Hemodynamics of renal artery stenosis
Transcript of Hemodynamics of renal artery stenosis

Hemodynamic Rounds
Hemodynamics of Renal Artery Stenosis
Josh Todd, MD and George A. Stouffer,*MD
' 2008 Wiley-Liss, Inc.
Key words: HEMO, hemodynamics; PVD, peripheral vascular disease; QVA, quantitativevascular angiography
CASE PRESENTATION
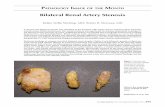
A 55-year-old white female with hypertension, dia-betes mellitus, hyperlipidemia, and a history of 4-ves-sel coronary artery bypass grafting 10 years previouslypresented with complaints consistent with crescendounstable angina. Angiography revealed severe diseasein a saphenous vein graft to an obtuse marginal thatwas treated with placement of a Cypher drug elutingstent (Cordis Corporation, Miami Lakes, FL). Becauseof uncontrolled hypertension (158/77 mm Hg) despitetreatment with three antihypertensives (enalapril, ateno-lol, and furosemide), she underwent screening renal an-giography that showed a 95% proximal left renal ar-tery stenosis (Fig. 1). The translesional pressure gradi-ent, measured by placing a Wavewire (0.014 inch indiameter, Volcano Therapeutics, Rancho Cordova, CA)into the distal renal artery, showed a systolic peak-to-peak gradient of 74 mm Hg and a mean gradient of 24mm Hg. The patient underwent an uncomplicatedangioplasty and stent placement to the left renal artery2 days after intervention on the saphenous vein graft.The patient’s blood pressure improved and remained
controlled during 27 months of follow-up. She contin-ued to be treated with enalapril, furosemide, and ateno-lol at the same doses as prior to renal stenting.
RENAL HEMODYNAMIC PHYSIOLOGY
Blood flow to the kidneys constitutes �25% of thecardiac output, and the kidneys consume 10% of thetotal oxygen requirements for the body [1,2]. Distribu-tion of blood flow to the renal parenchyma is con-trolled by an autoregulatory system that maintainsglomerular capillary pressure at 60–70 mm Hg andthereby regulates glomerular filtration rate. In additionto intrarenal mechanisms, renal blood flow is regulatedby systemic mechanisms including the renin–angiotensin
system, efferent sympathetic nerve activity, vasopressinproduction, and stimulation of natriuretic peptides [2].
PATHOPHYSIOLOGY OF RENALARTERY STENOSIS
In 1934, Goldblatt et al. demonstrated that constric-tion of renal arteries in dogs produced sustained hyper-tension, and this result led to the establishment of alink between a renal peptide (eventually identified asrenin) and hypertension [3]. Since that time, two dis-tinct animal models of renovascular hypertension havebeen extensively studied and provide much of our cur-rent understanding of the pathophysiology of renal ar-tery stenosis.In the 2 kidney, 1 clip (2K1C) model, the renal artery
on one side is partially occluded thus providing a modelof unilateral renal artery stenosis. The pathophysiologyin the 2K1C model varies over time. The ‘‘acute phase’’occurs immediately after surgical induction of renalartery stenosis, and is characterized by activation of therenin–angiotensin system and a rapid rise in blood pres-sure. Treatment with angiotensin receptor blockers, an-giotensin converting enzyme inhibitors, or genetic dis-ruption of the angiotensin system blunts the increase in
The authors have no financial conflicts of interest to disclose.
Division of Cardiology, University of North Carolina, ChapelHill, NC
*Correspondence to: George A. Stouffer, Division of Cardiology,
CB 7075, University of North Carolina, Chapel Hill, NC 27599-
7075. E-mail: [email protected]
Received 26 November 2007; Revision accepted 24 December 2007
DOI 10.1002/ccd.21508
Published online 16 June 2008 in Wiley InterScience (www.
interscience.wiley.com).
' 2008 Wiley-Liss, Inc.
Catheterization and Cardiovascular Interventions 72:121–124 (2008)

blood pressure. Removal of the renal artery clip or ne-phrectomy of the ischemic kidney normalizes bloodpressure. In some models, persistent renal ischemiacauses the animal to enter a phase in which hypertensionis less renin-dependent and where the response to re-moval of the clip is more variable.
1 Kidney, 1 Clip Model
In the 1 kidney, 1 clip (1K1C) model, the renal ar-tery on one side is partially occluded and the contralat-
eral kidney is removed, thus providing a model ofglobal renal ischemia.There are transient increases in serum renin and
Ang II levels over the initial few days. SuppressingAng II formation during this period delays but doesnot prevent the onset of hypertension, implicating fac-tors other than the renin–angiotensin system. Followingthis phase, increases in intravascular volume (andpotentially other factors) maintain hypertension despitea decrease in renin and Ang II levels.
