Dissolution presentation by subhakanta Dhal
-
Upload
subhakanta-dhal -
Category
Education
-
view
1.943 -
download
1
description
Transcript of Dissolution presentation by subhakanta Dhal

(In context To BCS,FDA prospective, Release kinetics)
PART-1
Presented bySUBHAKANTA
A Brief concept To Dissolution Method Development
aALKEM
R & D

Dissolution:
• “ Dissolution is the amount of drug substance from a dosage form that goes into solution per unit time under standard conditions of solid /liquid interface, temperature and solvent composition.”
• Takes two steps one is liberation (drug release from dosage form) and convection process ( drug transport within the dissolution media).USP <1088>:
• “No product, including suspensions and chewable tablets, should be developed without dissolution or drug release characterization where a solid phase exists." and
• “Dissolution testing is required for all solid oral Pharmacopeias dosage forms in which absorption of the drug is necessary for the product to exert the desired therapeutic effect. Exceptions are for tablets meeting a requirement for completeness of solution or for rapid (10 to 15 minutes) disintegration for soluble or radiolabeled drugs.”
aALKEM
R & D

Dissolution History:• 1945–1950 Disintegration official in Brit Pharmacon and USP• 1962 PMA Tablet Committee proposes 1% solubility threshold• 1967 USP and NF Joint Panel on Physiological Availability chooses dissolution as a test
chooses an apparatus• 1970 Initial 12 monograph standards official• 1971–1974 -Variables assessment; more laboratories, three Collaborative Studies by PMA and
Acad. Pharm. Sci• 1975 -First calibrator tablets pressed; First Case default proposed to USP• 1978 Apparatus 2—Paddle adopted; two Calibrator Tablets adopted• 1979 New decision rule and acceptance criteria• 1981 Policy adopted January, includes the default First Case, monograph proposals published
in June• 1982 Policy proposed for modified-release dosage forms• 1984 Revised policy adopted for modified-release forms• 1985 Standards now in nearly 400 monographs; field considered mature; Chapter <724>
covers extended-release and enteric-coated• 1990 Harmonization: apparatus 4—Flow- through adopted; Apparatus 3 Apparatus 5, 6, 7 for
transdermal drugs• 1995 Third Generation testing proposed—batch phenomenon; propose reduction in alibration
test number• 1997 FIP Guidelines for Dissolution Testing of Solid Oral Products; pooled analytical samples
allowed aALKEM
R & D

Dissolution History: contu…
aALKEM
R & D

Objective of Dissolution Studies:
To identify a QC dissolution test method to verify process and product consistency.
At Registration and beyond
To identify a test method that can provide an IVIVR, IVIVC, or other bio-relevant information.
II-III
To clearly establish the mechanism of invitro drug release and solubilization.
0-I
ObjectivePhase
To confirm manufacturing and product consistency.To evaluate the quality of product during its self life.To assess post approval changes and for BE studies.
To evaluate the rate of drug release.Monitoring products’release consistency. Assessing formulation changes.
Establishing IVIVC or IVIVR.
During Commercial stage
During development stage
aALKEM
R & D

What Does Dissolution Measure?
Solid drug particle stagnant layer (h) with aconcentration = Cs
bulk solution with aconcentration = CT
Bulk Solvent
)()( 1 tsts cckccvhD SkR −=−=
Modified Noyes and Whitney Equation
aALKEM
R & D

Mechanism of Dissolution
aALKEM
R & D

Mechanism of Dissolution
aALKEM
R & D

Factor affecting dissolution• A. PHYSICOCHEMICAL FACTORS • Drug solubility and dissolution rate• Particle size and effective surface area• Polymorphism and amorphousim• Salt form of the drug• pKa of the drug and pH – (pH partition hypothesis )
• B. PHYSIOLOGICAL FACTORS• .Gastric emptying time• Intestinal transit time• Gastrointestinal pH• Disease states• Blood flow through the GIT• Gastrointestinal contents: • Pre-systemic metabolism
• C. PHARMACEUTICAL FACTOR• Manufacturing variables• Pharmaceutical ingredients (excipients / adjutants)• Nature and type of dosage form• Product age and storage conditions• Disintegration time (tablets / capsules)
aALKEM
R & D

