Disulfide bonds determine growth hormone receptor folding ... · neighbouring strands A and B of...
Transcript of Disulfide bonds determine growth hormone receptor folding ... · neighbouring strands A and B of...

3078 Research Article
IntroductionThe growth hormone receptor (GHR) is a type Itransmembrane protein of 620 amino acids, belonging to thesuperfamily of cytokine receptors. Other members of thisfamily are the prolactin receptor, the erythropoietin receptorand several interleukin receptors (Bazan, 1990; Grotzinger,2002). The crystal structure of the GHR in complex withgrowth hormone (GH) was solved in 1992 (de Vos et al., 1992).Recently, the unliganded GHR was crystallised (Brown et al.,2005). The GHR has an intracellular domain of 350 aminoacids, containing the Box1 and the UbE motifs (Frank et al.,1994; Sotiropoulos et al., 1994; Govers et al., 1999). Thetransmembrane domain of the GHR has 24 amino acids andthe extracellular domain 246. The extracellular domainconsists of two subdomains, connected through a hinge region.The N-terminal part of the extracellular domain is involved inligand binding, whereas the membrane-proximal part has astructural (supportive) function (de Vos et al., 1992; Chen etal., 1997). The extracellular domain of the GHR contains fiveputative N-glycosylation sites and seven cysteines (Leung etal., 1987; Fuh et al., 1990; Harding et al., 1994). Six cysteinesform intramolecular disulfide bonds (see Fig. 1), and theseventh, at position 241, may form an intermolecular disulfidebond (Zhang et al., 1999). After protein synthesis, high-mannose glycosylation and dimerisation in the ER, complexglycosylation occurs in the Golgi system (Gent et al., 2002).As the GHR has no intrinsic kinase activity, two Jak2 kinasesare recruited to the dimerised GHR. Upon ligand binding at thecell surface, a conformational change occurs, bringing theJak2 kinases in close proximity (Gent et al., 2003; Brownet al., 2005). The kinases phosphorylate the receptor,crossphosphorylate themselves, and start signal transduction
(Argetsinger et al., 1993; Argetsinger and Carter-Su, 1996).The fate of the GHR can be twofold: (1) the extracellulardomain can be proteolytically cleaved by TACE, ametalloproteinase, generating the GH binding protein (GHBP)(Zhang et al., 2000; Conte et al., 2002; Schantl et al., 2003).Presumably, GHBP serves as a buffer capable of binding freeGH in the blood (Baumann, 2001). The remaining part of theGHR is a target for presenilin-dependent �-secretase activity(Cowan et al., 2005). (2) The major portion of the ligandedor unliganded GHR internalises ligand-independently viaclathrin-coated pits (Sachse et al., 2001; van Kerkhof et al.,2001b; van Kerkhof et al., 2002). Internalisation depends onthe ubiquitin system; the UbE motif in the intracellular tail isrequired for this process (Strous et al., 1996; Govers et al.,1999). After internalisation, the GHR is degraded via theendosomal and lysosomal system (van Kerkhof et al., 2001a;van Kerkhof and Strous, 2001; Sachse et al., 2002).
Until now most research on the GHR was performed on themature GHR: its signalling, shedding and internalisation. Notmuch is known about the synthesis and folding of the GHR. Inthis study, we mutated the three disulfide bonds of theextracellular domain of the GHR to investigate their role infolding, dimerisation and activation of the GHR.
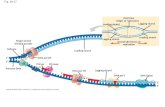
ResultsThe disulfide bonds of the GHRThe organisation of the N-terminal part of the extracellulardomain of the GHR is schematically depicted in Fig. 1. Thisfigure is based on the work of Bazan (Bazan, 1990), Bass etal. (Bass et al., 1991) and de Vos et al. (de Vos et al., 1992).The extracellular domain of the GHR consists of twosubdomains, each built of two antiparallel � sheets, one of four
The growth hormone receptor contains seven cysteineresidues in its extracellular domain. The six in the growthhormone binding domain form disulfide bonds, and helpthe receptor to gain its correct three-dimensional structure.In this study we replaced the cysteine for serine and alanineresidues and investigated their role in growth hormonereceptor folding, dimerisation and signal transduction.Folding and growth hormone binding capacity of the wild-type growth hormone receptor require less than twominutes for completion. Although less efficient, all mutantreceptors arrive at the cell surface as pre-formed dimers.
Disulfide bond C38-C48 is important for efficientmaturation. The middle disulfide-bond, C83-C94, isimportant for ligand binding. Removing disulfide bondC108-C122 has little effect without affecting signalling.When two or all disulfide bonds are changed, ligandbinding and activation are blocked. Dimerisation is delayedwhen all disulfide bonds are destroyed.
Key words: Growth hormone, Cytokine receptor, Folding,Dimerisation, ER, Disulfide bonds
Summary
Disulfide bonds determine growth hormone receptorfolding, dimerisation and ligand bindingMonique J. M. van den Eijnden, Liza L. Lahaye and Ger J. Strous*Department of Cell Biology, Institute of Biomembranes, University Medical Center Utrecht, Heidelberglaan 100, G02.525, 3584 CX Utrecht, TheNetherlands*Author for correspondence (e-mail: [email protected])
Accepted 3 May 2006Journal of Cell Science 119, 3078-3086 Published by The Company of Biologists 2006doi:10.1242/jcs.03036
Jour
nal o
f Cel
l Sci
ence

3079Disulfide bonds and functional GHR folding
(D,C,F,G) and the other of three strands (A,B,E). Both domainsare separated by a short linker sequence. Only the N-terminaldomain, which is shown in Fig. 1, contains cysteine residues.They form disulfide bonds as indicated: C38-C48 connectsneighbouring strands A and B of the three-stranded sheet (bond1). C83-C94 links strand D to strand E, thereby crosslinkingthe two � sheets (bond 2). The third disulfide bond, C108-C122, links strands F and G of the four-stranded sheet (bond3). The role of the disulfide bonds in achieving the tertiarystructure of the GHR was examined in this study.
