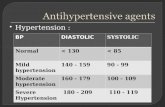
Erythrocytic protein kinase C activity in primary hypertension
Transcript of Erythrocytic protein kinase C activity in primary hypertension

Journal of Internal Medicine 1998; 243: 299–305
© 1998 Blackwell Science Ltd 299
Erythrocytic protein kinase C activity in primary hypertension
P. EK a , R. TOOMIK a , S. ERIKSSON a , G. FRITHZ c , G. RONQUIST b & L. ENGSTRÖM a
From the aDepartment of Medical Biochemistry and Microbiology, Biomedical Centre, University of Uppsala, and bDepartment of Clinical Chemistry,University Hospital, Uppsala, and the cDepartment of Internal Medicine, Central Hospital, Eskilstuna, Sweden
Abstract. Ek P, Toomik R, Eriksson S, Frithz G,Ronquist G, Engström L (University of Uppsala andUniversity Hospital, Uppsala; and Central Hospital,Eskilstuna, Sweden). Erythrocytic protein kinase Cactivity in primary hypertension. J Intern Med 1998;243: 299–305.
Objectives. Increased protein kinase C activity hasbeen reported in erythrocytes from patients with pri-mary hypertension and also from hypertensive rats.In this phenomenological study, we investigatedwhether a possible increased activity was the resultof an augmented amount of enzyme molecules or amore active enzyme.Design. Collect blood samples, separate erythrocytesfrom other blood cells. After partial purification ofprotein kinase C in the erythrocyte lysate, assay theenzyme activity under optimal conditions using aspecific peptide substrate.Setting. Central Hospital in Eskilstuna and UniversityHospital in Uppsala, Sweden.Subjects. Healthy individuals: 47 persons (20 women
and 27 men). Ten patients with untreated primaryhypertension.Main outcome measures. Erythrocytes were separat-ed from leucocytes and platelets by passing through acellulose column followed by repeated washings.Some proteins in the erythrocyte lysate interferingwith protein kinase C assay were removed by chro-matography on DEAE-cellulose.Results. The mean protein kinase C activity in ery-throcytes from healthy individuals was0.18 6 0.02 pmol [32P]phosphate min21 3 106 cells,regardless of sex and age. The corresponding valuefor patients with primary hypertension was0.16 6 0.04 pmol [32P]phosphate min21 3 106 cells.Conclusions. The amount of protein kinase C, mea-sured as the activity at optimal assay conditions, inerythrocytes from patients with primary hyperten-sion is not critical for the development of moderatehypertension.
Keywords: erythrocyte, hypertension, protein kinaseC.
Introduction
In spontaneously hypertensive rats and patients withprimary hypertension, intracellular Ca21 concentra-tion has been reported to be increased comparedwith controls [1–4]. Protein kinase C (EC 2.7.1.37),which is activated by lipids and – with regard to thegroup of conventional isozymes – also by Ca21, playsa major role in the regulation of several biologicalfunctions in various cell types [5]. Amongst manyother cells, the human erythrocyte is a source of pro-tein kinase C [6], the function of which is, however,obscure in this cell. Platelets have been shown tocontain the highest activity of the enzyme amongstseveral different types of mammalian cells or tissuesinvestigated [7].
In 1986 Takaori et al. reported that the activity of
protein kinase C in platelets from 12- and 20- week-old spontaneously hypertensive rats was considerablyincreased compared to that of age-matched nor-motensive rats [8]. When the blood pressure ofhypertensive rats was decreased or normalized withthree different types of antihypertensive drugs, theactivity of protein kinase C in the platelets was signif-icantly reduced and approached that of plateletsfrom normal rats [9].
Protein kinase C activity in the lysate of erythro-cytes of patients with primary hypertension and ofspontaneously hypertensive rats was similarlyincreased 1.6–2.0-fold compared to normotensivecontrols [10]. No notable changes in protein kinase Cactivity were revealed in erythrocytes from patientswith renal (secondary) hypertension. The activity ofthe enzyme was also claimed to be elevated in other
JINT255F

P. EK et al.300
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
tissues from hypertensive rats, e.g. brain [11] andaorta [12].
