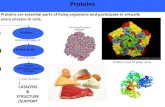
GLOBULAR PROTEINS. TYPES OF PROTEINS GLOBULAR PROTEINS FIBROUS PROTEINS.
-
Upload
dorothy-norah-cook -
Category
Documents
-
view
248 -
download
3
Transcript of GLOBULAR PROTEINS. TYPES OF PROTEINS GLOBULAR PROTEINS FIBROUS PROTEINS.

GLOBULAR PROTEINS

TYPES OF PROTEINS
• GLOBULAR PROTEINS• FIBROUS PROTEINS

• GLOBULAR PROTEINS

GLOBULAR HEMEPROTEINS
• Are a group of specialized proteins that contain heme.
• The two most abundant heme-proteins in humans are hemoglobin and myoglobin.
• The heme group function differently. Here, the heme group serves to reversibly bind oxygen

STRUCTURE OF HEME• Heme is a complex of protoporphyrin IX and ferrous iron (Fe²⁺).• The iron is held in the center of the heme molecule by bonds to
the four nitrogens of the porphyrin ring,• The heme Fe²⁺ can form two additional bonds on each side of the
porphyrin ring.• In myoglobin and hemoglobin, one of the positions is coordinated
to the side chain of a histidine residue of the globin molecule, whereas the other position is available to bind oxygen.


STRUCTURE AND FUNCTION OF MYOGLOBIN
• Myoglobin hemeprotein present in heart and skeletal muscle functions both as reservoir for oxygen and as a carrier of one oxygen molecule to the muscle cells when needed.
• Is composed of a single polypeptide chain, termed a monomer that transports.
• The interior of polypeptide chain in myoglobin is composed of non-polar amino acid with the exception of histidine.

STRUCTURE AND FUNCTION OF HEMOGLOBIN
• Hemoglobin is found extensively in red blood cells, where its main function is to transport four molecules of O₂ from the lungs to the capillaries of the tissues
• Hemoglobin can transport CO₂ from the tissues to the lungs, and carry four molecules of O₂ from the lungs to the cells of the body

• Hemoglobin is composed of 4 polypeptide chains making it a tetramer depending on the type of HbDepending on the globin chains:
HbA- α2 β2-Major Hb Adult Hb A2- α2 2-Minor Hb AdultHbF- α2 2 -Minor Hb Fetal22-Minor Embryonic Hb

• The adult Hb tetramer can be envisioned as being composed of two identical dimers (αβ)₁ and (αβ)₂ .
• Hydrophobic bond holds each α and β subunit while ionic or hydrogen bond hold each αβ dimer.
• The interior of the polypeptide chain contains mainly nonpolar amino acid as well as histidine.


12
Forms of Hemoglobin
An active “relaxed” (R) state High oxygen affinity
Binding of oxygen is facilitated
Oxygenated
An inactive “taut” or “tense” (T) state Low oxygen affinity
Resists the binding of oxygen
Deoxygenated

BINDING OF OXYGEN TO MYOGLOBIN AND HEMOGLOBIN
• Myoglobin can bind only one molecule of oxygen because it contains only one heme group
• In contrast, hemoglobin can bind four oxygen molecules—one at each of its four heme groups

Oxygen dissociation curve• A plot of % oxygen saturation (Y)
measured at different partial pressures of oxygen (Po₂)
• Curves for myoglobin and hemoglobin show important differences
• The curve illustrates that myoglobin has a higher oxygen affinity at all pO₂ values than does hemoglobin
• The partial pressure of oxygen needed to achieve half-saturation of the binding sites (P50) is approx 1mmHg for myoglobin and 26mmHg for Hb.
• The higher the oxygen affinity, the lower the P50
14

MYOGLOBIN
• The oxygen dissociation curve for myoglobin has a hyperbolic shape
• This reflects that myoglobin reversibly binds a single molecule of oxygen.
• Myoglobin binds oxygen released by Hb in the muscle and releases oxygen within the muscle cell in response to oxygen demands
HEMOGLOBIN
• The oxygen dissociation curve for hemoglobin is sigmoidal in shape, indicating that the subunits cooperate in binding oxygen
• Cooperative binding of oxygen by the 4 subunits of Hb means that the binding of an oxygen molecule at one heme grp increases the oxygen affinity of the remaining heme grps in the same Hb moleculeis referred to as heme-heme interaction
15


ALLOSTERIC EFFECTS• The ability of hemoglobin to reversibly bind to oxygen is
affected by:• a. pH of the environment• b. partial pressure of carbon dioxide (pCO₂)• c. the availability of 2,3-bisphosphoglycerate• d. partial press of oxygen (Po₂) thru heme-heme interactions• These are collectively called allosteric(“other sites”) effectors
cos their interaction at one site on the Hb molecules affects the binding of oxygen to heme groups at other locations on the molecule
• Note: the ability of myoglobin to bind oxygen is not influenced by allosteric effectors.