Folding of the GHRFirst, we investigated folding of the wild-type GHR (wtGHR)in a pulse-chase assay. The receptor was transiently transfectedin ts20 cells. To ascertain that no folding intermediates of theGHR were missed, immunoprecipitations were performed witha polyclonal antibody raised against the membrane-proximalcystosolic tail (Fig. 2A, left panel, anti-GHR-T). After a 10-minute pulse-label period (0-minute chase), the precursor (ER)form of the GHR is seen as a double band just above 100 kDa.During longer chase times, the double band slightly diminishedand shifted downwards in the gel. There are two additionalbands visible around 90 kDa which disappear after 60 minutes.The complex glycosylated, mature 130-kDa GHR (present atthe cell surface) appeared after 30 minutes of chase. After 240minutes of chase, no degradation can be seen.
To investigate how folding relates to the capacity of thenewly formed protein to bind ligand, we combined pulse-chaselabelling with a pull-down procedure using biotinylated GH(btGH) (Fig. 2A, right panel). In the case of the btGH pull-down, only GHR that bound GH will be isolated. In Fig. 2Aequal aliquots for both immunoprecipitation and btGH pull-down were used. After a pulse-label period of 10 minutes (0-minute chase) GH already bound GHR equally as well as theantibody. If folding into a GH-binding-competent moleculetakes more than 2 minutes, the first lane (0-minute chase, rightpanel) would be significantly less intense compared with thesecond lane (5-minute chase). However, both lanes are of equalintensity. Assuming that the anti-GHR-T antibody recognisedGHR immediately after completion, and that it takesapproximately 30 seconds to synthesise a GHR molecule, weconclude that folding of GHR into a GH-binding-competentmolecule occurs within 2 minutes. When the left and rightpanels are compared, at each time point both bands have thesame intensity (verified through image quantification, resultsnot shown) indicating that the two isolation methods areequally efficient. The only difference between the two panelsis that after immunoprecipitation, two additional bands runaround 90 kDa, which cannot be seen after btGH pull-down.
D
C
F
G
A
BE
2
3
1
Fig. 1. Schematic representation of the �-sheet and disulfide bondorganisation of the N-terminal portion of the extracellular domain ofthe GHR. The three-stranded �-sheet, with strands A,B,E, isindicated in dark grey. The four-stranded � sheet, with strandsD,C,F,G, is indicated in light grey. Disulfide bonds are connectedwith lines. Bond 1 is C38-C48, bond 2 is C83-C94 and bond 3 isC108-C122.
Fig. 2. Folding of the wild-type GHR. Transientlytransfected ts20 cells were pulse-labelled for 10minutes and chased for the indicated times.(A) Postnuclear lysates were split in equal amounts andeither immunoprecipitated with anti-GHR-T (leftpanel), or isolated with btGH and streptavidin beads(right panel). Samples were subjected to reducingSDS-PAGE. p, 110-kDa precursor form; m, 130-kDamature form. (B) After immunoprecipitation, sampleswere treated with EndoH (+) or mock treated (–). Allsamples were immunoprecipitated with anti-GHR-Tand subjected to reducing SDS-PAGE. (C) Cells werepre-incubated with MG132 for 1 hour andsubsequently treated with MG132 during pulse andchase periods (+), or mock treated (–). Samples wereimmunoprecipitated with anti-GHR-T and subjected toreducing SDS-PAGE. (D) After immunoprecipitation,samples were subjected to reducing (+) and non-reducing (–) SDS-PAGE. Relative molecular massstandards are indicated.
Jour
nal o
f Cel
l Sci
ence

3080
To investigate the nature of the 90-kDa bands, we treated thesamples with endoglycosidase H (Endo H) (Fig. 2B). Endo Hspecifically cleaves high-mannose N-linked glycans in theirpre-Golgi state. Upon treatment with Endo H, the two 110-kDabands shifted towards the 90-kDa bands, indicating that the110-kDa bands are Endo-H-sensitive forms of the GHR. As thelower bands did not shift, we conclude that they represent non-glycosylated forms of the GHR. The 130-kDa band was notsensitive to Endo H treatment, indicating that this GHR iscomplex glycosylated and is in or passed the Golgi complex.The untreated 110-kDa bands shifted downwards with time(Fig. 2A). Most likely, this is due to mannose trimming of themolecule, because after Endo H treatment both the 10-minuteand the 120-minute chase bands run at exactly the same height(Fig. 2B). Note that the 90-kDa bands do not bind GH (Fig.2A).
To explain the disappearance of the 90-kDa bands with time,we incubated the cells with MG132, a proteasome inhibitor(Fig. 2C). After 10 minutes of chase, no differences wereobserved. After 100 minutes of chase, the non-glycosylatedbands started to disappear in the non-treated cells. After 180minutes of chase the lowest bands almost completelydisappeared in the non-treated cells, whereas in the MG132-treated cells, the 90-kDa bands were still present. Thisindicates that the non-glycosylated, 90-kDa bands aresubjected to the ER-associated protein degradation (ERAD)system (Sommer and Wolf, 1997; Brodsky and McCracken,1999; Meusser et al., 2005) and that they do not convert tomature GHR. After 180 minutes of chase, no differences wereobserved between MG132-treated or non-treated cells in the110-kDa and 130-kDa forms, indicating that the glycosylatedGHR species are not sensitive to ERAD.
When reduced samples are compared with non-reducedsamples (Fig. 2D), the non-reduced (–DTT) samples runslightly lower. No folding intermediates were observed. Thismeans that the disulfide bonds, which are destroyed after DTTtreatment, create a more compact GHR and are already fullyformed after a 10-minute pulse period. The mannose-trimmingpattern is observed in both the reduced and non-reduced lanes.
In conclusion, the newly formed GHR polypeptide foldsquickly, without any discernable intermediates, into its correctprecursor form. GH recognises this form but not the non-glycosylated 90-kDa GHR species.