The relationships amongst the cytosolic-free Ca21,protein kinase C and the Na1/H+1 antiport in prima-ry hypertension have been discussed in a recentreview article [13]. Further study of enzyme activityin erythrocytes from hypertensive patients comparedto normotensive controls was of interest, becauseerythrocytes are easily available and very little hasbeen published on the subject. The activity of theenzyme should be proportional to the number ofenzyme molecules, if assayed under optimalconditions. However, the method for assaying proteinkinase C had to be modified because of the presenceof protein inhibitors and/or other proteins in crudetissue extracts which disturb enzyme assay and makeit unreliable, especially when histones are used asexogenous substrates. Such compounds might partlybe removed by DEAE-cellulose [see 14 for furtherreferences]. By using the synthetic peptide firstdescribed by Yasuda et al. [15], which is a specificsubstrate particularly for conventional protein kinaseC isozymes, the effect of the inhibitors can also becounteracted [14]. Further, because of the highprotein kinase C activity in platelets [7], it wasnecessary to remove the platelets from the erythro-cytes as completely as possible.
Thus, in the present paper the activity of proteinkinase C of erythrocytes from healthy individualsand from patients with untreated primary hyper-tension was assayed under strictly controlledconditions where the activity should be proportionalto the amount of the enzyme. The aim was to esti-mate the activity of the enzyme in erythrocytes fromhealthy individuals of different age and sex, andcompare that with the activity in cells from untreat-ed patients with hypertension. In contrast to whathas been described earlier, no increased enzymeactivity was detected in erythrocytes from hyperten-sive patients.
Methods
This study was approved by the ethical committee ofthe University of Uppsala, and informal consent wasgiven by the patients.
Materials
The compound g2[32P]ATP was purchased from
(Amersham, UK). Sigma (USA) supplied 1,2-dioleinand L-a-phosphatidyl-L-serine. CF-11 cellulose,diethyl amino ethyl cellulose (DE-52) and 3 MM paperwere obtained from Whatman (England). Percoll gelwas purchased from Kabi Pharmacia (Sweden) andthe peptide pGlu-Lys-Arg-Pro-Ser-Gln-Arg-Ser-Lys-Tyr-Leu from Peninsula Laboratories (England). Allother chemicals were of the highest purity commer-cially available.
Patients analysed for protein kinase C in erythrocytes
Ten hypertensive men (mean age 44.0, range35–61 years), mean body mass index 26.5 (range24.9–29.2) (kg m22) and seven normotensive malecontrol subjects were enrolled in the study. The crite-rion of hypertension was a diastolic blood pressure(DBP) of $ 105 mmHg recorded in the supine posi-tion after 10 min rest on at least three different occa-sions during the last three months. None of thepatients had received any antihypertensive drugs.There were no clinical or laboratory signs of sec-ondary hypertension. No one had any complicatingdisease and, in particular, there were no evidences ofdiabetes mellitus or impaired renal function. Meanserum cholesterol level was 5.9 (range 5.2–6.3)mmol L21. Alcohol abuse was denied and a meanserum g-glutamyltransferase value of 0.30 mkat L21
was found (range 0.20–0.46, the upper normal limitbeing 0.80 mkat L21). A family history of hyperten-sion was apparent in eight of the patients. Three ofthem had a history of both parents being hyperten-sive, whilst in another three, one of the parents washypertensive. For two other patients the parents wereat present normotensive with no ongoing anti-hypertensive medication. The mean systolic bloodpressure (SBP) was 166.5 (range 160–185) mmHgand the mean DBP was 106.9 (range 105–110)mmHg.
Control subjects
The control individuals were normotensive men andall had a diastolic blood pressure of , 90 mmHg onat least two occasions. They had a mean body massindex of 25.8, and were in excellent health as judgedfrom their medical history and routine laboratoryinvestigations.
At the time of patient(s) blood sample collection acontrol was always taken, and then the samples were

PROTEIN KINASE C IN HYPERTENSION 301
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
transported together from Eskilstuna CentralHospital to Uppsala. The analysis of both sampleswas done in parallel.