Factor affecting dissolution as GIT
aALKEM
R & D

Factor affecting dissolution as GIT
all types of drugs are absorbed, about half of the absorbed druggoes directly into the systemic circulation and the other half to the liver
much smaller surface areaRectum
all types of drugs are absorbed but to a lesser extentsmall surface areaLarge intestine
major site for absorption of all types of drugs (lipophilic, neutral, acidic or basic)
very large surface areaSmall intestine
lipophilic, neutral and acidic drugs absorbed but lesser than that from intestine
not too large a surface areaStomach
lipophilic, neutral and basic drugs are absorbed directlysmall surface areaMouth
4-12 hr4-12 hr5.5-6.54-12 hr4-12 hr5.5-6.5proximal colon
60 min60 min7.5(7-8)60 min120 min7.5(7-8)lower.ileum
60 min60 min7.2(6.5-7.5)60 min60 min7.2(6.5-7.5)upper.ileum
60 min60 min6.8(6-7.2)60 min30 min6.8(6-7.2)lower jejunum
60 min60 min5.5(5.5-7)60 min30 min6.5(5.5-7)upper jejunum
< 10 min< 10 min5(4-7)< 10 min< 10 min6(4-7)duodenum
2-4 hr2-10 hr4(3-6)30 min60 min1.8(1-3)Stomach
MRT (pellets)MRT (Tablet)PHMRT (pellets)MRT (Tablet)PHsite
fed conditionfasting
aALKEM
R & D

Method Development- Approach:
Drug Characterization(Solubility,Permeability etc)
BCS Class Dosage form GI Physiology
Dissolution method
Media Apparatus Test duration
aALKEM
R & D

Intrinsic Drug Dissolution :
• It is the rate of dissolution of a pure pharmaceutical active when conditions such as surface area, temp, agitation – stirring speed, pH and ionic strength of the dissolution medium are kept constant.
Particle size:Dissolution rate is typically influenced by particle size and wettability. The influence of wettability on the dissolution rate of pharmaceutical powder was studied by Lippold and ohm. Example: wetting agent: Polysorbate 80.
Ionization • The amount of drug that exists in unionized form is a function of dissociation constant (pKa)
of the drug and pH of the fluid at the absorption site. for weak acid: HA H+ + A-acid base
pH = pKa + log [A-][HA]
%( )
( )drugionized xpH pKa
pH pKa=+
−
−
101 10
100
for weak base: B + H+ BH+
pH = pKa + log [B]
[BH+]
%( )
( )drugionized xpKa pH
pKa pH=+
−
−
101 10
100
aALKEM
R & D
Drug Characterization:

Nature of drug
Weakly acidic, weakly basic, free base, salt etc.
aALKEM
R & D
Drug Characterization
0.1 99.9 ≥ 3
1 99 2 to 3
10 90 ≥ 1
50 50 0
90 10 -1 to 2
99 1 -2 to -3
99.9 0.1 ≥ -3
Bases Acids
Percentage of IonizationpH-pKa
Solubility of weak bases is often best in the stomach, that of weak acids in the small intestine.

