Evaluation ofSeverity in Aplastic Anemia by MR Imaging
Transcript of Evaluation ofSeverity in Aplastic Anemia by MR Imaging

J Korean Radiol S∞ 1999;40:347-354
Evaluation ofSeverity in Aplastic Anemia by MR Imaging l
Jeong Mi Park, M.D. , Gye Yeon Lim, M.D. , Euy Neyng Kim, M.D. , Jae Mun Lee, M.D. DongWool‘ Kim, M.D. 2, Chi Wha Han, M.D. 2, Chun Choo Kim, M.D.2
Purpose : To evaluate the role of bone marrow (BM) magnetic resonance (MR) imaging for assessment ofthe severity of aplastic anemia(AA).
Materials and Methods : Eighty patients with AA, ranging in age from 16 to 44 years underwent MR imaging. Fifty four patients had clinically severe AA(SAA), while in 26 the condition was moderate(MAA). Sagittal Tl -weighted images (Tl WI) and short tau inversion recovery (STIR) images of lumbar vertebral BM were analysed. Bulk Tl , T2 and rho values (msec) were also measured, with mixed sequences.
Signal intensity (SI) on both Tl WI and STIR was classified into four patterns according to the amount of fatty marrow : pattern 1 , homogeneous fatty marrow ; II , fatty marrow with focal cellular nodules; m, mixed fatty and cellular marrow ; N, cellular marrow with focal fatty nodules. These SI patterns and bulk Tl , T2 and rho values ofthe lumbar BM were compared with the clinical severity of AA.
Results: On both Tl WI & STIR sequences, MR imaging oflumbar vertebral BM in patients with AA showed various SI patterns.
Pattern 1 , II and m were much frequently seen in the SAA group (48 of 54 patients on Tl WI and 43 of 54 on STIR) and pattern N was common in the MAA group(16 of 26 patients on Tl WI and 18 of 26 on STIR). The SI patterns of AA seen
on both Tl WI and STIR sequences closely correlated with clinical severity(x2 test, p=O.OOOI)
Bulk Tl value was significantly different between SAA and MAA(SAA: 382.82 msec ::!::: 113.91; MAA: 517.99msec ::!::: 15 1. 92; t test, p=O.OOOl).
Conclusion : The SI pattern seen on MR imaging, and Tl relaxation time of lumbar spinal BM can be useful for assessing the severity of AA.
Index words : Bone marrow, MR Anemia, aplastic
Aplastic anemia (AA) refers to a diverse group ofpotentially severe marrow disorders characterized by peripheral pancytopenia and marrow that is largely devoid of hematopoietic cells; while retaining the basic marrow architecture or stroma, these are replaced
IDepartment of Rad iology, St. Mary ’ s Hospital Catholic Medical College The Catholic University
' Department of Intern al Medicine, St. Mary’s Hospital Catholic Medical College The Catholic University Received August 4, 1998; Accepted October 30 , 1998
Address reprint requests to : Jeongmi Park, M.D., Department of Radiology, 5t.
Mary ’s Hospita l The Catholic Un iversity, I 62, Youido-dong, Yongdungpo-gu 5eoul, Korea. Tel. 82-2-3779-127 1 Fax.82-2-783-5288 E-mai l. jmpark @cmc.cuk .ac.kr
by large amounts offat(I, 2). Once diagnosis of AA is firm, the most important
task is to assess its severity; the treatment of choice for severe AA (SAA) under the age of 20 is bone marrow transplantation, while moderate AA (MAA) is treated conservatively or with antilymphocytic globulin(I , 2). Although bone marrow (BM) biopsy is the standard method for assessing BM cellularity in AA, this is difficult to quantitate accurately and may not be representative ofthe entire BM(3 - 5).
Magnetic re5onance(MR) imaging has been proposed as a noninvasive method for the evaluation of BM
깎
1 J

Jeong Mi Park. et al : Evaluation of Severity in Aplastic Anemia by MR Imaging
composition(6 - 14). High fat content of the marrow results in high signal intensity (SI) on Tl weighted images(Tl WI), allowing the noninvasive evaluation of cellularity in patients with AA (7, 8, 10 - 12, 14- 16).