Disulfide bond mutations and foldingThe GHR contains seven cysteine residues in the extracellulardomain. Six of them form intramolecular disulfide bonds, asshown in Fig. 1. We mutated these cysteine residues into serineor alanine residues. The cysteine-to-alanine mutations aredisruptions of entire disulfide bonds. The cysteine-to-serinemutations are single point mutations, to investigate thedifference between removing an entire disulfide bond anddestroying one side of a bond. To determine the effect of thevarious mutations on the kinetics of folding of the GHR, wetransiently transfected the wild-type and mutant receptors intots20 cells, pulse-labelled the cells for 10 minutes, and analysedthe GHRs by immunoprecipitation after a short (15-minute)and a long (180-minute) chase period (Fig. 3A). In most casesthe precursor forms are slightly smaller at 180 minutes of chasecompared with a 15-minute chase time. As explained before,this is probably due to mannose trimming and occurs with all
Journal of Cell Science 119 (15)
mutants. No folding intermediates were observed. All mutantGHRs matured less efficiently than the wtGHR. Notably, withthe exception of the C018S mutant, all of the mature, mutantreceptors migrated as more compact species.
In Fig. 3B, the maturation efficiencies are shown, quantifiedas the ratio of mature to total at 180 and 240 minutes of chase,and presented relative to the wtGHR maturation efficiency. Themutant receptors have maturation efficiencies between 26%and 68% when compared with the wtGHR. The mutants thatmature least efficiently lack the first disulfide bond (Fig. 3B,mutants A, AB, AC, C485). The mutants that mature best lackthe third disulfide bond (Fig. 3B, mutants C, C108S). Weconclude that the first disulfide bond, C38-C48, is importantfor efficient maturation of the GHR.
Fig. 3. Maturation efficiency of wild-type and mutant GHRs.(A) Folding and maturation of wild type (WT) and mutated GHRstransiently transfected in ts20 cells. Cells were pulse labelled for 10minutes and chased for 15 or 180 minutes (as indicated). Sampleswere immunoprecipitated with anti-GHR-T and subjected toreducing SDS-PAGE. p, 110-kDa precursor form; m, 130-kDamature form; A, GHR(C38A-C48A); B, GHR(C83A-C94A); C,GHR(C108A-C122A); AB, AC, BC and ABC are combinations ofmutations A,B and/or C. Relative molecular mass standards areshown on the right. (B) Quantification of the maturation efficienciesof the wild-type and mutant receptors. The amount of radioactivitywas determined using ImageQuant (Molecular Dynamics). Theamount of mature GHR species (130 kDa) was divided by theamount of precursor (110 kDa) and mature GHR species at 180 and240 minutes chase and represented as a percentage relative to thematuration rate of wtGHR. The values represent the mean ± s.d. of atleast two different experiments.
Jour
nal o
f Cel
l Sci
ence

3081Disulfide bonds and functional GHR folding
Disulfide-bond mutations and ligand bindingIn order to determine the capability of the mutant receptors tobind ligand, we used btGH to pull down the mutant GHRs aftera 90-minute and 240-minute chase (Fig. 4A). The two timeperiods allow analysis of both rapid- and slow-folding GHRs.In all cases the amount of radioactivity was compared withand expressed as the radioactive GHR species afterimmunoprecipitation. We observed no differences between the90- and 240-minute chases. Fig. 4B shows the quantified data.In the wild-type situation, the receptor immunoprecipitationsignal and the btGH signal were equal and confirmed the datain Fig. 2A. btGH bound mutant A at 19% of the wild-typelevel, mutant C at 32% and mutant B was not recognised (Fig.4B). The C48S mutant was recognised only by a very smallamount of ligand (7%). btGH bound C108S at 37% of the wild-type level, but the C83S mutant was not recognised. Mutatingtwo or more disulfide bonds destroyed the possibility ofbinding ligand. Apparently, the middle disulfide bond, C83-C94, is important for ligand recognition.
Disulfide bond mutations and dimerisationTo examine whether the disulfide bond mutations can interferewith dimerisation of the receptors, we performed co-immunoprecipitation assays. Ts20 cells were transiently co-transfected with wild-type or mutant GHRs, either in afull-length (FL) form or as a truncated, epitope-taggedGHR(1-369; HA-His6-Myc) (369-HA). Fig. 5A containslysates of the samples and Fig. 5B immunoprecipitations.Immunoprecipitations were performed with anti-GHR-C,recognising only full-length GHRs. Detection of the westernblots was performed with an anti-HA antibody, showing theepitope-tagged truncated receptors. These receptors can onlybe seen in Fig. 5B when heterodimer formation occurs betweenfull-length and truncated receptors. To exclude the possibilitythat the interaction occurred after lysis, lysates from ts20 cellsexpressing full-length wild-type and mutant receptors weremixed with lysates from ts20 cells expressing 369-HA-taggedwild-type and mutant receptors. Under those conditions, nointeraction was observed (Fig. 5B, M lanes). Reprobing the
same blot with the GHR antibody Mab5revealed similar amounts of precipitated full-length GHR species (Fig. 5C). Apparently, alldisulfide bond mutations are able to formdimers. Comparison of the ratios of mature toprecursor before and after the pull-downprocedure reveals whether the differentmutants dimerise with equal efficiencies.When the ratios were compared between directlysates (Fig. 5A) and co-immunopecipitations(Fig. 5B), they were all approximately thesame, except for the ABC mutant. Here, theratio doubled after immunoprecipitation.Because the amount of mature receptors doesnot change, there is relatively less precursorform of the ABC mutant immunoprecipitatedin the dimers. This indicates that dimerisationis delayed for this mutant, whereas for thewtGHR and the other mutant GHRs, allprecursor forms have dimerised with equalefficiencies.
Fig. 4. Ligand-binding capacities of the mutantreceptors. (A) Transiently transfected ts20 cellswere pulse-labelled for 10 minutes and chased for90 or 240 minutes as indicated. Samples were splitinto equal amounts and immunoprecipitated (IP)with anti-GHR-T or isolated with btGH andstreptavidin beads (btGH) and subjected toreducing SDS-PAGE. Receptor species areindicated as in Fig. 3. p, 110-kDa precursor form;m, 130-kDa mature form. Relative molecular massstandards are shown on the right.(B) Quantification of the ligand binding capacity ofwild-type and mutated receptors. The amount ofradioactivity was determined using ImageQuant(Molecular Dynamics). Precursor form intensitiesof the wild-type and mutant receptors after btGHpull-down were divided by precursor formintensities of the wild-type and mutant receptorsafter immunoprecipitation and represented as apercentage. The values represent the mean ± s.d. ofat least two different experiments.
