Dye diffusion in human dentine
-
Upload
dj-anderson -
Category
Documents
-
view
216 -
download
1
Transcript of Dye diffusion in human dentine

Arch. oral Biol. Vo1.7, pp.505-512. 1962. Pergamon Press Ltd. Printed in Ct. Britain.
DYE DIFFUSION IN HUMAN DENTINE
D. J. ANDERSON and G. A.‘RONNING*
Physiological Laboratory, Guy’s Hospital Medical School,
London S.E. 1
Abstract-With freshly extracted teeth, observations on the diffusion of dyes from an exposed surface of dentine inwards to the pulp and from the pulp outwards, have shown that cutting the dentine can reduce the permeability of the tubules compared with that of tubules exposed by fracturing the dentine. Possible causes of this difference in permeability have been investigated. The significance of these observations to experiments on sensation in teeth is discussed.
INTRODUCTION
THE EXPERIMENTS to be reported were performed during an investigation into the
mechanism of sensation in human dentine. In experiments in which various sub-
stances were applied in solution to dentine (ANDERSON and RONNING, 1962) it was
found that solutions with high osmotic pressures could cause pain and it seemed
possible that this might be due to the stimulation of a receptor mechanism in dentine
or pulp by a sudden fluid disturbance transmitted through the dentinal tubules.
Whatever the nature of the receptor mechanism may be, the experiments of ANDERSON, CURWEN and HOWARD (1958), ANDERSON and RONNING (1962) and ANDERSON and
NAYLOR (1961, 1962) have shown that it is not excited as easily as would be expected
were it composed of nervous elements in the dentinal tubules so placed that stimulating
solutions had easy access when applied to the dentine. Before concluding that such nervous elements are not present, it seemed desirable to find out whether the insensi-
tivity of dentine to such substances as acetylcholine and potassium chloride, which
are known to evoke pain elsewhere, might be due to failure of the stimulating solutions
to reach nervous elements, rather than to the absence of such elements from the
dentine. With this object in view, experiments were designed to investigate the
permeability of dentinal tubules using various dyes in aqueous solution.
PART I
METHODS AND MATERIALS
In view of the connexion between these experiments and an investigation into sensation in dentine, the main objective was to study the diffusion of dye through
dentinal tubules opened by the method of cavity preparation employed in that investigation, namely cutting the dentine with a slow-running tungsten carbide bur
under a stream of cold water. However, although experiments on sensation necessarily
* Present address: University of Oregon Dental School, Sam Jackson Park, Portland 1, Oregon, U.S.A.
505

506 D. J. ANDERSON AND G. A. RONNING
take place in vivo, it was not considered practicable to test dye diffusion in vivo and therefore all experiments on dye diffusion took place on extracted teeth. In two
experiments the dentine was exposed before extraction and the teeth were placed in dye immediately after extraction. In the majority of experiments, however, both exposure of the dentine and dyeing took place after extraction and the teeth were kept
in 0.9 g/l00 ml NaCl during the period of i-2 hr which elapsed between extraction of
the teeth and commencement of the experiment. In addition to a slow-running
tungsten carbide bur under a stream of cold water, two other methods of exposing dentine were employed. These were cutting with a Carborundum disc under a stream
of cold water and fracturing with a hammer and chisel. In the latter method a shallow
groove was cut into the enamel round the base of a cusp with a diamond disc to limit the quantity of tissue to be removed by the chisel. The object of exposing dentine by
this means was to avoid blockage of the tubules or other changes in the tubule contents which might result from cutting the tissue. The slow-running tungsten carbide bur
was used for exposure of dentine both in vivo and in vitro; the other two methods
were employed only in vitro. All the experiments were conducted on sound premolar
teeth removed for orthodontic reasons.
Diffusion was studied from an exposed surface of dentine inwards towards the
pulp cavity and from the pulp cavity outwards. To study dye diffusion from the
surface inwards, dentine was exposed by cutting or by fracturing and the crown of the
tooth was immersed for 30 min in a concentrated solution of dye in distilled water. The pulp contents and remainder of the tooth were left intact for this experiment.
At the end of the 30 min the tooth was removed from the dye, rinsed in water, dried
and then bisected longitudinally in the bucco-lingual plane. No attempt was made to
control the concentration of dye, because in every experiment, except one, conclusions
about dye diffusion were based on a comparison between different areas of the same
tooth exposed to the same concentration of dye solution.
To study diffusion from the pulp cavity outwards, the root was cut off just below
the cemento-enamel junction and the coronal pulp tissue was removed. Then the
main bulk of the tooth tissue of the crown mesial and distal to the pulp chamber was
cut away with a Carborundum disc under a stream of water, leaving a thin plate of dentine above and surrounding the pulp chamber and a thin cap of enamel sur- mounting the dentine (see Fig. 1). Crystals of dye were then inserted into the empty
pulp chamber and moistened with a small quantity of distilled water. Modelling clay
placed over the apical end of the pulp cavity prevented evaporation of the dye
solution. Observations of dye diffusion through the tubules, and the effect on it of opening the tubules at their peripheral portions by cutting or by fracturing, could be
made with a microscope and transmitted light. Various dyes have been used; acidic, basic and of both large and small molecular
weights, but all are commonly described as “vital staining dyes”. Those used were
methyl blue, methylene blue, crystal violet, trypan blue, Evans blue, brilliant cresyl blue, patent blue V and vital new red. Since in early experiments the behaviour of all
seemed to be similar, most of the experiments were conducted with Evans blue (T 1824) with a molecular weight of 960.