DETERMINATION OF THE SEVERITY OF RENALARTERY STENOSIS
There is a high prevalence of renal artery atheroscle-rosis in patients undergoing cardiac catheterization. Invarious studies, moderate renal artery stenosis (�50%)was present in 6–19% of patients with severe stenosis(�70%) was found in 3–12% of patients [4,5]. Butwhile angiography is the ‘‘gold-standard’’ for determin-ing the anatomic significance of a renal artery lesion,estimating the physiological significance of renal arterydisease remains problematic.Translesional pressure gradients, the difference
between the aortic and the poststenotic renal arterypressure, have been measured at the time of surgeryand percutaneously using either a small catheter or apressure-tipped guidewire. The pressure drop across anarterial lesion is dependent upon blood flow in a rela-tionship defined by the equation P 5 R 3 F (where P5 pressure, R 5 resistance and F 5 flow). Transle-sional pressure gradients can be measured either underbasal conditions or following stimulation of renalblood flow. Various agents including nitroglycerin, pa-paverine, dopamine, adenosine, acetylcholine, andfenoldopam have been used to increase renal bloodflow. Renal flow reserve, as measured in arteries with-out renal artery stenosis, varies between 1.5 and 2 [6].Mounier-Vehier found that renal flow reserve, as meas-ured during papaverine-induced hyperemia, was 1.1 atbaseline in 19 patients with unilateral stenosis. Afterstent placement, renal flow reserve improved immedi-ately and was the same as observed in the contralateralkidney [7].Translesional pressure gradients can be measured
utilizing either a pressure-wire or catheter. Placing acatheter across a lesion, however, introduces inaccur-acy due to the obstruction caused by the catheter itself.For example, in a 6-mm renal artery with a 65% ste-nosis, a 4 French catheter will obstruct 42% of theexisting lumen. Thus in a lesion of borderline signifi-cance, the catheter could obstruct the lumen diameterenough to result in an artifactual pressure gradient.The accuracy of translesional pressure gradients
Fig. 1. Renal artery stenosis—Angiogram in an AP projectionshows a severe lesion involving the ostium of the left renal ar-tery (A). Translesional pressure gradient, measured using apressure-tipped guidewire, is shown in panel (B). An angio-gram in an AP projection following stent placement is shownin panel (C).
122 Todd et al.
Catheterization and Cardiovascular Interventions DOI 10.1002/ccd.Published on behalf of The Society for Cardiovascular Angiography and Interventions (SCAI).

increases when very thin pressure wires, rather thancatheters, are used. In a study by Garcia and Corroza,pressure wire measurements were compared to thecatheter-derived (4F and 5F) measurements for transle-sional pressure gradients during hyperemia in periph-eral arteries [8]. The results obtained in 20 peripheralarteries after nitroglycerin-induced hyperemia showedthat the catheter-based measurement averaged 28 mmHg, whereas the pressure wire gradient had a mean of12 mm Hg. In all cases, the catheter-derived pressuregradient was higher than the pressure wire gradient.One of the earliest measurements of the usefulness
of translesional pressure gradient in patients with renalartery stenosis was performed in the operating room inpatients undergoing either surgical revascularization ornephrectomy [9]. Messerli et al. performed direct aor-tic and renal artery needle puncture and compared thedata to blood pressure response. In an average of 4.3years of follow-up, good responders (defined asrelieved of hypertension without medications) and fairresponders (reduction in blood pressure of >20 mmHg without medications) had mean systolic pressuregradients >40 mm Hg. Poor responders encompassed awide-range of pressure gradients with 64% of patientshaving gradients >40 mm Hg and 36% of patientshaving gradients <40 mm Hg. In a study more reflec-tive of current practice in which pressure gradients areprimarily determined using percutaneous techniques,Colyer et al. found a good correlation between mini-mal lumen diameter and translesional systolic pressuregradient as determined with a pressure wire (r2 50.801). The correlation was much weaker when 4Fcatheters were utilized to measure pressure gradient (r2
5 0.360) [10]. The correlation between translesionalsystolic pressure gradient and percent stenosis was notstrong, regardless of the method used.A study of 46 renal artery stenoses in 38 patients
(mean stenosis of (51 6 17)% with a range of 12–85%) found that systolic and mean translesional pres-sure gradients, measured using a pressure-wire, corre-lated with stenosis severity, systolic blood pressure,and serum creatinine [11]. These investigators con-cluded that a systolic pressure gradient greater than 20mm Hg reliably correlated with an angiographic steno-sis of �50% and identified patients who would benefitfrom renal artery revascularization. This study, how-ever, did not include any follow-up data on blood pres-sure response or other outcomes.Studies that have compared angiographic stenosis