Heme-heme interaction
• Loading and unloading oxygen: the cooperative binding of oxygen allows Hb to deliver more oxygen to the tissues in response to relatively small changes in the partial pressure of oxygen.

Significance of the sigmoidal oxygen dissociation curve
• The steep slope of the ODC over the range of oxygen conc that occur between the lungs and tissues Allows Hb to carry and deliver oxygen efficiently from sites of high to sites of low pO₂ unlike the hyperbolic dissociation curve.

Bohr effect
• Refers to the change in oxygen binding to hemoglobin when exposed to changes in pH and pCO₂
• ↓ in pH• ↑ in pCO₂• Both of which causes a
right ward shift of the ODC AND vice versa
20

BINDING OF CO₂
• Binding of CO₂ (carbaminohemoglobin) results in a decrease in hemoglobin affinity for oxygen by stabilizing the taut form of Hb


EFFECTS OF 2,3-BISPHOSPHOGLYCERATE ON OXYGEN AFFINITY
2,3 BPG is the most abundant organic phosphate in RBC.
It is an important regulator of Hb binding with O2.


• 2,3-bisphosphoglycerate decreases the oxygen affinity of hemoglobin by binding to deoxyhemoglobin stabilizing the taut form of Hb
• Shift of the oxygen-dissociation curve: • a. hemoglobin from which 2,3BPG has been
removed has a high affinity for oxygen shifting the curve to the left.
• However, the presence of 2,3BPG in RBCs significantly reduces the affinity of hemoglobin for oxygen, shifting the curve to the right
• This reduced affinity enables oxygen to be efficiently delivered to tissues

2,3 BPG concentration increasesin response to chronic hypoxia incases of anemia or at high altitudes.
Severely ill patients maybe seriously compromised if transfused with large quantities of 2,3-BPG-’stripped blood.’

BINDING OF CO(carbon monoxide)• Carbon monoxide (CO) binds tightly (high affinity)
but reversibly to hemoglobin iron , forming carbon monoxyhemoglobin or carboxyhemoglobin (HbCO)
• This shifts the curve to the left• The affinity of hemoglobin for CO is 220 times
greater than for oxygen• CO poisoning is treated with 100% oxygen therapy
which facilitates the dissociation of CO from the hemoglobin

• TYPES OF HEMOGLOBIN

Globin Gene Family

Hb Variants
Synthesized more in people with diabetes mellitus
occurs 12 weeks after
birth
Val of chain

Fetal Hb
Hb Gower 1 - embryonic Hb - 2 2
HbF - major Hb - 22
- binds 2,3 BPG weakly

• Binding of 2,3BPG to HbF:• Under physiologic conditions, HbF has a higher affinity
for oxygen than does HbA, as a result of HbF’s binding only weakly to 2,3BPG (cos 2,3BPG binds to the beta chains of hemoglobin whereas HbF lacks beta chain)
• The higher oxygen affinity of HbF facilitates the transfer of oxygen from the maternal circulation across the placenta to the RBCs of the fetus.

Hemoglobinopathies
Sickle-cell anemia (HbS)Hemoglobin C disease (HbC)Thalassemia

Sickle Cell Disease
- caused by a point mutation
- autosomal recessive
- 2S2
- infant does not begin showing symptoms of the disease until sufficient HbF has been replaced by HbS so that sickling can occur

SYMPTOMS OF SICKLE CELL• Sickle cell disease is characterized by lifelong episodes of pain
(crises), chronic hemolytic anemia and increased susceptibility to infections usually beginning in early childhood.
• Other symptoms include: acute chest syndrome, stroke and splenic and renal dysfunction.
• The lifetime of a HbS is approx 20 days, compared with 120 days for normal RBC
• Heterozygotes have one normal and one sickle cell gene (HbAS). These individuals have sickle cell trait and usually do not show symptoms like patients with sickle cell disease and can have a normal life span.
• In the deoxygenated state, HbS aggregates, forms fibrous strands that reduce RBC membrane flexibility and promote sickling.


HbS
1.Sickled cells block the flow of blood in the narrow capillaries which leads to anoxia in the tissue causing pain and hemolytic anemia.2.High altitudes, increased pCO2, decreased pH and increased 2,3 BPG in erythrocytes increases chances of sickling.3.Treatment: adequate hydration, analgesics, aggressive antibiotic therapy, transfusion, hydroxyurea4.Side-effects: hemosiderosis (transfusion), infection, weak immune system

ADVANTAGE OF HETEROZYGOUS CARRIERS
• Heterozygotes for sickle cell gene are less susceptible to malaria caused by plasmodium falciparum because the RBC in heterozygotes have a shorter lifespan than normal, the parasite cannot complete the stage of its development.

HEMOGLOBIN C DISEASE
• Patients homozygous for hemoglobin C generally have a relatively mild, chronic hemolytic anemia

HEMOGLOBIN SC DISEASE• In this disease, some β-globin chains have the sickle cell mutation,
whereas other β-globin chains carry the mutation found in HbC disease.
• Patients with HbSC disease are doubly heterozygous (compound heterozygote) because both of their β-globin genes are abnormal, although different from each other.
• The clinical course of adults with HbSC disease differs from that of sickle cell disease, in which patients generally have painful crises beginning in childhood.
• They are usually well and undiagnosed until they suffer an infarctive crisis.
• This crisis often follows childbirth or surgery and may be fatal.

• In this disease, there are two point mutations occuring at the sixth position of the β-globin chain.
• On one beta-globin chain, valine replaces glutamate(S)
• On the other beta-globin chain, lysine replaces glutamate(C)

Thalassemias
A group of inherited hemolytic disorders characterized by reduced synthesis of one or more globin genes.
1. -thalassemia – mutation in one or all of 4 genes for the -chain. Silent carriers, -thalassemia-trait, hemoglobin H disease, hydrops fetalis (deletion)
2. -thalassemia – thalassemia major (cooley anemia) (o), thalassemia minor (1) (point mutation)

-Thalassemias
functional genotype -chain productiongenes
Normal 4 / 100%Silent carrier 3 / - 75%-thalassemia trait 2 -/ - 50%HbH 1 -/-- 25%
Hb Bart’s, 4 0 --/-- 0%(Hydrops fetalis)

METHEMOGLOBINEMIA (HbM)
• Methemoglobin is a form of hemoglobin in which the iron atom is in the more oxidized ferric (Fe3+) state rather than the normal ferrous (Fe2+) state.
• – Methemoglobin is not capable of binding oxygen, so it is normally reduced back to its functional state by an enzyme-mediated mechanism in the RBC.

• • Hereditary methemoglobinemia arises from a deficiency of the enzyme that catalyzes this reduction,
• NADH-cytochrome b5 reductase.• • This is a benign condition that causes patients to appear
cyanotic and have mild symptoms, such as• headache and fatigue.• • Acquired methemoglobinemia may occur in response to
Drugs like nitrites which oxidize hemoglobin to methemoglobin, producing cyanosis.

• Methemoglobinemias are characterized by chocolate cyanosis (a brownish-blue coloration of the skin and membranes) and chocolate colored-blood as a result of the dark-colored methemoglobin

FUNCTIONS OF PROTEINS• As enzymes• Transport of small molecules and ions e.g., hemoglobin is a carrier of
oxygen while lipoproteins are carriers of lipids.• Structural elements: e.g.,• 1. cell membrane: glycoproteins• 2. skin and bone: collagen.• Hormonal regulation:• 1.some hormones are protein in nature e.g., insulin• 2. cellular receptors that recognize hormones are proteins.• Defense mechanism:• 1. antibodies (immunoglobulins) are protein in nature• 2. keratin found in skin and other tissues is protein that protect against
mechanical and chemical injury.• Blood clotting: coagulation factors (factors 2, 7, 9 & 10)• Storage: ferritin, which is the storage form of iron.• Control of genetic expression: activators, receptors and many regulators of
genes are protein in nature.