DYE DIFFUSION IN HUMAN DENTINE 507
PULP CAVITY
FIG. 1. Diagram of tooth crown prepared for observation of diffusion of dye from pulp cavity outwards. The stippled areas show the portions of the tooth removed.
RESULTS
Inward difSusion of dye through cut and fractured dentine Forty teeth were used in this series in which dentine was exposed in vitro by cutting
off one cusp with a carborundum disc under water and fracturing the other with a
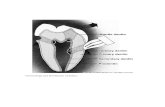
chisel. Fig. 2 shows a premolar crown bisected after treatment with Evans blue dye
for 30 min. Whatever the dye used, it was consistently found that inward penetration
occurred freely through fractured dentine but not through cut dentine. For this
reason, it was possible by fracturing both cusps of an upper premolar tooth to use one
as a control while investigating possible causes of the apparent block to diffusion by
applying various treatments to the other.
Outward dijiision of dye through cut and fractured dentine Figs. 3 and 4 show two preparations made in the manner already described and
cleared in phenol for photography. After its insertion into the pulp chamber the dye
rapidly diffused towards the periphery, but appeared to fall short of the amelo-
dentinal junction and the ends of tubules that had been cut with a Carborundum
disc (see Fig. 3). The dye appeared to reach the ends of tubules opened by fracturing
(see Fig. 4).
Inward dijksion of dye through dentine cut with a cooled tungsten carbide but Since the procedure followed in experiments on sensation was to expose dentine
with cooled slow-running tungsten carbide burs, it was necessary to find out whether this had any effect on dye diffusion. In nineteen freshly extracted sound premolar
teeth a small cavity was cut through the occlusal.or buccal surface to expose dentine and one cusp was removed by fracturing. The crown was then immersed in dye for 30 min. Diffusion occurred across all the fractured control surfaces and across ten of the surfaces exposed by cutting with a bur. Of the remaining nine cut surfaces there was no diffusion across six and only slight diffusion across three.