• Partition coefficientThe partition coefficient is the ratio of concentrations of un-ionized compound between the two solutions. The logarithm of the ratio of the concentrations of the un-ionized solute in the solvents is called log P.
• Higher the value, more the hydrophilic and faster the dissolution in aqueous fluids. • If log p>4 then the drug is very lipophilic, which is practically estimated by shake flask method.
Usually intestinal permeability increases with liphophilicity but decreases with molecular weight or H-bonding properties.
• Low LogP( below 0) injectable• Medium (0-3) oral• High(3-4) Transdermal• Very High (4-7)toxic build up in fatty tissues.• Drugs should be designed with the lowest possible Log P
• Effective surface area of the drug particles .When the particle size of a certain mass of a drug is reduced the surface area is increased, hence, if particle size is reduced dissolution rate increases .Two types of surface area can be defined:Absolute surface area: Which is the total area of solid surface of any particle, andEffective surface area: Which is the area of solid surface exposed to the dissolution medium
aALKEM
R & D
Drug Characterization

aALKEM
R & D
Drug Characterization
Lipnski rule of 5 (an orally active drug has no more than one violation of the following criteria)
• Not more than 5 hydrogen bond donors (nitrogen or oxygenatoms with one or more hydrogen atoms)
• Not more than 10 hydrogen bond acceptors (nitrogen or oxygenatoms)
• An octanol-water partition coefficient log P of less than 5 • A molecular weight under 500 daltons. Exception to Exception to LipnskiLipnski rule of 5rule of 5
1. Compound classes that are substrates for biological transporters:2. Antibiotics3. Fungicides-Protozoacides -antiseptics4. Vitamins5. Cardiac glycosides.

BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS)
IVIVC Level A expected
VariableVariableBasic
Little or no IVIVCVariableVariableAcidic
Case by caseLittle or no IVIVCLow ,Dependent on site andNarrow absorption window
Low & SiteIndependent
4
permeabilityIVIVCLevel A expected
High & SiteIndependent
HIGH, & Site
Independent
3
dissolutionIVIVCLevel C expected
High, Dependent on site and
Narrow absorption window
low & SiteIndependent
2
Gastric emptying
IVIVC Level Aexpected
High & SiteIndependent
High & Site independent
1
Absorption control
IVIVCPermeabilitySolubilityClass
aALKEM
R & D

Solubility A drug substance is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over the pH range of 1-7.5. A Drug is said to be very rapidly dissolving when NLT 85% is dissolved in 15 min using usp-1 at 100RPM (or 50 rpm )in volume 500mlin each media :0.1N Hcl OR SGF WITHOUT ENZYME ,PH4.5,PH 6.8 OR SIF .
Permeability:A drug substance is considered to be highly permeable when the extent of absorption is at least 90% in comparison to an i.v. reference dose .
Methods used for determination of permeability include: a. Mass balance studies, Absolute bioavailability studies and Intestinal perfusion methods
in human b. In vivo or in situ intestinal perfusion in a suitable animal model c. In vitro permeability methods using excised intestinal tissues d. Mono layers of suitable epithelial cells e.g. Caco-2 cells or TC-7 cells .
BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS) CONT….
aALKEM
R & D

.
aALKEM
R & D
BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS) CONT….
ADDITIONALLY APPROACH OF BCS
The dose number (Do)- IT is the ratio of the dose to the amount of drug that will dissolve in 250 mL of range 1 to 8. Ideally, this ratio should be below 1 if full dissolution is to be possible in principle.
• The absorption number (A n) -IT is the ratio of the transit time to the absorption time (1/absorption rate constant). Ideally, this should exceed 1.
• The dissolution number (D n)-IT is the ratio of the transit time to the dissolution time (1/dissolution rate constant). Ideally, it should exceed 1..

.
BIOPHARMACEUTICS CLASSIFICATIONSYSTEM (BCS) APPROACH FOR CLASS I/III…..
aALKEM
R & D

.
aALKEM
R & D
BIOPHARMACEUTICS CLASSIFICATIONSYSTEM (BCS) APPROACH FOR CLASS II/IV…..