We prospectively evaluated the role of BM MR imaging in assessing the severity of AA.
Materialsand Methods
In 80 patients with AA (44 men and 36 women) MR imaging was used for prospective evaluation. Their average age was 27 (range, 16 - 44) years. BM aspirates and biopsy specimens were taken from the posterior iliac crest within 6 weeks (mean 8 days) of the MR imaging study. Marrow cellularity was determined from the biopsy and estimated to the nearest 10 % by a clinical pathologist. In peripheral blood , absolute neutrophil counts(ANC), corrected reticulocyte count (CRC) and platelet count (PC) were determined.
AA has been classified as severe when at least two of the following three criteria are present: (1) anemia with a CRC < 1 percent ; (2) ANC < 500 per cubic millimeter; and (3) PC < 20,000 mm3
• In addition, marrow hypocellularity must account for less than 25 percent of marrow space( l, 2). By these criteria our patients were classified as 54 SAA and 36 MAA.
On the basis of hepatic and renal function, and immunologic and cytogenetic studies, underlying diseases causing pancytopenia were excluded. Not all patients received a blood transfusion or underwent immunotherapy.
A 8
MR imaging was performed using a O. 5 Tesla MR unit(Gyroscan T5, Philips, Netherlands). Sagittal T1 weighted(TR/TE=560/30msec) and short tau inversion recovery (STIR, TR/TE/TI, 1,400/30/1 20 msec) images of the lumbar spine were obtained . Image parameters were 5mm thickness with a 0.5-mm intersection gap, 205 X 256matrix, four signal acquisitions, and a 350 mm field ofview onall pulse sequences. In 68 patients, coronal Tl WI of the pelvis (TR/TE 560/30 msec) with 6mm slice thickness and 0.6mm intersection gap was obtained.
Two bone radiologists evaluated the SI pattern on T1-weighted and STIR images, and reached a consen-sus.
On T1 WI, diffusely homogeneous high SI was classÍfied as pattern 1, hyperintense background with less than 25 % hypointense nodules as pattern 11, extensive homogeneous bright and low SI as pattern III, and diffuse hypointense SI with scattered bright nodules less than 25 % as pattern IV. On STIR with reversed SI, pattern 1 included diffusely homogeneous low SI, pattern II hypointense background with less than 25 % of bright SI nodules, pattern III mixed patchy bright and low signal areas, and pattern IV diffusely high SI with less than 25 % hypointense area.
According to SI patterns seen on T1 WI and STIR, BM was categorized as follows : 1, homogeneous fatty marrow; II, fatty marrow with focal cellular nodules; III, mixed fatty & cellular marrow; 값ld IV, cellular marrow with focal fatty nodules(Figs. 1, 2, 3 & 4), On T1-weighted images, low signal was less than or equal
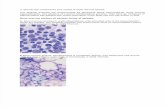
Fig. 1. Pattern 1, completely homogeneous fatty marrow, in a 32-yearold woman with severe AA. A. Sagittal Tl -weighted MR image (560/30) of the lumbar spine reveals homogeneous bright signal intensity in the vertebral bone marrows. B. Sagittal STIR image (1, 400/30 /1 20) of the lumbar spine reveals reversed homogeneous low signal intensity.
- 348 -

J Korean Radiol Soc 1999; 40 : 347-354
to the signal of paravertebral muscle, and high signal was equal to or greater than the signal of subcutaneous fat. On STIR images with fat suppression, marrow SI was rated as high or low in relation to the paravertebral muscle. Patterns 1, 11, 111 were indicative of a marked decrease of BM cellularity, and pattern IV as a moderate decrease.