508 D. .I. ANDERSON AND G. A. RONNING
Inward diffusion of dye through dentine cut with a cooled tungsten carbide but-, before extraction of the teeth
Cavities were cut in nine.sound premolar teeth in vivo using a water-cooled slow- running tungsten carbide bur; the teeth were then extracted and immediately placed
in dye for 30 min. Dye diffusion did not occur in three, it was slight in four and well
marked in two teeth.
DISCUSSION OF RESULTS IN PART 1
It appears from these results that there is a range of permeability to dyes shown by dentine exposed by different methods in vitro and in vivo. This observation raises
doubts as to the accessibility of possible receptor mechanisms in the dentinal tubules
to materials applied to the dentine in experiments on sensation and may account for the variations in sensitivity shown between subjects. Further information about the
mechanism of sensation would be gained if dentine could be tested after exposure by
fracturing-an infrequent possibility, or if the apparent block to diffusion could be
either prevented or produced consistently. Therefore, further experiments were performed in an attempt to learn more about the mechanism of the block.
PART 2
METHODS AND MATERIALS
The effect of heat on dye difSusion To test the possibility that heat was the cause of the block, fifteen freshly extracted
premolars were divided into three groups of five and in every tooth one cusp was
removed by fracturing. A copper-constantan thermocouple junction was placed in
contact with the dentine, in the centre of the exposed area, and a jet of water at
various temperatures was played on to the area for 2 min; this being the time taken to cut off a cusp with a Carborundum disc. After this treatment the other cusp was
removed by fracturing and a jet of water at room temperature was played on it; then the crown was immersed in dye for 30 min. In all five teeth in which one cusp
was treated with water at 55”C, dye diffusion occurred through both heated and
control areas of dentine. After treatment with water at 7O”C, diffusion occurred in two but not in three of the heated areas, but when water at 85°C was applied to one
cusp, the whole tooth was hot to touch and dye diffusion failed to occur in both test and control areas in all five teeth.
It therefore seemed possible that heat was the cause of the block to dye diffusion, but this view is acceptable only if it could be shown that, in spite of the use of water for cooling purposes, the temperature rose sufficiently, i.e. between 70 and 85”C, at the interface between the tooth and the cutting instrument. Information in the
literature about the temperature of dentine immediately beneath a cutting instrument could not be found, since the interest of previous investigations has been in the effect of heat on the pulp tissue rather than the dentine. As is shown in Figs. 2 and 3,
the block to dye diffusion was restricted to a narrow band of dentine just below the cavity and for this reason a temperature recording instrument must occupy a similar position to provide data relevant to this problem.

DYE DIFFUSION IN HUMAN DENTINE 509
To record the temperature in dentine just in advance of a cutting instrument a
thermistor was used in the manner described by NAYLOR (1961). A premolar tooth was cut horizontally through the cemento-enamel junction and the coronal pulp
tissue was removed. The bead of a thermistor calibrated through the range 30-100 ‘C was placed in contact with the pulpal roof in a small recess cut with a round bur and
it was held in position with sticky-wax. The tooth and in-dwelling thermistor were
placed in a chamber surrounded on all sides except the top by a waterbath at 37°C. When the cavity was cut into the dentine from the occlusal surface, the bur was
advanced until the thermistor was destroyed or dislodged. The thermistor output was amplified and displayed on an oscilloscope screen. Five freshly extracted teeth were
used and in all of them a cavity was prepared with a slow-running tungsten carbide
bur: in four of them iced water was used during cutting, in the fifth no cooling was
attempted. In the four teeth cooled during cavity preparation the temperature of the
thermistor fell slightly soon after the experiment began and in two it remained at
about 25°C until the thermistor record ceased with contact with the bur. In the remaining two, there was a terminal rise in temperature to 42’C in one and 36’C in
the other just before contact. The record from the uncooled tooth showed a steady
rise in temperature throughout cavity preparation, reaching a maximum of 8S’C
just before the experiment ceased. Since water-cooling was employed during the
cutting of dentine with burs, it seems unlikely that the subsequent failure of dye diffusion was caused by heat.
The eflect of tooth debris on dye d$iision Another possible cause of the block to dye diffusion was mechanical sealing of the
tubules with tooth debris. To test this possibility eight freshlyextracted upper premolar
teeth were used and both cusps were removed by fracturing. A paste of powdered
dentine, enamel and water was rubbed on to one cusp surface with a slow-running
burnishing instrument applied intermittently for 3 min. The paste was kept moist and renewed at frequent intervals. No dye-diffusion occurred after this treatment in two
of the treated cusps and in the other six diffusion was much less than in the control
untreated cusps, occurring only through tubules at the margin of the treated area, close to the enamel as shown in Fig. 5. This region was not accessible to the rounded
burnisher.
This observation, however, did not rule out the possibility that friction alone without tooth debris might cause the block to diffusion. To investigate this possibility nine teeth were taken and both cusps were removed by fracturing. The exposed dentine of one cusp was subjected to a slow-running burnisher applied intermittently under
a stream of iced water for 3 min. The otherwise untreated cusp was subjected to the same stream of water. In all nine teeth the dye diffused through the untreated control
area, but no diffusion, or diffusion only at the edges, occurred through the burnished area.
From these experiments it seems clear that whatever precautions are taken in cutting dentine, a block to dye diffusion is a possible result. It might be possible to compare sensitivity with dye permeability in individual teeth, but this would require