Preparation of erythrocytes
To obtain the data on the normal level of proteinkinase C activity, both in platelets and erythrocytes,blood was also obtained from healthy blood donorsfrom the blood centre of the University Hospital ofUppsala. It was collected in vacutainer/venojecttubes containing EDTA and stored at 14°C for18–24 h. It was ascertained that the activity oferythrocyte protein kinase C remained on the samelevel during 72 h, if untreated blood was stored at14°C. One volume of whole blood was mixed withone volume of buffer A (10 mmol L21 potassiumphosphate, pH 7.4/150 mmol L21 NaCl) containing0.1 mmol L21 EDTA. In order to remove platelets andleucocytes, this suspension was passed through a15 mL cellulose CF-11 column [16], equilibrated inbuffer A/0.1 mmol L21 EDTA and eluted with thesame buffer on the same day using a flow rate of0.7 mL min21. The eluate was collected in 10 mLtubes containing 2 mL buffer A/5 mmol L21 EDTA.After mixing, the cells were centrifuged at 310 3 gfor 10 min. The washing procedure with 2 mL bufferA/0.1 mmol L21 EDTA was repeated twice. The cellswere then suspended in buffer B (50 mmol L21
Tris/HCl buffer, 0.1 mmol L21 EDTA, pH 7.4) to 4.5 3
106 cells mL21 (see below) and disrupted by freezingin liquid nitrogen and thawing, which was repeatedtwice immediately before the assay for protein kinaseC activity. After specific staining of the cell types andmicroscopic inspection, it was shown that it wasalmost exclusively erythrocytes in the suspension(see Results).
Determination of the number of blood cells
The number of erythrocytes, determined withCoulter Counter, was related to the concentration ofcyanmethemoglobin in the cell homogenate. It wasfound that 4.5 3 106 cells mL21 corresponded to anabsorbance of 0.410 in the cyanmethemoglobinassay at 540 nm. It was also ascertained that theMCHC varied little amongst the 10 hypertensivepatients, mean value being 339 g L21 6 8.7 (SD),range 326–348 g L21 (normal value 320–360 g L21).The MCHC for the healthy individuals and controlpersons was assumed to be within normal range.
Partial purification of protein kinase C from erythrocytes
The purification was carried out at 14°C. DE-52 gelwas equilibrated and suspended in buffer B so that750 mL suspension after centrifugation for 5 s at 12000 3 g in a 2 mL Eppendorf tube resulted in500 mL pelleted gel. After removal of the liquid,250 mL of the pelleted, frozen and thawed erythro-cytes was added, together with 750 µL of buffer B.The content was mixed with a glass rod and then,gently, by rotation for 15 min. It was centrifuged for5 s at 12000 3 g, the supernatant was removed andthe pellet was resuspended with 750 mL buffer B,rotated and centrifuged as before. After removal ofthe supernatant, 500 mL buffer B/167.5 mmol L21
NaCl was added to elute protein kinase C from the gel.After mixing with a glass rod, the tubes were incubat-ed without rotation for 15 min, centrifuged for 30 sat 12000 3 g and 400 mL of the supernatant wasremoved to new tubes, from which samples weretaken for immediate analysis of protein kinase C.
Partial purification of protein kinase C from platelets
Blood was collected in citrate vacutainer tubes fromhealthy individuals. Platelet-rich plasma had to beprepared within 30 min by centrifugation at roomtemperature at 150 3 g for 15 min in order to pre-serve the protein kinase C activity. An aliquot of theplatelet-rich plasma was removed for platelet count-ing in a Thrombocounter C®. The remaining platelet-rich plasma was immediately frozen in liquidnitrogen and analysed for protein kinase C activitythe same day, after another freezing and thawingcycle. It had to be diluted with at least 4 volumes ofbuffer B also containing 1 mmol L21 dithiothreitol, toavoid inhibition by components in the plasma (datanot given).
Protein kinase C assay
The assay was performed as described before [17]using the protein kinase C specific peptide pGlu-Lys-Arg-Pro-Ser-Gln-Arg-Ser-Lys-Tyr-Leu, first used byYasuda et al. [15]. Six samples from each preparationwere tested, both in the presence and absence of pep-tide. The reaction product was applied onto 2 3 2 cmpieces of phosphocellulose paper, which were washedwith 75 mmol L21 ice cold phosphoric acid as in [17].It was ascertained that the reaction was interruptedwhilst it was still linear. The values were found to bestable over time.

P. EK et al.302
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
Statistics
The activity values obtained were statisticallyprocessed using Student’s t-test (95% confidencelevel). The data are shown as mean value 695% con-fidence limits (CI).
Results
Separation of erythrocytes from white blood cells andthrombocytes
Whole blood was passed through a cellulose column[16]. Approximately 20% of the platelets coelutedwith the erythrocytes. These were almost totallyremoved by repeated washings of the cells, but theremainder amounted to about 0.5% of the initialplatelet count. The obtained purified erythrocyte sus-pension contained approximately 4.5 3 1012 ery-throcytes L21.