Jour
nal o
f Cel
l Sci
ence

3082
Cell surface appearance of the mutant receptorsThe expression of wild-type and mutant GHRs at the cellsurface was measured by flow cytometry analysis. As anegative control, empty-vector-transfected cells were used,visible in each panel of Fig. 6 as a solid histogram. Themonoclonal antibody Mab5 is able to detect cell surfaceexpression of the wtGHR (Fig. 6, upper left panel, openhistogram). The mutant receptors (open histograms) are namedin each panel. Compared with the empty vector control, all themutants show a shift to the right, indicating expression at thecell surface. For mutants A, B, AB, AC and C48S the shift isvery small, but this is in agreement with Fig. 3, which showedthat these mutants mature least well. As the flow cytometry
Journal of Cell Science 119 (15)
method is dependent on a conformationally sensitive antibody,the actual amount of mutant receptors at the cell surface maybe higher. To control this, we performed proteinase K digestionand analysed the disappearance of the mature form of theGHRs on western blot (results not shown). For all mutantreceptors, the extracellular domain disappeared uponproteinase K treatment. We conclude that for all mutants, the130 kDa mature GHR arrives at the cell surface.
Signalling capacities of the mutant receptorsFinally, we examined whether the mutant receptors that areligand-binding competent, GHR(C48S), GHR(C108S), mutantA and mutant C, are also able to initiate signal transduction.This should result in tyrosine phosphorylation of the receptorsafter btGH and not after btGH antagonist (btGHA) treatmentand pull-down. The mutant receptors C48S and A have such alow amount of mature receptors, that it was not possibleto obtain sufficient mature receptor to detect tyrosinephosphorylation. Fig. 7A shows the lysates of the wtGHR,mutant C108S and mutant C, transiently transfected in ts20cells. Equal amounts of receptors were used for the pull-downs.In Fig. 7B the isolated proteins were detected with the anti-GHR-C antibody, showing efficient pull-down of only themature species with both btGH and btGHA. After 15 minutesof incubation with btGH, a phosphorylated tyrosine (PY)signal was visible for all three receptor species at the sameheight as the mature GHR (Fig. 7C). No PY signal was visiblein the cells incubated with btGHA. In conclusion, even thoughdisulfide bond C108-C122 is partly or completely changed, thereceptors that mature and arrive at the cell surface are stillcapable of initiating signal transduction.
DiscussionThe functional relevance of the three disulfide bonds of theGHR was investigated in this study. The results can be clarifiedby the crystal structure of the receptor (see Fig. 1). C83-C94connects both sheets of domain 1, whereas the other two bondsconnect strands within one sheet. Therefore, this disulfide bondis probably most important and, indeed, when disrupting C83-C94, the mutated GHR is no longer capable of binding itsligand. Bond C38-C48 is more conserved than C108-C122 andprobably needs to fold first, to allow the rest of the extracellulardomain to form. This could explain its role in maturationefficiency. The minor effects of mutating C108-C122 areexplained by the fact that this disulfide bond is not conservedamong the cytokine receptor family (Bazan, 1990). In thesalmon GHR it is also absent (Fukada et al., 2004). When thecysteine-to-alanine mutations are compared with the cysteine-to-serine mutations, no major differences are observed. Thetrend in maturation efficiency is the same, with the firstdisulfide bond, C38-C48 having the lowest and the third havingthe highest maturation efficiency. The middle disulfide bondloses GH recognition in both situations, whereas C108-C122can still be activated in both types of mutation. In this study,mutating one or two cysteines of a disulfide bond does notcause different effects. The membrane-proximal domain of theextracellular part of the GHR contains one unpaired cysteineat position 241. This cysteine may form a disulfide bond witha dimerising GHR but was not investigated in this study (Zhanget al., 1999).
To our knowledge, only one patient has been described with
Fig. 5. Dimerisation of the GHR mutations. Full-length (FL) wild-type (WT) and mutant GHRs were transiently transfected in ts20cells, either together with GHR(1-369; HA-6xHis-Myc) (369-HA)bearing the different mutations or with empty vector (EV). 369-HAreceptors were also co-transfected with EV. After lysis, full-lengthreceptors were immunoprecipitated with anti-GHR-C and separatedby reducing SDS-PAGE. (A) Immunoblotting of lysates with anti-HA antibody. Transfection combinations (+) are indicated. MutantGHRs as indicated in Fig. 3. (B) Immunoblotting of anti-GHR-Cimmunoprecipitates with anti-HA antibody. (C) Efficiency ofimmunoprecipitation was determined by reprobing the blot shown inB with anti-GHR antibody Mab5. M, mix of lysate from ts20 cellstransiently transfected with full-length GHR (mutants) and emptyvector with lysate from ts20 cells transiently transfected with 369-HA GHR (mutants) and empty vector; m, mature GHR; p, precursorGHR; IP, immunoprecipitation; WB, immunoblotting. Relativemolecular mass standards are shown on the left.
Jour
nal o
f Cel
l Sci
ence

3083Disulfide bonds and functional GHR folding
a missense mutation at a cysteine position (C38S) (Sobrier etal., 1997). This person had Laron syndrome, with detectableplasma GH-binding activity (Laron et al., 1971). According tothe authors the mutation affected a highly conserved residue,which is a key element of secondary structure, involved in theedification of the GH-binding site. Our data confirm theseresults. In addition, even though we mutated the opposite siteof the disulfide bond and removed the entire bond, our resultspredict a reduced amount of GHRs at the plasma membrane.The mutant GHR can bind GH, it may even be able to signal,but it matures with such a low efficiency that its signaltransduction capacities will probably be insufficient.
