Evaluation Of Methods For Measurement Of Cellular Microparticles & Exosomes In Human Disease...
-
Upload
samantha-bowman -
Category
Documents
-
view
213 -
download
0
Transcript of Evaluation Of Methods For Measurement Of Cellular Microparticles & Exosomes In Human Disease...

Evaluation Of Methods For Measurement Of Cellular Microparticles & Exosomes In Human Disease
Introduction
•Circulating blood contains a variety of cell membrane microparticles (MPs) derived from many different cell types, including platelets, white cells, red cells and endothelial cells.
•Cellular particles are divided into two types:
Microparticles (100nm – 1M), released from cells during activation or apoptosis.
Exosomes or nanoparticles (40nm – 100nm) which are formed from internalised endocytic vesicles that are subsequently secreted from the cell.
•MPs are involved in cell to cell interactions and cell signalling, possibly by transferring functional molecules between different cell types.
•MPs have been implicated in a large number of vascular pathologies where they have been found to be elevated, raising the possibility of their use as potential prognostic and diagnostic markers for numerous disease states.
•Their use as biomarkers for pre-eclampsia, haemostatic disorders and cancer is currently being investigated as part of the BRC programme.
Dragovic, R.Aa, Harrison Pb, Sheldon Hc, Harris Ac, Ratoi Ma, Dobson Pa and Sargent I.L.a
aWomens’ Health Theme (NDOG & Dept of Engineering Science),
bBlood Theme (Oxford Haemophilia & Thrombosis Centre), cCancer Theme (WIMM)
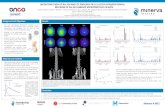
ELECTRON MICROSCOPY
Current Methods of DetectionELISA
FLOW CYTOMETRY Analysis carried out on biological samples. Multiple markers can be used to simultaneously distinguish particles of different cellular origins. Disadvantages: Limit of sensitivity is ~200nm. Cannot be used to detect small MPs or exosomes
Ultra centrifugation of biological samples is used to pellet cellular particles and separate them from soluble proteins. Antibodies specific for the particles of interest are used to capture the particles on the ELISA plate. Disadvantages: Only one particle marker can be studied at one time and this method cannot discriminate between MPs and exosomes.
C NP PE0
25
50
75
***
*****
ST
BM
(ng
/ml) Total placental MP levels
measured in the plasma of non-pregnant (C), normal pregnant (NP) and pre-eclamptic (PE) women using a specific ELISA
Electron microscopy of exosomes from cultured U87 tumour cell line
Ultracentrifugation of culture supernatants or plasma to pellet cellular particles allows visualisation by electron microscopy. Disadvantages: Subjective, not quantitative and only limited phenotyping possible.
LSRII analysis of a Mixture of 190nm, 400nm and 660nm polystyrene particles
660nm
190nm
400nm
660nm190nm
400nm
Pre-eclamptic plasma double labelled with Annexin V-APC and CD61-FITC
0.9% 2.2%
96.1% 0.8%
200nm
Becton Dickinson LSR-II Flow Cytometer

Resolves particles between 5nm – 75M in the same sample by density gradient sedimentation. Disadvantages: time taken to analyse samples
CPS DISC CENTRIFUGE
CPS disc centrifuge analysis of pre-eclamptic (PE), normal pregnant (NP) and non-pregnant (C) plasma.
New TechnologyNANOSIGHT
Nanoparticle Tracking Analysis (NTA) is a light scattering method for determining particle size and concentration of monodisperse and polydisperse samples. Accurately sizes particles based on analysis of their Brownian motion. Dynamic range of 20nm – 1M.
Beckman Coulter N5 Submicron Particle Analyser
DYNAMIC LIGHT SCATTERING
Sample Name: STBMComments: Operator: Erol Uman SOM / SOP name: Fingerprint.somTemperature: 25.0°C Diluent: WATERStart Time: 10-Apr-08 01:56:35 PM End Time: 10-Apr-08 02:13:09 PM
Angle:Run Time:
30.1 °200 s
62.6 °200 s
90.0 °200 s
Different Angle Unimodal Distribution Results for Repetition 1
70 2370 370 670 970 1270 1570 1870 2170
Size (nm) [linear]
30.1°62.6°90.0°
Inte
nsit
yIn
tens
ity
Size [nm] Linear
30º60º90º
DLS analysis of Placental (Syncytiotrophoblast) MPs
DLS sizes particles through analysis of their Browninan motion. The N5 Submicron Particle Analyser has a dynamic range of 3nm – 5M. Disadvantages: Samples must be of a very narrow size range. Unable to accurately size highly polydisperse samples.
238nm
Placental (Syncytiotrophoblast) MPs
189nm 390nm 659nm
Mixture of 190nm, 400nm and 660nm polystyrene particles
50nm
50nm polystyrene beads
Conclusions
PE Plasma
NP Plasma
C Plasma
100nm 300nm
Mixture of 100nm and 300nm polystyrene beads
Mixture of 100nm and 300nm polystyrene beads post filtration
100nm
0.2M Filter
Normal Pregnant Plasma
150nm
Pre-eclamptic Plasma
87nm
Non Pregnant Plasma
84nm
• These methods are currently being evaluated as effective tools to measure MPs and exosomes in human disease.
• NTA is a new robust technique that accurately determines particle size and concentration. It has many advantages in resolving particle size distribution profiles which are not possible with other current methodologies.