Protein kinase C activity in purified platelets fromhealthy individuals
There was no difference between females(11.7 6 2.4 pmol [32P]phosphate min21 and 106 cells,11 subjects tested) and males (10.7 6 2.4 pmol[32P]phosphate min21 and 106 cells, nine subjects test-ed) displaying a mean value of 11.2 6 1.6 pmol[32P]phosphate min21 and 106 cells. Platelets were dif-ficult to handle, and possible artefacts in the proteinkinase C activity could depend on the method ofplatelet isolation. Therefore the patient study wasfocused on erythrocytes.
Protein kinase C activity in purified erythrocytes fromhealthy individuals
The mean protein kinase C activity amounted to0.03 pmol min21 3 106 cells (Fig. 1, background sub-tracted) when measured directly in the erythrocytelysate. The lysate was separated on a DEAE- celluloseusing the conditions in reference 14 to get an indica-tion of the presence of proteases, phosphatases, com-petitive substrates and/or inhibitors in theerythrocyte. The protein kinase C activity that elutedbetween 50 and 150 mmol L21 NaCl (Fig. 1, assays 3and 4, blood samples collected from one person) washigher than the activity measured directly in the celllysate, which supported this hypothesis. Data pre-sented in Fig. 1 show that the protein kinase C activi-ty amounted to 0.10 pmol min21 3 106 cells after
subtraction of the phosphorylation in the absence ofpeptide (endogenous phosphorylation), a value morethan three times higher than the activity measureddirectly in the cell lysate. A batch-wise separation incombination with step-wise elution was elaboratedoriginating from the chromatography on DEAE-cel-lulose in order to get maximal protein kinase C activi-ty. It was also found that the enzyme activity wasproportional to sample volume.
The activity of protein kinase C was determined inhealthy females and males using the partially puri-fied protein kinase C (see above) from erythrocytes.An age- and sex-distribution of the activity is givenin Fig. 2 and the corresponding mean values aregiven in Table 1. As is apparent from Fig. 2 and Table1, no statistically significant difference existedbetween the sexes, nor did age influence the activity.The mean normal value was 0.18 6 0.02 pmol[32P]phosphate incorporated min21 and 106 erythro-cytes. For one male the protein kinase C activity wasdetermined 10 times during half a year. The valueswere found to be stable over time (0.12 6 0.02 pmol[32P]phosphate incorporated min–1 and 106 cells). Theinterassay variability was found to be negligible. The
Fig. 1 Bar graph shows the activity of protein kinase C inerythrocytes from healthy individuals. Assays 1 (n 5 2) and2 (n 5 4) were performed using red cell lysate (RBC-L). Partlypurified protein kinase C (PP-PKC) from erythrocytes was used forassays 3 and 4 (n 5 4). Assays 1 and 3 (light bars) were performedin the absence of pGlu-Lys-Arg-Pro-Ser-Gln-Arg-Ser-Lys-Tyr-Leu(endogenous phosphorylation) and assays 2 and 4 (dark bars) inthe presence of peptide. Samples for assays 3 and 4 were takenfrom one person; n denotes the number of samples tested.

PROTEIN KINASE C IN HYPERTENSION 303
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
contaminating platelets contributed to the erythro-cytic protein kinase C activity by 1.5%, as calculatedfrom the amount of platelets present in purified ery-throcyte fraction and the platelet protein kinase Cactivity (see above).
Activity of protein kinase C in patients with primaryhypertension
We determined the protein kinase C activity in ery-throcytes from patients suffering from primaryhypertension. The samples had to be transported for4 h and therefore the blood from a matching controlwas simultaneously transported and tested. Proteinkinase C activity in erythrocytes of 10 patients was
0.16 6 0.04 pmol [32P]phosphate min21 and 106
cells, in erythrocytes of seven control persons0.20 6 0.07 pmol [32P]phosphate min21 and 106
cells. The results are included in Fig. 2. There was nosignificant difference in protein kinase C activitybetween controls, patients and healthy individuals(the latter shown in Table 1). Moreover, the values forthe normotensive controls and the correspondingpatients were compared by paired Students’s t-testand no difference was found.
Discussion
The calcium distribution pattern is disturbed in ery-throcytes from patients with untreated primaryhypertension [18, 19]. This could be the result of analtered plasma membrane permeability for calciumions and a greater exchangeable pool of cytosolic-free calcium in these cells [18, 19]. The free calciumconcentration has likewise been claimed to be elevat-ed in platelets of patients with untreated primaryhypertension [1]. Primary hypertension has beenattributed to an abnormality in the balance or distri-bution of the sodium ion leading to an increasedsodium content in various cells including erythro-cytes [20, 21]. Despite a widely recognized correla-tion between sodium metabolism and hypertension,the underlying mechanisms at a molecular level havenot been elucidated [13]. Some circulating factorscould, however, be involved, such as endocrine orparacrine substances, e.g. endothelins.