510 D. J. ANDERSON AND G. A. RONNING
extraction of the teeth and the opportunities for this type of experiment, as also for
testing the sensitivity of freshly fractured dentine, must be rare. It was therefore
decided to make a different approach to the problem by trying to block the tubules consistently and to test their sensitivity before and after the blocking procedure. Since
cutting the dentine with a bur did not consistently produce a block to dye diffusion, it
was necessary to adopt some additional procedure. HOWE’S (1917) ammoniacal silver
nitrate when applied to dentine and followed with eugenol causes the formation of a precipitate of silver; it is a protein precipitant and is also said to diminish sensitivity
(GROSSMAN, 1934); accordingly this technique was chosen and tested.
The efSect of AgN03 and eugenol on dye dijksion For this experiment fourteen freshly extracted premolar teeth were used. Both
cusps were fractured in eight teeth and one surface was exposed to Howe’s silver nitrate for 2 min, followed by eugenol for 3 min. After drying, the whole crown was
immersed in dye for 30 min. In a further six teeth, one cusp was fractured and a
cavity was cut into the dentine from the occlusal or buccal surface with a water- cooled slow-running bur. The dentine of the cavity floor was then treated as above
with AgNO, and eugenol and after drying was exposed to dye for 30 min. In none of the fourteen teeth was there any dye diffusion through the AgNO,-treated dentine
while the dye did diffuse through all the untreated control areas. After exposure of
the teeth to light it became clear that the silver nitrate had penetrated throughout the
entire course of the dentinal tubules. Diffusion of AgNO, was tested in vivo in five
teeth to be extracted for orthodontic reasons. Cavities were cut according to the technique already described, and Howe’s silver nitrate was applied for 2 min followed
by eugenol for 3 min. The teeth were then extracted and after sectioning the crowns
longitudinally, it was seen that the solution had crossed the cut surface and penetrated
almost to the pulp in four of the five teeth. In the fifth tooth there was slight un- suspected caries at the base of the cavity and this was well stained, but there was only
slight diffusion through the sound dentine of the remainder of the cavity floor. The use of silver nitrate in sensitivity experiments will be discussed in a subsequent paper.
Molecular weight and d(ffision The lowest molecular weights used in the dye diffusion experiments were those of
brilliant cresyl blue and methylene blue with values of 318 and 320 respectively. It
has been shown that AgNO, will penetrate a cut surface of dentine both in vitro and in vivo and on the assumption that AgNOB is fully ionized, the barrier to diffusion
appears to be set above the particle size of silver with a molecular weight of 107.
In the experiments of ANDERSON and NAYLOR (1962) the largest molecular weight was that of 5-hydroxytryptamine which, as the creatine sulphate, is 405. On the basis
of the dye diffusion experiments it may be presumed that this substance would not penetrate a cut surface of dentine, whereas KC1 would be free to do so. The molecular weights of histamine, tryptamine and acetylcholine fall in the range 180-200 and no data are available on the diffusion of these substances through dentine. Indirect evidence based on molecular weight was obtained, however, by the use of picric acid with a molecular weight of 229. The yellow colour of solutions of this substance made