In the pelvis, the pattern of SI was classified as L homogeneous fatty marrow, or 11, fatty marrow with
A B
A 8
variable cellular nodules(Fig. 5) Spin density(rho), and spin-lattice and spin-spin
relaxation time (Tl and T2, respectively) of BM were measured using a mixed sequence(17) : a multi-echo spin echo sequence interleaved with a multiple-echo inversion recovery sequence(TE/TRSE/TI/TRIR 50/ 760/3 1O/2,290msec) of lumbar spine sagittal images. This sequence automatically provided calculated Tl and T2 images in which SI was directly proportional to
Fig. 2. Pattern II, fatty marrow with focal cellular nodules, in a 44-yearold woman with severe AA. A. Sagittal Tl -weighted MR image (560 /30) of the spine shows inhomogeneous bright signal intensity with low signal nodules in the vertebral bone marrows B. Sagittal STIR image (1, 400/30 /1 20) shows hypointense background with variable bright portions in the vertebral bone marrows.
Fig. 3. Pattern IIL mixed fatty and cellular marrow in large regions , in a 29-year-old man with severe AA A. Sagittal Tl -weighted MR image (560/30) of. the spine demonstrates patchy bright and low signal intensity in large regions of the vertebral bodies. B. Sagittal STIR image (1, 400/30/120) clearly delineates reversed large bright and dark areas in the vertebral bodies.
- 349 -

Jeong Mi Park. et al : Evaluation of Severity in Aplastic Anemia by MR 1m행ing
T1 and T2 values. The number of acquisitions for this sequence was two. Values for rho, T1 and T2 were measured by placing a cursor over a region of interest (4 to 9 single voxels) in the vertebral bodies ofT11-L5.
These S1 patterns and rho, T1 & T2 values oflumbar spines were compared with the clinical severity of AA.
Kendall ’s correlations and the chi-square and t test were used for statistical analysis.
Results
The MR imaging findings ofBM in patients with AA showed variable patterns(Table 1).
On Tl W1, the majority of patients with SAA (48 of 54) displayed a hyperintense background with a variable amount of low signal nodules(patterns 1, II and m). The other six patients with SAA showed pattern 1V. On STIR, major patterns in SAA (43 of 54 patients) were 1, II, and III. On T1 W1 and ST1R, most MAA cases (16 of 26, and 18 of 26, respectively) showed pattern 1V. The S1 patterns on T1 W1 and STIR images were significantly different between SAA and MAA(Table 2). Clinical severity was accurately predicted by T1W1 in 80 % of cases, and by STIR images in 76 %.
There was relatively good correlation between S1 patterns seen on T 1 W1 and STIR images and cellularity
A B
Fig. 4. Pattern IV, cellular marrow with focal fatty nodules, in a 31-yearold woman with moderate AA. A. Sagittal Tl-weighted MR image (560/30) of the spine shows diffuse hypointense background with mottled bright foci in the vertebral bone marrows. B. Sagittal STIR image (1,때0/30/120) demonstrates diffusely high signal intensity with small foci of low signals in the vertebral bone marrows.
A B Fig. 5. Pelvic marrow patterns on coronal T 1 weighted MR image(560/30) A. Bone marrow ofthe pelvis shows diffuse homogeneous high signal intensity (Pattern 1) in a 31-year old woman wÍth severe AA. B. 34-year-old woman with moderate AA shows hyperintense background bone marrows with multiple foci oflow signal intensity(Pattern II)
m ”

J Korean Radiol Soc 1999; 40 : 34? - 354
seen after BM aspiration and biopsy(Table 3). On TIWI, BM in the pelvis was almost invisible or
showed a variable amount of cellular nodules with a hyperintense background offatty marrow ; this was ir respective ofthe clinical severity of AA(Table 4).
Rho, Tl and T2 values are shown in Table 5. Bulk Tl values were significantly different between SAA and MAA.
Discussion
Bone marrow aspiration and/or biopsy (usually of the posterior iliac crest) remains the primary means of examining BM. Areas of normal or even increased hematopoiesis may, however, coexist with hypocellular fatty marrow ; iliac crest sampling alone might, therefore, not reflect the true state of BM function. In each sampling, the degree ofpancytopenia varied slightly.