In this study, transient transfections were used. We foundsome differences between the stable and the transient systemregarding GHR turnover, being prolonged in the transientsystem. Van Kerkhof et al. found the stably transfected GHRto have a half-life of about 75 minutes (van Kerkhof et al.,2000; van Kerkhof et al., 2002). In the experiments describedhere, the half-life of the GHR is over 4 hours. In the pulse-chase assay (Fig. 2A), the intensity of the ER form of theGHR only slightly reduces in 4 hours (verified by imagequantification), whereas in the stable system, almost allprecursor forms become mature. Transient transfectionprobably overloads the ER and the glycosylation machinery to
such an extent that a large proportion of the precursor formsof the GHR never matures. Our MG132 experiments haveshown that in the first 180 minutes, the 110- and 130-kDaspecies of the GHR are not being degraded (Fig. 2C). But wedo see mannose trimming, especially after 60 minutes of chase,which signals the onset of ERAD (Sommer and Wolf, 1997;Brodsky and McCracken, 1999). If chase times are prolonged,we observe the disappearance of the wild-type precursor(results not shown).
The 90-kDa non-glycosylated species are also typical fortransient transfections. They seem to appear under strong ERoverload and are removed by ERAD relatively quickly. On theextent to which non-glycosylated GHRs are recognised by GH,Fuh et al. concluded that glycosylation of the GHR, producedin bacteria, is not important for ligand recognition (Fuh et al.,1990). The crystal structure of the GHR in complex with GHwas determined with non-glycosylated GHR, produced inbacteria (de Vos et al., 1992). Harding et al. and Szecowka etal. state that mutating glycosylation sites or interfering withglycosylation strongly reduce GH-binding capacities(Szecowka et al., 1990; Harding et al., 1994). The non-glycosylated GHR molecules in this study cannot berecognised by GH and run at exactly the same height as normalprecursor forms with the glycans removed (Fig. 2B,D). This
200
200
200
100 101 102 103 104
100 101 102 103 104
100 101 102 103 104
200
200
200
100 101 102 103 104
100 101 102 103 104
100 101 102 103 104
200
200
200
100 101 102 103 104
100 101 102 103 104
100 101 102 103 104
103 104
200
200
100 101 102
100 101 102 103 104
WT A B C
AB AC BC ABC
C48S C83S C108S
Fluorescence intensity
Cel
l num
ber
Fig. 6. Cell-surface appearance of the mutant receptors. Cells transiently expressing empty vector, wtGHR (WT) or the GHR mutants weresurface stained with monoclonal antibody Mab5 and subsequently with Cy5-conjugated goat-anti-mouse IgG. Living cells were gated andanalysed by flow cytometry. WtGHR and the mutants are indicated with open histograms and shown as an overlay with the negative control,empty vector transfected cells (solid histogram). Receptor species are indicated as in Fig. 3. The data shown are representative of threeindependent experiments.Jo
urna
l of C
ell S
cien
ce

3084
implies that the non-glycosylated species generated in the ER,although correctly folded, cannot bind GH.
Mutating disulfide bonds does not prevent dimerisation ofthe GHR. However, for the ABC mutant, a different mature toprecursor ratio was observed, implicating delayed dimerisation(Fig. 5). Thus, the ABC mutant matures relatively fast, doesnot bind GH, and has impaired dimerisation. This mightindicate that such a disordered extracellular domain hindersdimerisation. As no cysteine residues are present in the GH-binding domain of the ABC mutant, it is tempting to speculatethat protein disulfide isomerase might play a role in GHRdimerisation.
Gent et al. showed that dimerisation of the GHR occurs inthe ER, but these data were not quantified (Gent et al., 2002;Gent et al., 2003). With our quantified data we found that forthe wtGHR the mature to precursor ratio is equal when directlysates and co-immunoprecipitations are compared. This
Journal of Cell Science 119 (15)
proves that all ER forms are dimerised receptors. Probably,dimerisation occurs co-translationally or very early aftertranslocation into the ER lumen.
In conclusion, this study provides novel information on therole which disulfide bonds have in achieving a correctly folded,dimerised and functional GHR. Understanding how foldingand dimerisation govern GHR biogenesis will help inunderstanding how cells regulate GHR availability at the cellsurface. Since the two most conserved disulfide bonds arepresent in all cytokine receptors these results might explainstructure-function relationships in other family members.
Materials and MethodsMaterials and antibodiesAnti-GHR rabbit antisera generated against amino acid residues 271-320 (anti-GHR-T), 327-493 (anti-GHR-B) and 493-620 (anti-GHR-C) were raised aspreviously described (Strous et al., 1996; van Kerkhof et al., 2000). Monoclonalanti-HA antibody 16B12 was purchased from Eurogentec (Berkeley, CA). AntibodyMab5, recognising the extracellular domain of the GHR was obtained from AGEN(Parsippany, NJ). Monoclonal antibody 4G10, recognising phosphotyrosineresidues, was obtained from Upstate Biotechnologies (Lake Placid, NY). HumanGH was kindly provided by Eli Lilly & Co. Research Labs (Indianapolis, IN).Human GH antagonist B2036 (GHA), containing a G120K mutation in binding site2 and eight additional mutations that enhance the binding affinity of site 1(Cunningham et al., 1991; Ross et al., 2001), was a generous gift from William F.Bennet of Sensus Drug Development Cooperation (Austin, TX). Biotinylated GH(btGH) or GHA (btGHA) were created according to the manufacturer’s instructions.(Pierce, Rockford, IL) (Bentham et al., 1994). MG132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal) was from Calbiochem (San Diego, CA).
GHR mutants and cell linesDisulfide bond mutations of the GHR extracellular domain were created byQuikChange Site-directed Mutagenesis (Stratagene). Briefly, pcDNA3 plasmids(Invitrogen) encoding wild type rabbit GHR (wtGHR) and GHR(1-369; HA-His6-Myc) (369-HA) were used as a template in a polymerase chain reaction with 3� and5� oligonucleotides encoding for the C48S, C83S, C108S, C38A-C48A (A), C83A-C94A (B), C108A-C122A (C) mutations or combinations of mutations: A and B(AB), A and C (AC), B and C (BC), A, B and C (ABC). The oligonucleotides alsointroduced a silent mutation that either created or disrupted a restriction site. Allconstructs were verified by restriction analysis and sequencing. The construction ofa triple-epitope-tagged truncation mutant GHR(1-369; HA-His6-Myc) wasdescribed before (Gent et al., 2002).