(using visual estimates or quantitative angiography)with translesional pressure gradients have, in general,found a poor correlation for moderate stenoses. Joneset al. studied 22 patients with intermediate renal arterylesions, in whom translesional pressure gradient was
measured with a 0.014@ guidewire [12]. They found nocorrelation between visually determined angiographicstenosis and pressure gradient under basal conditions(r2 5 0.124) or under conditions of hyperemia (r2 50.101) in intermediate lesions. A poor correlationbetween quantitative angiography and either basalmean or hyperemic mean translesional pressure gra-dients was also found by Subramanian et al. in theirstudy of 13 patients with intermediate lesions [13].Clinical studies of translesional hemodynamics have
focused on pressure gradients across a stenosis, how-ever, the absolute value of renal perfusion pressureand/or the ratio of renal perfusion pressure to aorticpressure may be more important in regulating reninrelease. Animal studies suggest the existence of a criti-cal renal perfusion pressure below which the stretchsensitive receptors within the juxtaglomerular apparatusare activated [1]. In an elegant clinical study, deBruyne et al. examined renin release in 15 patientswho were undergoing intervention for unilateral renalartery stenosis [14]. Following relief of the stenosis,incremental balloon inflations were used to creategraded, artificial stenoses with the severity of the ste-nosis expressed as a ratio of the mean distal (renal per-fusion) pressure to the mean aortic pressure (Pd/Pa).Renin levels were measured in the central aorta andboth renal veins at 10-min intervals following sequen-tial increases in the artificial stenosis created by bal-loon. Plasma levels of renin from aortic bloodincreased, but not significantly, with increasing degreesof stenosis. There was, however, a progressive increasein renin levels in the vein of the stenotic kidney start-ing at a Pd/Pa ratio of 0.80. Surprisingly, renin levelsin the vein of the nonstenotic kidney also increased ata Pd/Pa of 0.50. In this study, renin levels did notincrease until Pd/Pa � 0.80, irrespective of the abso-lute value of translesional pressure gradient, and thusthe authors concluded that stenoses with Pd/Pa � 0.90are unlikely to be responsible for renovascular hyper-tension.
CURRENT RECOMMENDATIONS FORDETERMINING THE PHYSIOLOGICSIGNIFICANCE OF A RENAL ARTERY STENOSIS
There is general agreement that lesions �70% byangiography are hemodynamically significant, whereaslesions �50% have no hemodynamic effect. The he-modynamic significance of lesions �50% and �70%varies and ACC/AHA guidelines recommend the mea-surement of translesional pressure gradients in thesepatients to determine hemodynamic significance [15].There are limited data correlating translesional gra-dients to clinical outcomes and there is no consensus
Hemodynamics of Renal Artery Stenosis 123
Catheterization and Cardiovascular Interventions DOI 10.1002/ccd.Published on behalf of The Society for Cardiovascular Angiography and Interventions (SCAI).

on whether absolute systolic, peak systolic, or meanpressure should be used, whether the pressure shouldbe measured during a resting or hyperemic state, andwhat level constitutes a lesion that would benefit fromrevascularization.
RENAL FRACTIONAL FLOW RESERVE
Although not validated in large clinical studies orincluded in current guidelines, early work on renal frac-tional flow reserve (rFFR) has been promising, as itappears to have good predictive value in regards topatient outcomes. rFFR is a proportional determinant ofthe severity of a stenosis by comparing the pressure dis-tal to a stenosis with aortic pressure during hyperemia.In a study of 17 patients with unilateral renal artery
stenosis who underwent renal stenting [16], rFFRdetermined using papavarine predicted patient out-comes at 8–12 months. Blood pressure improved in86% of patients with rFFR <0.8 at 3 months, and 71%of patients at last follow-up. In comparison, bloodpressure improvement was observed in only 30% ofpatients with RFFR � 0.8 at 3 months and 10% at lastfollow-up. Of note, there was no difference betweenresponders and nonresponders in mean, systolic resting,or hyperemic translesional pressure gradients.
CONCLUSIONS
Renovascular hypertension has long been recognizedas a potentially curable form of secondary hyperten-sion. Recent data suggest that identification of hemo-dynamically significant lesions can be enhanced withmeasurement of translesional pressure gradients. Themost promising recent data involves the measurementof rFFR in order to better identify patients that willbenefit from revascularization.
REFERENCES
1. Rundback JH, Murphy TP, Cooper C, Weintraub JL. Chronic re-
nal ischemia: Pathophysiologic mechanisms of cardiovascular
and renal disease. J Vasc Interv Radiol 2002;13:1085–1092.