MR imaging is complementary to BM biopsy in that, although the former shows only the gross anatomy of
Table 1. Spinal Bone Marrow Patterns on T 1 WI and STIR in Patients with Aplastic Anemia
_____________ Pattern --------------- • “““ “ II III IV Total
Sequence ~
Tl WI STIR
24 31 26 19
22 80 29 80
3
6
1 : Homogeneous fatty marrow II : Fatty marrow with focal cellular nodules m : Mixed fatty and cellular marrow N : Cellular marrow with focal fatty nod ules STIR : short tau inversion recovery
Table 2. Comparison of Spinal Bone Marrow Patterns on Tl WI and STIR with Clinical Severity in Patients with Aplastic Anemia
Pattern TlWI STIR
Severity ~~ 1, 11, III IV 1, II, III IV
Severe 48 6 43 11
h‘oderate 10 16 8 18
58 22 51 29
x2, p=O.OOOl
the marrow, it can sample a large fraction of active marrow in a single, noninvasive, clinical study(5 - 13). Our results may suggest that MR imaging ofBM might be a suitable noninvasive method for estimating the cellularity of the entire marrow and classifying the severity of AA.
In normal adults, the distribution of hematopoietic and fatty marrow relative to age has been extensively described(18 - 22). Since the predominant sites of cellular marrow untillate in life are the lumbar spine, the pelvis, and the intertrochanteric area ofthe femur, these areas were included in our study.
Our protocol included sagittal Tl-weighted and STIR imaging ofthe lumbar spine and coronal Tl WI of the pelvis, areas in which there is, in adulthood , a reservoir of red marrow. Short Tl relaxation time (higher SI) of the fatty marrow allows noninvasive assessment by Tl WI of the amount and distribution of those tissues. STIR produces images characterized by yellow marrow SI being nulled, and therefore appearing
Table 3. Comparison of Spinal Bone Marrow Patterns on Tl WI & STIR with Cellularity in BM Biopsy in Patients with Aplastic Anemia
Pattern TlWI STIR
Cellular~ ________ 1, II, III IV 1, IL III IV
Below 25 % 53 10 46 17 Above 25 % 5 12 5 12
58 22 51 29
Kendall’ s correlation, r=0.56 on T1 WI and 0.48 on STIR, p=O.OOOL BM: bone marrow STIR : short tau inversion recovery
Table 4. Comparison of Pelvic Bone Marrow Patterns on Tl WI with Clinical Severity in Patients with Aplastic Anemia
_______________ Pattern ~ ranern II Total
Severity ~
Severe Moderate
%
m -%
경 4
-잉
깅 νm
-%
x2, p=0.015
Severity Pattern T1 *
TableS. Mean Values ofTL T2 and Rho (msec) in Patients with Aplastic Anemia
T2 Rho
Severe Moderate
382.82 :t 113.91 517.99 :t 15 1.90
* t-test, p=O.OOO 1
71.41 :t 14.83 80.22 :t 23.49
349.29 :t 91.38 35 1.1 2 :t 61.72
X
μ

Jeong Mi Park. et al : Evaluation of Severity in A미astic Anemia by MR 1m행ing
b1ack(13, 23 - 24). T1 and T2 contrast of tissues other than fat are additive, and contrast sensitivity is thus
greatly enhanced. The fat signa1 is highlighted on Tl WI and the cellu1ar portion is emphasized on STIR ; each patient cou1d therefore be classified as pattern 1, II or III on T1 WI and as IV on STIR. In predicting the
clinical severity of AA, the accuracy of T 1 WI and STIR varied on1y slightly(80 % and 76.2 %, respectively).
The sensitivity of MR imaging to marrow disease can be improved by a quantitative separation of fat and water signa1s, which is possib1e on chemica1 shift images and lH MR spectroscopy( 25 - 30).
In AA, MR imaging findings and the role of MR imaging in monitoring patients with AA to determine their response to therapy have been reported(8 - 9, 11 - 15, 27, 31). In untreated stages, marrow dem
onstrates diffuse high S1, reflecting the preponderance offatty marrow and the 1ack ofhematopoictic marrow.