Chinese hamster ts20 cells, bearing a thermolabile ubiquitin-activating (E1)enzyme, were used for experiments. Transient transfections were performed withFuGENE6 (Roche) according to the manufacturer’s description. 48 hours aftertransfection, cells were used for experiments. Cells were cultured at 30°C in MEM�(Gibco) supplemented with 10% fetal bovine serum (Sigma), 4.5 g/l glucose and100 U/ml penicillin/streptomycin (Gibco).
Pulse-chase assaySubconfluent ts20 cells, grown in 6 cm dishes, were used 48 hours post-transfectionfor pulse-chase analysis as described (Jansens and Braakman, 2003). The cells werewashed in phosphate-buffered saline (PBS) and pre-incubated in starvation mediumlacking methionine and cysteine for 15 minutes at 30°C. Cells were pulse-labelledfor 10 minutes with 125 �Ci/ml RedivueTMPRO-MIXTM L-[35S] in vitro celllabelling mix (Amersham Biosciences) and chased with excess cold methionine andcysteine and 1 mM cycloheximide in MEM� medium (with HEPES) at 30°C forthe indicated times. Incubations were stopped by transferring the cells to ice,aspirating the medium and adding ice-cold PBS, containing 0.5 mM MgCl2, 1 mMCaCl2 and 20 mM N-ethylmaleimide (NEM) (Sigma). The cells were lysed inice-cold lysis buffer containing 1% Triton X-100, 1 mM EDTA, 50 mM NaF,1 mM Na3VO4, 10 �g/ml aprotinin, 10 �g/ml leupeptin, 1 mMphenylmethylsulfonylfluoride (PMSF) and 20 mM NEM in PBS. Cell lysates werecentrifugated to pellet the nuclei and post-nuclear supernatants were used forimmunoprecipitation or btGH pull-down. GHR molecules were immunoprecipitatedwith anti-GHR antibodies in 1% Triton X-100, 1% SDS, 0.5% sodiumdeoxycholate, 1% BSA, 1 mM EDTA, 1 mM PMSF, 50 mM NaF, 1 mM Na3VO4,10 �g/ml aprotinin, 10 �g/ml leupeptin and 20 mM NEM. Immune complexes wereisolated with protein-A-conjugated agarose beads (Repligen, Waltham, MA).Alternatively, cell extracts were incubated with 100 ng btGH. btGH-GHRcomplexes were isolated with Immunopure immobilised streptavidin (Pierce).Immunoprecipitates or btGH-GHR complexes were subjected to SDS-PAGE. Gelswere stained in a Coomassie Brilliant Blue solution, destained in 10% acetic acid,10% methanol, dried, and visualised using a Molecular Dynamics Phosphorimager.
Fig. 7. Signalling capacities of the mutant receptors. Transientlytransfected ts20 cells expressing wtGHR (WT), GHR(C108S) orGHR(C108A-C122A) (C) were treated for 15 minutes with 8 nMbtGH (GH) or btGHA (GHA) at permissive temperature (30°C) andlysed. (A) Immunoblotting of lysates with anti-GHR-C. After lysis,btGH(A)-GHR complexes were isolated with streptavidin beads andsubjected to reducing SDS-PAGE. (B) Immunoblotting ofcomplexes with anti-GHR-C. (C) The same blot as in Bimmunoblotted with anti-phosphotyrosine (anti-PY) antibody. m,mature GHR; p, precursor GHR; IP, immunoprecipitation; WB,immunoblotting. Relative molecular mass standards are shown onthe left.
Jour
nal o
f Cel
l Sci
ence

3085Disulfide bonds and functional GHR folding
The amounts of radioactivity were determined using ImageQuant (MolecularDynamics). Endoglycosidase H (Endo H) treatment (Boehringer): Afterimmunoprecipitation, 15 �l of 100 mM sodium acetate pH 5.5 and 0.2% SDS wasadded, the beads were resuspended and boiled for 5 minutes. Next, 15 �l of 100mM sodium acetate pH 5.5, 1 mM PMSF, 1 mM Na3VO4, 10 �g/ml aprotinin, 10�g/ml leupeptin, 2% Triton X-100 and 0.0025 U Endo H were added and thesamples were incubated for 90 minutes at 37°C. Control samples were treated inthe same way, except for the addition of Endo H. After incubation, samples wereprepared for reducing SDS-PAGE.
Co-immunoprecipitationCells were grown in 6 cm dishes and used 48 hours post-transfection. Dishes wereput on ice and washed three times with ice-cold PBS. The cells were lysed in ice-cold co-immunoprecipitation (co-ip) buffer containing 0.5% Triton X-100, 1 mMEDTA, 50 mM NaF, 1 mM Na3VO4, 10 �g/ml aprotinin, 10 �g/ml leupeptin and 1mM PMSF in PBS. Cell lysates were centrifuged to pellet the nuclei and post-nuclear supernatants were mixed as indicated and used for immunoprecipitationwith anti-GHR-C. Immune complexes were isolated with protein-A-conjugatedagarose beads (Repligen). The immunoprecipitates were washed twice with co-ipbuffer and twice with PBS. The proteins were subjected to reducing SDS-PAGE andtransferred to Immobilon-FL polyvinylidenedifluoride (PVDF) membrane(Millipore). Blots were immunostained with the indicated antibodies followed byAlexa Fluor 680- (Molecular Probes) or IRDye800 (Rockland, Gilbertsville, PA)-conjugated goat-anti-mouse antibodies. Detection and quantification wereperformed with an Odyssey System (LI-COR Biosciences, Lincoln, NE). Whenindicated, blots were reprobed after stripping twice for 15 minutes with strippingbuffer (25 mM glycine pH 2.0 and 1% SDS in H2O).