The aim of the present work was to assay the pro-tein kinase C activity in erythrocytes under optimalconditions with a linear relationship between activityand amount of enzyme solution added to estimateany possible difference in the amount of the enzymebetween untreated patients with primary hyperten-
Fig. 2 Scatterplot shows the activity of partially purified proteinkinase C in erythrocytes of healthy individuals, patients withprimary hypertension and normotensive controls. Open symbolscorrespond to patients. m Females 20–25 years old (n 5 12); j females . 45 years old (n 5 8); . males 20–25 years old(n 5 8); d males 40–45 years old (n 5 12); h patients (n 5 10);and r controls (n 5 7).
Table 1 The activity of partially purified protein kinase C in human erythrocytes from healthy individuals
pmol [32P]phosphate min213 106 cells
Parameter 20–25 years 40–45 years .45 years All
Females 0.16 6 0.03 0.16 6 0.02 0.16 6 0.02(n 5 12) (n 5 8) (n 5 20)
Males 0.18 6 0.06 0.20 6 0.03 0.20 6 0.03(n 5 8) (n 5 12) (n 5 20)
Females and 0.18 6 0.02males (n 5 40)
Values are expressed as mean 6 95% CI.

P. EK et al.304
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
sion and normotensive controls to pinpoint an aetio-logical molecular basis for this disease. The activitymeasured under these strictly controlled conditionsshould be proportional to the number of enzymemolecules. The reproducibility and stability of theanalytical procedure used in this study allowed us toconclude that no clear-cut difference between thesetwo groups could be observed. There might still be adifference between healthy individuals and hyperten-sive patients in vivo, since enzyme activity is modifiedby several factors in a complicated fashion, e.g. thepresence of inhibitors and different degrees of activa-tion by calcium ions and lipids, which additionallyinfluence the intracellular localization of the enzyme.
Since we found about 60 times higher enzymeactivity per cell in platelets than in erythrocytes(under optimal conditions in the absence of endoge-nous inhibitors) it was important to separate thesecells from each other as completely as possible. Beforethe assays of the enzyme in erythrocytes, it was par-tially purified in order to remove inhibitors and otherinterfering compounds, such as phosphatases andproteases.
The activity of protein kinase C in erythrocytesfrom healthy individuals was determined for bothyounger and elderly females and males, the valuebeing 0.18 pmol min-1 and 106 erythrocytes. It wassomewhat surprising that there was no difference inthe amount of enzyme in erythrocytes from all theseindividuals regardless of sex or age. Furthermore, nodifference was found between this group and patientswith primary hypertension. The reason there was noincrease of enzyme in the erythrocytes from thepatients as reported by others [10] is difficult to state.In the report by Kravtsov et al. [10] the patients had adiastolic pressure with a range 110–135 mmHg,which was distinctly higher than that for the patientsin our study (range 105–110 mmHg). Hence, thedegree of hypertension could be involved. Because ofa well-developed out-patient care system, individualswith untreated higher blood pressure than thoseused in our study are exceedingly rare in Swedishhospitals and, consequently, not available for investi-gation. In addition, the difference between ourresults and those in the study of Kravtsov et al. couldalso be the result of the diversity in the experimentalmethods used. For instance, it is not mentioned intheir report whether the erythrocytes were checkedfor contamination with other blood cells, e.g.platelets, which we invariably found nearly impossi-
ble to remove by the simple washing procedure theyused. Furthermore, histones, which are unspecificsubstrates, were used as an exogenous substrate inthe assay of protein kinase C, whilst we used a specif-ic peptide.
We therefore conclude that the amount of proteinkinase C, measured as the activity at optimal assayconditions, in erythrocytes from patients with prima-ry hypertension is not critical for the development ofmoderate hypertension.
Acknowledgements
This study was supported by grants from the SwedishMedical Research Council (No. 13X–50), from theSödermanland County Council and the HållénFoundation (Skandinaviska Enskilda Banken).
References1 Erne P, Bolli P, Burgisser E, Buhler FR. Correlation of platelet
calcium with blood pressure: effect of antihypertensive thera-py. N Eng J Med 1984; 310: 1084–8.