With treatment, foci of 10w signa1 intensity representing is1ands ofhematopoietically active tissue appear in the yellow marrow. Amano and Kumazaki (32) classi
fied BM patterns in ap1astic anemia but did not evaluate the re1ationship between BM patterns and the clini
ca1 severity of AA. It is, in addition, important to be aware ofthe exist
ence of foca11ow S1 areas of hematopoiesis in the marrow ofpatients with AA; these hematopoietic areas are thus not confused with areas of fibrosis , metastatic deposits, or other patho1ogic processes(14, 15, 33).
According to Negendak et al., the detection of either foca1 or diffuse 10ss of bright signa1 of aplastic marrow, in the absence of hemosiderosis, may provide evidence for clona1 disease(31). They did not, however, consider
the matter ofintermixed hematopoietic marrow within fatty marrow. 1n our patients, variab1e initia1 S1 and S1
patterns classified according to the amount of remaining or hyperplastic marrow, and fatty marrow estimated on T1 W1 and STIR images, correlated closely with the clinica1 severity of AA.
T1 and T2 relaxation times of BM in hemato1ogic disorders and norma1 vo1unteers have been measured
(11 , 12, 18, 29, 33-35). Long Tl re1axation time of water-rich hematopoietic tissue may be influenced by marrow fat , which has a shorter T1 va1ue. Some resear chers have reported a correlation between cellu1arity and T1 re1axation time in patients with benign and malignant infiltrative disease of vertebra1 marrow(11). Others, howe
were 10wer than normal marrow T1 values measured by others(17, 18). SAA and MAA revea1ed statistically
significant differences in T1 va1ues. Potentia1 sources of error in the determination of T1 relaxation time in
clude imperfections in slice profile, variable sampling points for calcu1ations of T1 , inhomogeneity in the static magnetic field over the R01s, and inhomogeneities in the RF pu1se(34).
1n conclusion, S1 patterns, as seen on MR imaging, and the T1 relaxation time of BM can be usefu1 for assessing the severity of AA.
References
1. Rappeport JM, Bunn HF. Bone marrow failure: A까astic anemia
and other primary bone marrow disorder. In: Wilson JD eds. Harrison' s princψle of internal medicine. 12th ed. Vo l. 2. New York: McGraw-Hill, 1991: 1568-1570
2. Camitta BM. Thomas ED, Nathan DG. et a1. Severe aplstic anemia: a prospective study of the effect of early bone marrow σanspl따ltation on actue mortality. Blood 1976; 48 : 63-70
3. Nissen C. The pathophysiology of aplstic anemia. Semin Hem
atol 1991; 28(4): 313-318 4. Keisu M, Ost A. Diagnoses in patients with severe pancytop
enia suspected of having aplastic anemia. Eur J Haematol 1990; 45: 11-14
5. Hartsock RJ. Srnith EB, Petty CS. Norrna1 variations with aging of the arnounts of hematopoietic tissue in bone marrow frorn the anterior iliac crest. Am J Clin Pathol 1965; 43 : 326-331
6. Cohen MD. Klatte EC, Baehner R, et a1. Magnetic resonance imaging of bone marrow disease in children. Radiology 1984; 151: 715-718
7. Wisrner G. Rosen BR, Buxton R, Stark DD, Brady TJ. Chernical shift imaging of the bone marrow: preliminary experience. AJR
1985; 145: 1031-1037 8. Olson DO, Shields AF, Scheurich CJ, Porter BA, Moss AA.