Flow cytometry Ts20 cells were dislodged 48 hours post-transfection from 6 cm dishes with a celldissociation solution (Sigma) according to the manufacturer’s description. The cellswere incubated on ice with monoclonal antibody Mab5 and Cy5-conjugated goat-anti-mouse secondary antibody (Jackson Immunolaboratories) in PBS and 5% heat-inactivated FCS. Cells were assayed on a FACSCalibur flow cytometer (BectonDickinson) with a live gate to exclude dead cells. Setting of the live gate waschecked by the addition of propidium iodide.
Tyrosine phosphorylationTs20 cells transiently transfected with wild-type GHR or GHR mutants were grownin 10 cm dishes and incubated for 15 minutes in the presence of 8 nM btGH orbtGHA at 30°C. After incubation, cells were put on ice and washed three times withice-cold PBS, supplemented with 0.4 mM Na3VO4. Cells were lysed in the sameice-cold lysis buffer as described above. btGH(A)-GHR complexes were isolatedwith immobilised streptavidin beads (Pierce), subjected to reducing SDS-PAGEand transferred to Immobilon-FL PVDF membrane (Millipore). Blots wereimmunostained with the indicated antibodies followed by Alexa Fluor 680-conjugated goat-anti-rabbit (Molecular Probes) or IRDye800-conjugated goat-anti-mouse (Rockland) antibodies. Detection was performed with an Odyssey system(LI-COR).
We thank Eva Rijkers for excellent technical assistance with FACSanalysis, René Scriwanek and Marc van Peski for assistance with thefigures, and Ineke Braakman, Jürgen Gent and Peter van Kerkhof forhelpful suggestions. The investigations were supported in part by theResearch Council for Earth and Life Sciences (A.L.W.) with financialaid from the Netherlands Organization for Scientific Research (NWO-814.02.011).
ReferencesArgetsinger, L. S. and Carter-Su, C. (1996). Mechanism of signaling by growth
hormone receptor. Physiol. Rev. 76, 1089-1107.Argetsinger, L. S., Campbell, G. S., Yang, X. N., Witthuhn, B. A., Silvennoinen, O.,
Ihle, J. N. and Carter-Su, C. (1993). Identification of JAK2 as a growth hormonereceptor-associated tyrosine kinase. Cell 74, 237-244.
Bass, S. H., Mulkerrin, M. G. and Wells, J. A. (1991). A systematic mutational analysisof hormone-binding determinants in the human growth hormone receptor. Proc. Natl.Acad. Sci. USA 88, 4498-4502.
Baumann, G. (2001). Growth hormone binding protein 2001. J. Pediatr. Endocrinol.Metab. 14, 355-375.
Bazan, J. F. (1990). Structural design and molecular evolution of a cytokine receptorsuperfamily. Proc. Natl. Acad. Sci. USA 87, 6934-6938.
Bentham, J., Aplin, R. and Norman, M. R. (1994). Histochemical detection of bindingsites for human growth hormone using biotinylated ligand. J. Histochem. Cytochem.42, 103-107.
Brodsky, J. L. and McCracken, A. A. (1999). ER protein quality control andproteasome-mediated protein degradation. Semin. Cell Dev. Biol. 10, 507-513.
Brown, R. J., Adams, J. J., Pelekanos, R. A., Wan, Y., McKinstry, W. J., Palethorpe,
K., Seeber, R. M., Monks, T. A., Eidne, K. A., Parker, M. W. et al. (2005). Modelfor growth hormone receptor activation based on subunit rotation within a receptordimer. Nat. Struct. Mol. Biol. 12, 814-821.
Chen, C., Brinkworth, R. and Waters, M. J. (1997). The role of receptor dimerizationdomain residues in growth hormone signaling. J. Biol. Chem. 272, 5133-5140.
Conte, F., Salles, J. P., Raynal, P., Fernandez, L., Molinas, C., Tauber, M. and Bieth,E. (2002). Identification of a region critical for proteolysis of the human growthhormone receptor. Biochem. Biophys. Res. Commun. 290, 851-857.
Cowan, J. W., Wang, X., Guan, R., He, K., Jiang, J., Baumann, G., Black, R. A.,Wolfe, M. S. and Frank, S. J. (2005). Growth hormone receptor is a target forpresenilin-dependent {gamma}-secretase cleavage. J. Biol. Chem. 280, 19331-19342.
Cunningham, B. C., Ultsch, M., De Vos, A. M., Mulkerrin, M. G., Clauser, K. R. andWells, J. A. (1991). Dimerization of the extracellular domain of the human growthhormone receptor by a single hormone molecule. Science 254, 821-825.
de Vos, A. M., Ultsch, M. and Kossiakoff, A. A. (1992). Human growth hormone andextracellular domain of its receptor: crystal structure of the complex. Science 255, 306-312.
Frank, S. J., Gilliland, G., Kraft, A. S. and Arnold, C. S. (1994). Interaction of thegrowth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase.Endocrinology 135, 2228-2239.
Fuh, G., Mulkerrin, M. G., Bass, S., McFarland, N., Brochier, M., Bourell, J. H.,Light, D. R. and Wells, J. A. (1990). The human growth hormone receptor. Secretionfrom Escherichia coli and disulfide bonding pattern of the extracellular domain. J. Biol.Chem. 265, 3111-3115.
Fukada, H., Ozaki, Y., Pierce, A. L., Adachi, S., Yamauchi, K., Hara, A., Swanson,P. and Dickhoff, W. W. (2004). Salmon growth hormone receptor: molecular cloning,ligand specificity, and response to fasting. Gen. Comp. Endocrinol. 139, 61-71.
Gent, J., van Kerkhof, P., Roza, M., Bu, G. and Strous, G. J. (2002). Ligand-independent growth hormone receptor dimerization occurs in the endoplasmicreticulum and is required for ubiquitin system-dependent endocytosis. Proc. Natl.Acad. Sci. USA 99, 9858-9863.
Gent, J., van den Eijnden, M., van Kerkhof, P. and Strous, G. J. (2003). Dimerizationand signal transduction of the growth hormone receptor. Mol. Endocrinol. 17, 967-975.
Govers, R., ten Broeke, T., van Kerkhof, P., Schwartz, A. L. and Strous, G. J. (1999).Identification of a novel ubiquitin conjugation motif, required for ligand-inducedinternalization of the growth hormone receptor. EMBO J. 18, 28-36.
Grotzinger, J. (2002). Molecular mechanisms of cytokine receptor activation. Biochim.Biophys. Acta 1592, 215-223.
Harding, P. A., Wang, X. Z., Kelder, B., Souza, S., Okada, S. and Kopchick, J. J.(1994). In vitro mutagenesis of growth hormone receptor Asn-linked glycosylationsites. Mol. Cell Endocrinol. 106, 171-180.
Jansens, A. and Braakman, I. (2003). Pulse-chase labeling techniques for the analysisof protein maturation and degradation. Methods Mol. Biol. 232, 133-146.
Laron, Z., Pertzelan, A., Karp, M., Kowadlo-Silbergeld, A. and Daughaday, W. H.(1971). Administration of growth hormone to patients with familial dwarfism with highplasma immunoreactive growth hormone: measurement of sulfation factor, metabolicand linear growth responses. J. Clin. Endocrinol. Metab. 33, 332-342.
Leung, D. W., Spencer, S. A., Cachianes, G., Hammonds, R. G., Collins, C., Henzel,W. J., Barnard, R., Waters, M. J. and Wood, W. I. (1987). Growth hormone receptorand serum binding protein: purification, cloning and expression. Nature 330, 537-543.
Meusser, B., Hirsch, C., Jarosch, E. and Sommer, T. (2005). ERAD: the long road todestruction. Nat. Cell Biol. 7, 766-772.
Ross, R. J., Leung, K. C., Maamra, M., Bennett, W., Doyle, N., Waters, M. J. andHo, K. K. (2001). Binding and functional studies with the growth hormone receptorantagonist, B2036-PEG (Pegvisomant), reveal effects of pegylation and evidence thatit binds to a receptor dimer. J. Clin. Endocrinol. Metab. 86, 1716-1723.
Sachse, M., van Kerkhof, P., Strous, G. J. and Klumperman, J. (2001). The ubiquitin-dependent endocytosis motif is required for efficient incorporation of growth hormonereceptor in clathrin-coated pits, but not clathrin-coated lattices. J. Cell Sci. 114, 3943-3952.
Sachse, M., Urbe, S., Oorschot, V., Strous, G. J. and Klumperman, J. (2002).Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting towardlysosomes. Mol. Biol. Cell 13, 1313-1328.
Schantl, J. A., Roza, M., Van Kerkhof, P. and Strous, G. J. (2003). The growth hormonereceptor interacts with its sheddase, the tumour necrosis factor-alpha convertingenzyme (TACE). Biochem. J. 377, 379-384.
Sobrier, M. L., Dastot, F., Duquesnoy, P., Kandemir, N., Yordam, N., Goossens, M.and Amselem, S. (1997). Nine novel growth hormone receptor gene mutations inpatients with Laron syndrome. J. Clin. Endocrinol. Metab. 82, 435-437.
Sommer, T. and Wolf, D. H. (1997). Endoplasmic reticulum degradation: reverse proteinflow of no return. FASEB J. 11, 1227-1233.
Sotiropoulos, A., Perrot-Applanat, M., Dinerstein, H., Pallier, A., Postel-Vinay, M.C., Finidori, J. and Kelly, P. A. (1994). Distinct cytoplasmic regions of the growthhormone receptor are required for activation of JAK2, mitogen-activated protein kinase,and transcription. Endocrinology 135, 1292-1298.
Strous, G. J., van Kerkhof, P., Govers, R., Ciechanover, A. and Schwartz, A. L.(1996). The ubiquitin conjugation system is required for ligand-induced endocytosisand degradation of the growth hormone receptor. EMBO J. 15, 3806-3812.
Szecowka, J., Tai, L. R. and Goodman, H. M. (1990). Effects of tunicamycin on growthhormone binding in rat adipocytes. Endocrinology 126, 1834-1841.
van Kerkhof, P. and Strous, G. J. (2001). The ubiquitin-proteasome pathway regulateslysosomal degradation of the growth hormone receptor and its ligand. Biochem. Soc.Trans. 29, 488-493.
Jour
nal o
f Cel
l Sci
ence

van Kerkhof, P., Govers, R., Alves dos Santos, C. M. and Strous, G. J. (2000).Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J. Biol. Chem. 275, 1575-1580.
van Kerkhof, P., Alves dos Santos, C. M., Sachse, M., Klumperman, J., Bu, G. andStrous, G. J. (2001a). Proteasome inhibitors block a late step in lysosomal transportof selected membrane but not soluble proteins. Mol. Biol. Cell 12, 2556-2566.
van Kerkhof, P., Sachse, M., Klumperman, J. and Strous, G. J. (2001b). Growthhormone receptor ubiquitination coincides with recruitment to clathrin-coatedmembrane domains. J. Biol. Chem. 276, 3778-3784.
van Kerkhof, P., Smeets, M. and Strous, G. J. (2002). The ubiquitin-proteasome
pathway regulates the availability of the GH receptor. Endocrinology 143, 1243-1252.
Zhang, Y., Jiang, J., Kopchick, J. J. and Frank, S. J. (1999). Disulfide linkage of growthhormone (GH) receptors (GHR) reflects GH-induced GHR dimerization–associationof JAK2 with the GHR is enhanced by receptor dimerization. J. Biol. Chem. 274,33072-33084.
Zhang, Y., Jiang, J., Black, R. A., Baumann, G. and Frank, S. J. (2000). Tumornecrosis factor-alpha converting enzyme (TACE) is a growth hormone binding protein(GHBP) sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced)GH receptor proteolysis and GHBP generation. Endocrinology 141, 4342-4348.
Journal of Cell Science 119 (15)3086
Jour
nal o
f Cel
l Sci
ence