2. Dworkin LD, Brenner BM. The renal circulations. In: Brenner
BM, editor. The Kidney. Philadelphia, PA: Saunders; 2004.
3. Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies in
experimental hypertension. I. The production of persistent eleva-
tion of systolic blood pressure by means of renal ischemia.
J Exp Med 1934;59:347.
4. Cohen MG, Pascua JA, Garcia-Ben M, Rojas-Matas CA, Gabay
JM, Berrocal DH, Tan WA, Stouffer GA, Montoya M, Fernan-
dez AD, Halac ME, Grinfeld LR. A simple prediction rule for
significant renal artery stenosis in patients undergoing cardiac
catheterization. Am Heart J 2005;150:1204–1211.
5. White CJ. Catheter-based therapy for atherosclerotic renal artery
stenosis. Prog Cardiovasc Dis 2007;50:136–150.
6. Manoharan G, Pijls NH, Lameire N, Verhamme K, Heyndrickx
GR, Barbato E, Wijns W, Madaric J, Tielbeele X, Bartunek J,
de Bruyne B. Assessment of renal flow and flow reserve in
humans. J Am Coll Cardiol 2006;47:620–625.
7. Mounier-Vehier C, Cocheteux B, Haulon S, Devos P, Lions C,
Gautier C, Carre A, Beregi JP. Changes in renal blood flow
reserve after angioplasty of renal artery stenosis in hypertensive
patients. Kidney Int 2004;65:245–250.
8. Garcia LA, Carrozza JP Jr. Physiologic evaluation of translesion
pressure gradients in peripheral arteries: Comparison of pressure
wire and catheter-derived measurements. J Interv Cardiol 2007;
20:63–65.
9. Messerli FH, Genest J, Nowaczynski W, Kuchel O, Cartier P,
Rojo-ortega JM, Schurch W, Honda M, Boucher R. Hyperten-
sion with renal arterial stenosis: Humoral, hemodynamic and
histopathologic factors. Am J Cardiol 1975;36:702–707.
10. Colyer WR Jr, Cooper CJ, Burket MW, Thomas WJ. Utility of
a 0.014’’ pressure-sensing guidewire to assess renal artery trans-
lesional systolic pressure gradients. Catheter Cardiovasc Interv
2003;59:372–377.
11. Gross CM, Kramer J, Weingartner O, Uhlich F, Luft FC, Wai-
gand J, Dietz R. Determination of renal arterial stenosis severity:
Comparison of pressure gradient and vessel diameter. Radiology
2001;220:751–756.
12. Jones NJ, Bates ER, Chetcuti SJ, Lederman RJ, Grossman PM.
Usefulness of translesional pressure gradient and pharmacologi-
cal provocation for the assessment of intermediate renal artery
disease. Catheter Cardiovasc Interv 2006;68:429–434.
13. Subramanian R, White CJ, Rosenfield K, Bashir R, Almagor Y,
Meerkin D, Shalman E. Renal fractional flow reserve: A hemo-
dynamic evaluation of moderate renal artery stenoses. Catheter
Cardiovasc Interv 2005;64:480–486.
14. de Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J,
Bartunek J, Vanderheyden M, Heyndrickx GR. Assessment of
renal artery stenosis severity by pressure gradient measurements.
J Am Coll Cardiol 2006;48:1851–1855.
15. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA,
Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB,
Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ,
White J, White RA, Antman EM, Smith SC Jr, Adams CD,
Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs
AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA
2005 Practice Guidelines for the management of patients with
peripheral arterial disease (lower extremity, renal, mesenteric,
and abdominal aortic): A collaborative report from the American
Association for Vascular Surgery/Society for Vascular Surgery,
Society for Cardiovascular Angiography and Interventions, Soci-
ety for Vascular Medicine and Biology, Society of Interventional
Radiology, and the ACC/AHA Task Force on Practice Guide-
lines (Writing Committee to Develop Guidelines for the Man-
agement of Patients With Peripheral Arterial Disease): Endorsed
by the American Association of Cardiovascular and Pulmonary
Rehabilitation; National Heart, Lung, and Blood Institute; Soci-
ety for Vascular Nursing; TransAtlantic Inter-Society Consensus;
and Vascular Disease Foundation. Circulation 2006;113:e463–
e465.
16. Mitchell JA, Subramanian R, White CJ, Soukas PA, Almagor Y,
Stewart RE, Rosenfield K. Predicting blood pressure improve-
ment in hypertensive patients after renal artery stent placement:
Renal fractional flow reserve. Catheter Cardiovasc Interv 2007;
69:685–689.
124 Todd et al.
Catheterization and Cardiovascular Interventions DOI 10.1002/ccd.Published on behalf of The Society for Cardiovascular Angiography and Interventions (SCAI).