Magnetic resonance imaging of the bone marrow in patients with leukemia. aplastic anemia, and lymphoma. Invest Radiol
1986; 21 : 540-546 9. Kangarloo H, Dietrich RB, Taira. RT, et al. MR imaging of bone
marrow in children. J Comput Assist Tomogr 1986 ; 10: 205-209 10. Daffner RH, Lupetin AR, Dash N, Deeb Z, Sefczek RJ, Schapiro
RL. MRI in detection of malignant infiltration of bone marrow. AJR 1986; 146: 353-358
11. Nyman R. Rhen S. Glimelius B. Magnetic resonance imaging in diffuse malignant bone marrow disease. Acta Radiol 1987; 28 199-205
12. Mckinstry CS, Steiner RE. Young AT, Jones L. Swirsky D .. Aber v. Bone marrow in leukemia and aplastic anemia: MR imaging before, during. and after treatment ‘ Radiology 1987; 162: 701-707
13. Vogler JB IIT, Murphy W A. Bone marrow imaging. Radiology
1988; 168: 679-693 14. Steiner RM. Mitchell DG. Rao VM, Schweitzer ME. Magnetic
resonance imaging of diffuse bone marrow. Radiol Clin North
Am 1993;31 :383-409 15. Kaplan PA. Asleson RJ. Klassen LW, Duggan MJ. Bone marrow
patterns in aplastic anemia:observations with 1.5-T MR irnaging. Radiolo.‘gy 1987; 164: 441-444
m “

J Korean Radiol Soc 1999 ;40:347- 354
16. Mitchell DG, Burk DL Jr, Vinitski S, Rifkin MD. Thy biop
hysical basis of tissue contrast in extracranial MR imaging. AJR
1987; 149: 831-837
17. BC Vande Berg, J Malghem, 0 Devuyst, BE Maldague, MJ Lam
bert. Anorexia nervosa: Correlation between MR appearance of
bone marrow and severity of disease. Radiology 1994; 193
859-864
18. Dooms Gc, Fisher MR, Hricak H, Richardson M, Crooks LE, Genant HK. Bone marrow imaging: Magnetic resonance studies
related to age and sex. Radiology 1985; 155: 429-432
19. Kricun ME. Red-yellow marrow conversion: its effect on the 10-
cation of some solitary bone lesions. Skeletal Radiol 1985; 14:
10-19
20. Dunnill MS, Andersion JA, Whitehead R. Quantitative histo
logical studies on age changes in bone. J Pathol Bacteriol 1967;
94 ‘ 275-291
21 ‘ Ricci C, Cova M , Kang YS , et al. Normal age-related patterns of
cellular and fatty bone marrow distribution in the axi꾀 skel
eton: MR imaging study. Radiolo,밍 1990;177 ’ 83-88
22. Moore Sc, Dawson KL. Red and yellow marrow in the femur
Age related changes in appearance at MR imaging. Radiology
1990; 175 : 219-223
23. Bydder GM, Young IR. Clinical use of the partial saturation and
saturation recovery sequences in MR imaging. J Comput Assist
Tomogr 1985; 9 : 1020-1032
24. Bydder GM, Young IR . MR imaging: Clinical use of the
inverson recovery sequence. J Comput Assist Tomogr 1985; 9
659-675
25. Dixon WT. Simple proton spectroscopic imaging. Radiolo.정
1984; 153: 189- 194
26. Brateman L. Chemical shift imaging:A rev iew. AJR 1986;146 ’
971-981
27. Rosen BR, Fleming DM, Kushmer DC, et 허. Hematologic bone
marrow disorders: Quantitative chemical shift MR imaging
Radiology 1988; 169 : 799-804
28. Guckel F, Brix G, Semmler W , et 따. Systemic bone marrow
disorders: characterization with proton chemical shift imaging.
