Energy. DEFINITION the ability to cause a change common measurement unit = Joule (J)
Energy Energy is the ability to do work or transfer heat. Units of Energy: -Joule (J) 1 J = 1 kg-m 2...
-
Upload
augustus-parker -
Category
Documents
-
view
214 -
download
0
Transcript of Energy Energy is the ability to do work or transfer heat. Units of Energy: -Joule (J) 1 J = 1 kg-m 2...

EnergyEnergy is the ability to do work or
transfer heat.
Units of Energy:-Joule (J) 1 J = 1 kg-m2/s2

EnergyKinetic Energy: Energy of an object in motion
Potential Energy: Energy of an object with respect to the position of the object in relation to another object

EnergyChemical Energy: Energy produced or absorbed by the formation or breakage of chemical bonds.
Thermal Energy: Heat Energy

Law of Conservation of Energy
The energy can NOT be created or destroyed.

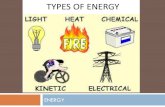
Some Types of EnergyKinetic
Thermal (the internal energy of an object due to the kinetic energy of its atoms and/or molecules)Mechanical (the energy associated with motion and position of an object)
PotentialChemical (energy due to chemical reaction)Gravitational (object can “fall”)Elastic (energy stored as a result of deformation of an elastic object)

Chemical Energy Graphs
Exothermic RxnΔ H is negativeHeat is released
Endothermic RxnΔ H is positiveHeat is absorbed

Conservation of EnergyEnergy cannot be created or destroyed.When we see energy change, it is not lost, just transferred, perhaps converted to another type of energy

Heat EnergyTemperature = Measurement of the average kinetic energy of molecules in a substance.Heat = Energy that is transferred from one substance to another.Internal energy = Total amount of energy a substance contains. (Most I.E. is kinetic.)More molecular movement = more kinetic energy = higher temperature

Temperature measurement
Temperatures are measured using the Celsius and Kelvin scales.Kelvin is based on the amount of energy in a substance. At 0 Kelvin, there is no movement, no kinetic energy. This temperature is called absolute zero.Recall: K = C + 273.

Transfer of HeatCan occur by1. Conduction2. Convection3. Radiation
Is measured as a temperature change in a substance.Heat is measured in Joules (like work).

Conduction is heat energy transferred when one substance comes in contact with another.
Metal spoon in boiling liquidDepends on collision between molecules of a substance.

Radiation is the transfer of energy by infrared waves.
Sun warming your skin: The molecules in your skin vibrate faster when struck by IR waves.For radiation, matter is not needed to transfer heat energy.Radiant energy is any energy transferred by radiation.

Convection is heat energy transferred by movement of a fluid.Ex: Warm air from a wood-burning stoveA convection current is the pattern of heat energy movement. Warm fluid expands and is less dense than surrounding fluid: Warm rises and cool sinks.Convection currents continue to form as long as there is a heat source.

CaloriesAnother way to measure heat1 calorie = 4.186 J1 calorie = the amount of heat needed to raise 1 g of water 1°C.Food Calories are actually kilocalories.When calorie is spelled with capital C, it is a food calorie.A resting 150-lb person gives off heat at a rate of ~1200 Calories in 24 hours.

Thermal Properties of MaterialsLocal surface temperatures on the Earth’s
surface depend on:Reflectivity
Is the proportion of radiation that is reflectedReflected energy does not raise temperature
Specific heat capacityQuantity of thermal energy needed to raise the temp of 1 g of a material by 1°C
Quantitative application: q = mcΔT

Specific heatTo calculate the energy transferred to or by a substance, use this formula:
q = mcΔT
q = energy in Jm= mass in gramsc = specific heat for the substance (J/g-°C)ΔT = change in temperature (tfinal – tinitial) in °C

Specific Heat Capacities (Cp) for Selected Materials at 20°C
Material Cp (Joules/g-°C)
Air 1.00
Water (l) 4.18
Carbon dioxide 0.839
Aluminum 0.902
Brass 0.380
Copper 0.386
Gold 0.126
Iron 0.448
Lead 0.128
Silver 0.233
Zinc 0.387
Granite 0.79
CaCO3 0.852
Stainless steel 0.51
Glass 0.84
Ice (-10°C) 2.05
Ethyl alcohol 2.45
Asphalt 0.92
Sandy clay 1.38
Quartz sand 0.83
Concrete 0.88
Tipler, Paul A., Physics for Scientists and Engineers, 4th Ed., W.H. Freeman, (1999). & engineeringtoolbox.com

Enthalpy: ΔH
• Enthalpy is the amount of heat content used or released in a system at constant pressure
• Mathematically:Sum of ΔH products – sum of ΔH
reactants = ΔH for the reaction (ΔHrxn)

Enthalpy ExampleChemical reactions: When bonds are broken, the energy in those bonds is available to be used in the products or is released as heat. Example = combustion reaction, such as for glucose:
C6H12O6 + 6O2 6 CO2 + 6H2O ΔH = - 2814 kJ
ΔH = 6(ΔHfCO2) + 6(ΔHfH2O) –(ΔHC6H12O6) -6(ΔHfO2) (values from table)
ΔH = 6(-393) + 6(-286) –(-1260) -6(0) = -2814 kJ
Recall that the negative ΔH means that 2803 kJ are released in the combustion of 1 mole of glucose.ΔH is negative: This is an exothermic reaction.

Heat and MatterAdding heat energy increases the motion of a substance’s molecules.Phase changes occur when energy changes.When ice melts, the temperature changes only when ice has melted.

Thermal ExpansionThermal expansion occurs when heat causes the molecules of a substance to spread out.Occurs in solids, liquids and gases.Examples include:
Roads and bridges in the hot sunBoiling liquids Air in a tire (After driving, the friction increases the heat and tire expands.)
Exception: Water expands as it cools between 4°C and 0°C.

Phase ChangesConsider water to remember the words for most phase changes:
Ice
Water
Steam
SOLID
LIQUID
GAS
Melt
Evaporate
Freeze
Condense
Note that all phases still water (H2O). These are PHYSICAL changes.

Phase Changes, Cont.Another word for changing to the gaseous state is vaporization.Vaporization includes evaporation (molecules leave the liquid’s surface) AND sublimation (solid to gas, such as dry ice, CO2)

Physical and Chemical Changes
1st test: Is something new made?Does the new substance have a different chemical formula than the original substance? If yes, then it is a chemical change.Examples of new substances:
Gas (bubbles)Energy (heat/light)Precipitate (solid – may be a different color)
Remember- If the substance only changes phase, it is a physical change.















![Toolbox 4 ENERGY UNITS, CONVERSIONS, THERMO- … · Toolbox 4 ENERGY UNITS, CONVERSIONS, ... Length Meter m Mass Kilogram kg ... [J] Joule is a SI unit of work or energy.](https://static.fdocuments.us/doc/165x107/5abf57957f8b9a8e3f8e26aa/toolbox-4-energy-units-conversions-thermo-4-energy-units-conversions-.jpg)


