar molecule
Symmetry- all sides are the same
Polar bonds Polar molecule
Asymmetry- has different sides
δ-
δ-
δ-
δ-
δ-
δ+δ+
negative side
Positive side
If the electrons are not distributed equally, the molecule is said to be
polar.
The molecule has a negative end and
a positive end.
POLAR MOLECULES INTERACT!!
A partial positive charge (+) is attracted to
negative ions
and
negative partial charges (-) of other polar bonds.
A partial negative charge (-) is attracted to
positive ions
and
partial positive charges (+) of other polar bonds.
POLAR MOLECULES INTERACT!!
+
The electrons are not distributed evenly so the water molecule is polar. The negative end of the
molecule is the oxygen end. O is more electronegative
than H and pulls the negative electrons toward itself. Also, there are two
lone pairs around oxygen.
negative end
positive end