DYE DIFFUSION IN HUMAN DENTINE 511
it possible to observe the extent of its diffusion through dentinal tubules. To avoid
the possibility of decalcification by picric acid, the solution was brought to pH 7 by the addition of solid sodium carbonate.
Ten freshly extracted sound teeth were prepared by cutting a small cavity through the buccal enamel with a water-cooled tungsten carbide bur to expose dentine, and in
addition one cusp was fractured in every tooth. The crowns were then immersed for
30 min in the neutralized picric acid. After this the teeth were washed and dried and then bisected buccolingually. In all except two, the yellow colour had diffused through
the dentinal tubules to the pulp from both the fractured and cut surfaces. No evidence was obtained on the diffusion of this solution through dentine in viva.
DISCUSSION
The results obtained in this investigation indicate that cut dentine differs in
permeability from fractured dentine and while no evidence is offered to show how the
difference is brought about, it seems unlikely to be due entirely to the effect of heat or
tooth debris. The fact that a solution of Evans blue dye with a molecular weight of
960 frequently failed to cross a cut surface of dentine does not mean that smaller
molecules and water are prevented. However, experiments with other dyes early in
the series showed that this failure of diffusion occurred with brilliant cresyl blue and methylene blue with molecular weights of 318 and 320 respectively. At a lower level,
the penetration of AgNO, shows that if the barrier depends on molecular weight it must be set higher than that of silver at 107. From this, it seems unlikely that the
failure of KCI to evoke pain was due to its inability to reach receptor mechanisms
made inaccessible to larger molecules as a result of the diffusion barrier revealed by
dyes. Results obtained with picrate suggest that molecular weight would not prevent
acetylcholine, histamine and tryptamine from penetrating cut dentinal tubules,
although the diffusion of 5-hydroxytryptamine as the creatine sulphate might be
limited by its large molecular weight. However, electrical charge and molecular shape
may also be significant factors in the diffusibility of stimulating substances through dentinal tubules and no firm conclusion about specific substances can be drawn
without more direct evidence. Attempts to obtain this are being made.
Acknowledgement-This investigation was supported by U.S.P.H.S. Research
Grant D-1037 from the National Institute of Dental Research, National Institutes of Health, Bethesda, Maryland, U.S.A.
REFERENCES
ANDERSON, D. J., CIJRWEN, M. P. and HOWARD, L. V. 1958. The sensitivity of human dentine. J. dent. Res. 37, 669-677.
ANDERSON, D. J. and NAYLOR, M. N. 1961. Sensation in human dentine. J. dent. Res. 40, 1275. (Abstract).
ANDERSON, D. J. and NAYLOR, M. N. 1962. Chemical excitants of pain in human dentine and dental pulp. Arch. oral Biol.7,413-415.

512 D. J. ANDERSON AND G. A. RONNING
ANDERSON, D. J. and RONNING, G. A. 1962. Osmotic excitants of pain in human dentine. Arch. oral Biol. 7, 513-523.
HOWE, P. R. 1917. Metallic impregnation of dentine. Dent. Cosmos 59, 891-904.
GROSSMAN, L. I. 1934. The treatment of hypersensitive dentine. J. Amer. dent. Ass. 21, 20X1-2053.
NAYLOR, M. N. 1961. Cold sensation in human dentine. J. dent. Res. 40, 1277 (Abstract).
PLATE 1
FIG. 2. Tooth crown bisected bucco-lingually after exposure of the dentine to dye. No due has diffused through the cut surface (on the left). Dye has diffused from the fractured surface (on the right) to reach the pulp. Evans blue dye.
FIG. 3. Low power photomicrograph showing dye diffusion from pulp cavity falling short of a cut surface of dentine. Evans blue dye. Approx. x6.
FIG. 4. Low power photomicrograph showing dye diffusion from pulp cavity outwards reaching the end of tubules exposed by fracturing (on the left), but falling short of the ends of tubules exposed by cutting (on the right). Evans blue dye. Approx. x6.
FIG. 5. As Fig. 2, except that both cusps were fractured. Before immersion in the dye, the dentine on the left was rubbed with a burnisher under a paste of powdered dentine and water. Evans blue dye.

DYE DIFFUSION IN HUMAN DENTINE