J Comput Assit Tomogr 1990; 14: 633-642
29. Jensen KE, Jensen M , Grundtrig P, Thomsen C, Karle H, Hen
riksen O. Localized in vivo proton spectroscopy of the bone
marrow in patients "With leukemia. Magn Reson Imaging 1990; 8
: 779-789
30 ‘ Ballon D, Jakubowski A, Gabrilove J , et al. In vivo measure
ments of bone marrow cellularity using volume-Iocalized pro
ton NMR spectroscopy. Magn Reson Med 1991 ; 19: 85-95
31. Negendak W , Weissman , D, Bey TM, et al. Evidence for clonal
disease by magnetic resonance imaging in patients with hypop
lastic marrow disorders. Blood 1991; 78(11): 2872-2879
32. Amano Y, Kumazaki T. Proton MR imaging and spectroscopy
evaluation of aplstic anemia: three bone marrow patterns. J
Comput Assist Tomogr 1997; 21 : 286-292
33. Smith SR, Willams CE, Davies JM, Edwards RHT. Bone marrow
disorders: characterization with quantitative MR imaging. Radi
ology 1989; 172: 805-810
34. Jensen KE, Srensen PG, Christoffersen P, Henriksen 0 , Karle H.
Magentic resonance irnaging of the bone marrow in patients
with acute leukemia during and after chemotherapy. Acta
Radio11990;31 :361-369
35. Depaoli L, Davini 0 , Foggetti MD, et 꾀 Evaluation of bone
marrow cellularity by magnetic resonance imaging in patients
with myelodysplastic syndrome. Eur J Haematol 1992; 49 :
105-107
m
끽

Jeong Mi Park. et al : Evaluation of Severity in Aplastic Anemia by MR 1m행ing
대한밤시선의학호|지 1999;40:34?-354
자기공명영상을 이용한 재생불량성 빈혈의 중증도 평가1
l가톨릭대학교 의과대학 방사선과 2가톨릭대학교 의과대학 내과
박정미 · 엄계연 · 검의녕 · 이재문 · 검동욱2. 한치화2. 검춘추2
목 적 : 재생불량성 빈혈환자(aplstic anemia, AA)의 중증도(severity)를 평가하는데 있어서 골수 자기공명
(MR)영상의 역할을 알아보고자 하였다.
대상 및 방법 · 중증 재생불량성 빈혈 (severe AA, SAA) 54명, 경증 재생불량성빈혈 (moderate AA, MAA) 26명
총 80명의 재생불량성 빈혈환자의 요추부골수 MR영상소견을분석하였다.남자 44명,여자 36명이었고연령분
포는 16세에서 44세로 평균 27세였다.
요추골수의 시상면 T1 강조 빛 단시간반전회복(STIR) 영상에서 골수의 신호강도를 분석하였고 mixed se
quence를 이용하여 요추골수의 T1, T 2 이완시간 및 양자밀도이완시간을 측정하였다.
지방신호강도의 양에 따라 골수의 신호강도를 4가지 유형으로 분류하였는데 유형 I 은 균일한 지방골수를 보
언 경우, 유형 n는 지방골수내에 부분적으로 세포성골수를 갖는 경우, 유형 m은 지방골수와 세포성골수가 균
일하게 분포하는경우,유형 W는세포성골수내에 부분적으로지방골수가있는경우로하였다.
요추골수의 T1 강조 및 STIR 영상에서 이들의 신호강도 유형과 T1, T 2 및 양자밀도 이완시간을 재생불량성
빈혈의 임상적 중증도와 비교하였다.
결 과 : T1 강조 및 STIR 영상에서 골수의 신호강도는 상당히 다양한 유형으로 관찰되였으며 54명의 SAA 환
자중 48명은 T1장조영상에서 유형 1 , IT , m의 신호강도를 보였으며 STIR 영상에서 43명이 유형 1 , IT , m로
관찰되 었다. 반면 유형 W는 MAA환자에서 좀더 흔하게 나타나서 T1 강조영상에서는 26명중 16명, STIR 영상
에서는 18명이 유형 N로관찰되였다.
이들 T1 강조 및 STIR 영상에서 관찰되는 골수신호강도의 유형은 재생불량성 빈혈의 임상적 중증도와 통계
학적으로 유의한 차이를 보였다(x2 test, p=O.OOO1). T1 이완시간은 질병의 엄상적 중증도와 통계학적으로 유의
한 차이를 보였다(SAA ; 382.82msec::!: 113.91, MAA ; 517.99msec::!: 151.92, t test, p=O.OOO1).
결 론 ; 골수 MR 영상의 신호강도 유형 및 T1 이완시간은 재생불량성 빈혈의 임상적 중증도를 평가하는데
유용하였다.
않