2 Sugiyama T, Yoshizumi M, Takaku F, Urabe H, Tsakakoshi M,Kasuya T. The elevation of the cytoplasmic calcium ions invascular smooth muscle cells in SBR: measurement of the freecalcium ion in single living cells by laser microfluorospectrom-etry. Biochem Biophys Res Commun 1986; 141: 340–45.
3 Copper RS, Shamsi N, Katz S. Intracellular calcium and sodi-um in hypertensive patients. Hypertension 1987; 9: 224–9.
4 Lechi A, Lechi C, Bonadonna G, Sinigaglia D, Corranidi P,Polignano R et al. Increased basal and thrombin-induced freecalcium in patients with essential hypertension. Hypertension1987; 9: 230–35.
5 Blobe GC, Stribling S, Obeid LM, Hannun YA. Protein kinase Cisoenzymes: regulation and function. Cancer Surveys 1996;27: 213–48.
6 Palfrey HC, Waseem A. Protein kinase C in the human ery-throcyte. J Biol Chem 1985; 260: 16021–9.
7 Minakuchi R, Takai Y, Yu B, Nishizuka Y. Widespread occur-rence of calcium-activated, phospholipid-dependent proteinkinase in mammalian tissues. J Biochem 1981; 89: 1651–4.
8 Takaori K, Itoh S, Kanayama Y, Tekeda T. Protein kinase Cactivity in platelets from spontaneously hypertensive rats(SHR) and normotensive Wistar-Kyoto rats (WKY). BiochemBiophys Res Commun 1986; 141: 769–73.
9 Takaori K, Inariba H, Itoh S, Inoue T, Kanayama Y, Takeda T.Chronic antihypertensive drug treatment decreases proteinkinase C activity in platelets from SHR. Clin Exp Hypertens1990; 12: 1063–75.
10 Kravtsov GM, Dulin NO, Postnov YV. Activity of protein kinaseC in erythrocytes in primary hypertension. J Hypertension1988; 6: 853–7.
11 Kravtsov GM, Dulin NO, Postnov YV. Protein kinase C activityin brain tissue of spontaneously hypertensive rats.Byulleten’Eksperimental’noi Biologii i Meditsiny 1989; 108:42–4.

PROTEIN KINASE C IN HYPERTENSION 305
© 1998 Blackwell Science Ltd Journal of Internal Medicine 243: 299–305
12 Murakawa K, Kohno M, Yasunari K, Yokokawa K, Horio T,Takeda T. Possible involvement of protein kinase C in the main-tenance of hypertension in spontaneously hypertensive rats. JHypertension 1988; 6: S157–9.
13 Aviv A. Cytosolic Ca21, Na1/H1 antiport, protein kinase C trio inessential hypertension. Am J Hypertens 1994; 7: 205–12.
14 Ekman P, Eller M, Ragnarsson U, Engström L. Two methods toavoid the effect of endogenous protein inhibitors during theassay of protein kinase C activity in tissue extracts. PrepBiochem 1992; 22: 165–75.
15 Yasuda I, Kishimoto A, Tanaka S, Tominaga M, Sakurai A,Nishizuka Y. A synthetic peptide substrate for selective assay ofprotein kinase C. Biochem Biophys Res Commun 1990; 166:1220–27.
16 Beutler E, West C, Blume K-G. The removal of leukocytes andplatelets from whole blood. J Lab Clin Med 1976; 88: 328–33.
17 Humble E, Heldin P, Forsberg P-O, Engström L.Phosphorylation of human fibrinogen in vitro by calcium-acti-vated phospholipid-dependent protein kinase from pig spleen. JBiochem 1984; 95: 1435–43.
18 Ronquist G, Frithz G. Decreased 45calcium uptake in red cells ofpatient with primary hypertension. Acta Med Scand 1988; 224:445–9.
19 David-Dufilho M, Astarie C, Pernollet A-G, Del Pino M,Levenson J, Simon A, Devynck M-A. Control of the erythrocytefree Ca21 concentration in essential hypertension. J Hypertens1992; 19: 167–74.
20 Friedman SM. Cellular ionic perturbations in hypertension. JHypertens 1983; 1: 109–14.
21 Losse H, Wehmayer H, Wessels F. Der Wasser undElektrolytgehalt von Erythrocyten bei arterieller Hypertonie.Klin Wochenschr 1960; 38: 391–8.
Received 18 May 1997; accepted 11 September 1997.
Correspondence: P. Ek, Department of Medical Biochemistry andMicrobiology, Biomedical Centre, University of Uppsala, Box 575,S–751 23 Uppsala, Sweden (fax: 146 18 471 49 75; e-mail:[email protected]).