Method Development as per FDA Prospective
aALKEM
R & D

FDA ROLE in DISSOLUTIONEnforcement of USP
• Publishes Guidances• Co-Sponsors workshops with AAPS• Task force
– Gelatin working group• Perform off shelf testing• Perform validation on NDA methods• Inspections• Recalls• Approve products• Publishes the Orange Book
– Approved drug products with therapeutic equivalence evaluations• AB….• www.fda.gov/cder/ob/default.htm
aALKEM
R & D

FDA Guidances• Dissolution Testing of Immediate Release Solid Oral Dosage Forms - 1997• Extended Release Oral Dosage Forms:Development, Evaluation, and Application of
In Vitro/In Vivo Correlations• SUPAC-IR: Immediate-Release Solid Oral Dosage Forms: Scale-Up and Post-
Approval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
• SUPAC-MR: Modified-Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
• Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on Biopharmaceutics Classification System -2000
• 483 warnings– Failure to investigate Out of Specification results– Do not have or follow SOP’s– Inadequate calibration program or compliance– Bubbles in vessel observed– Unvalidated methods or computer programs
aALKEM
R & D

FDA PERSPECTIVE
aALKEM
R & D

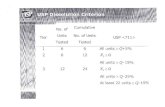
Apparatus
United Sates of Pharmacopiea:-Apparatus 1 Rotating basket Tablets/Capsules -Apparatus 2 Paddle assembly Tablets/Capsules -Apparatus 3 Reciprocating cylinder Escalating pH media -Apparatus 4 Flow-through cell Low soluble drugs -Apparatus 5 Paddle over disk Semisolids and transdermal -Apparatus 6 Cylinder Transdermal patches -Apparatus 7 Reciprocating holder Transdermal patches
European Pharmacopiea:/ British Pharmacopiea
-For solid dosage forms Paddle/basket/flow through cell -For transdermal patches Disk assembly method/cell method/rotating cylinder -For special dosage forms Chewing apparatus/Flow through apparatus
aALKEM
R & D

STRATEGY FOR METHOD DEVLOPMENT
aALKEM
R & D

aALKEM
R & D
STRATEGY FOR METHOD DEVLOPMENT

STRATEGY FOR METHOD DEVLOPMENT CONT…… Selection of Test Conditions
aALKEM
R & D

aALKEM
R & D
STRATEGY FOR METHOD DEVLOPMENT CONT…… Selection of Test Conditions

Choices of Media
aALKEM
R & D
VolumeMaximum Dissolvable Dose = V . CS / Sin kV = Dissolution medium volume,CS = Saturated solubility of the co m pound in the medium
Sink = Sink condition factor
Sink condition-It is volume of medium at least three times to required form saturated solution of a drug substance.
Some sources recommend 5x or even 10x.•If a medium fails to provide sink condition or said to be more discriminating Which can be provide some surfactant to the medium.
•Sink condition is a must and is a volume (not a ratio) in which drug could easily and accurately be dissolved . And be on the safe side, just increase the volume 20-30% than the volume required .
•Sink condition is mandatory; however, ratio of 3:1 (medium volume used vs medium volume required for saturation with drug) .

Choices of Media cont..
Volume: sink condition cont….
• Different techniques to improve sink conditions.
Addition of organic solvents to aqueous medium,Use of large dissolution volume,pH changesAddition of surfactants or their combinations Medium Volume
• 900 mL typical• 500 mL for low dosage strengths• 1000 mL for increased solubility, sink• 2 L, 4 L, 200 mL - special needs• Exceptions• low solubility compound-2000 to 4000ml• Highly potent drug -100 to 250ml.
aALKEM
R & D

• Acid
–Hydrochloric acid (0.1--0.001 N)
• Buffers (use USP preparation instructions)
–Acetate (pH 4.1--5.5; 0.05 M)
–Phosphate (pH 5.8--8.0; 0.05M)
• Water–Disadvantages
• No buffering capacity, can’t measure pH accurately
• Some regulatory bodies do not favor usage
• Different conductivity/pH depending on source
–Advantages
• Inexpensive, easy disposal
• Useful in correlations, provide good profile
Non-aqueous media
• -hydroalcholic media is used (e.g cortsone acetate tab)
•Measure pH before and after run
Medium Selection
aALKEM
R & D

Surfactants
aALKEM
R & D
•For low solubility compounds, adequate dissolution can not be obtained with aqueous solutions within the physiologic pH ranges. That’s why compounds, aqueous solution containing a surfactant may be used to enhance drug solubility.•A surfactant can be used as either a wetting agent or when the critical Micelle concentration (CMC)is reached, to stabilize the drug .•Addition of surfactant – Air bubble trapping
EXAMPLESodium lauryl sulfate,Polyoxyethylenesorbitan monolaurate (Tween),Cetyltrimethylammoniumbromide (CTAB ),Polyoxyl castor oil (Cremophor),Hexadecyltrimethylammonium bromide (HTAB),Polyethylene glycol tert-octphenyl ether (Triton),
Media-enzymes
Need for enzyme- for cmc formationCross linkage of gelatin capsule shellsExamplePepsin-SGF(PH-1.2)Pancreatin –SIF (PH-6.8)

STRATEGY FOR METHOD DEVLOPMENT
aALKEM
R & D

Selection of Test Apparatus• Basics for Apparatus
– Need low variability
– Good profile
– Should pick up changes
– Hydrodynamic aspects-
aALKEM
R & D

Typical Apparatus Speeds
Paddles– 50 rpm preferred-speed for BCS– 75 rpm to eliminate coning, variability– 25 rpm or more used for suspensions– Used with tablets or capsules with sinkers– 100 rpm or higher needs justification– 100 rpm used frequently with ER
• Basket– 50-100 rpm preferred– Over 100 rpm sometimes necessary– Used for floating dosage forms– Used for slowly disintegrating dosage forms• Reciprocating cylinder-15 rpm(capsule,tablet,suspension)• Paddle over disk-25rpm (TDS)
aALKEM
R & D

Temperature• Oral dosage forms -37 ± 0.5°c • Suppositories- 38 ± 0.5°c• TDS- 32 ± 0.5°c
• Chewing gum- 37 ± 0.5°c
Hydrodynamic Issues• Fluid Flow
– Basket mesh size– Coning with paddle– In-residence
probes/automation– Vessel irregularities
aALKEM
R & D

Deaeration
• Troubles from non-de -aerated medium– Slow down (barrier) or speed up
(buoyant)– Bubbles adhere to screens– Particles adhere to bubbles that
may be clinging to the vessel walls
– Basket carries down the bubble on surface
– Can break up cone– Surfactants not practical due to
foaming• Additionally air bubbles- cling to
apparatus & vessel wall.• Bubbles on the dosage unit→
buoyancy (↑) → dissolution (↑)
aALKEM
R & D
Methods

Sinkers• USP -- ”such as not more
than a few turns of a wire helix…”
• Uniformity is critical, especially when transferring method
• Sinker should be validated• Method should Compared
with sinker vs non sinker• Sinkers can be barriers to
dissolution.
20.55
8#3 and #4
30.7
10#1 and #2
40.8
12#0, elongated
Cork Bore Number
Diameter Size (cm)
Length of Wire (cm)
Capsule Shell Type
aALKEM
R & D

Test Duration and sampling interval• Rapidly Dissolving product (IR PRODUCT)- profile generated at 5-10 min,interval may necessary.
• Extended release product- At least 3 point of dissolution
• IR PRODUCTHighly soluble (class-I & III)-A single point NLT 85% in 60 min.
Poorly soluble (class-II & IV)-2 Point dissolution specification ,one at 15 min, other at 30,45,60 min
• When avg. dissolution from reference product does not reach 85% ,at testing time would be T/4,2T/4,3T/4,T
• INFINITY POINT FOR DEVLOPMENT STUDY- Help during formulation study at 150rpm for 1 hr or 2 hr depending
on type of dosage form.
. aALKEM
R & D

Observations
• