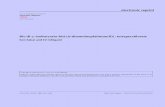
Exp 6 Calculation Preparation of bis(acetylacetonato)copper(II)
1
1st equation can be written in 2 forms a. ionic equation: Cu 2+ + 4NH 3 -> Cu(NH 3 ) 4 2+ b. chemical equation: Cu(NO 3 ) 2 + 4NH 3 -> Cu(NH 3 ) 4 (NO 3 ) 2 2nd equation Cu(NH 3 ) 4 2+ + 2NO 3 - + 2C 5 H 8 O 2 -> C 10 H 16 CuO 4 + 4NH 3 + N 2 + 3O 2 From the equations above, we can conclude that: 1 mol of Cu 2+ produces 1 mol of Cu(NH 3 ) 4 2+ and 1 mol of Cu(NH 3 ) 4 2+ produce 1 mol of C 10 H 16 CuO 4 no of mol of copper(II) nitrate = = y mol y mol of Cu(NO 3 ) 2 = y mol of C 10 H 16 CuO 4 Theoretical mass of C 10 H 16 CuO 4 = y mol x 263.764 g/mol = z g Percentage Yield =
-
Upload
john-baptist-john-bosco -
Category
Documents
-
view
191 -
download
6
description
Science
Transcript of Exp 6 Calculation Preparation of bis(acetylacetonato)copper(II)

1st equation can be written in 2 formsa. ionic equation: Cu2+ + 4NH3 -> Cu(NH3)4
2+
b. chemical equation: Cu(NO3)2 + 4NH3 -> Cu(NH3)4(NO3)2
2nd equationCu(NH3)4
2+ + 2NO3- + 2C5H8O2 -> C10H16CuO4 + 4NH3 + N2 + 3O2
From the equations above, we can conclude that:1 mol of Cu2+ produces 1 mol of Cu(NH3)4 2+
and
1 mol of Cu(NH3)4 2+ produce 1 mol of C10H16CuO4
no of mol of copper(II) nitrate =
= y mol
y mol of Cu(NO3)2 = y mol of C10H16CuO4
Theoretical mass of C10H16CuO4 = y mol x 263.764 g/mol = z g
Percentage Yield =


![Tris(ethylenediammonium) bis[(2-aminoethyl)ammonium] bis ...journals.iucr.org/e/issues/2010/05/00/wm2326/wm2326.pdf · Tris(ethylenediammonium) bis[(2-amino-ethyl)ammonium] bis[bis(l](https://static.fdocuments.us/doc/165x107/5e49e7fe0e042522d772f14a/trisethylenediammonium-bis2-aminoethylammonium-bis-trisethylenediammonium.jpg)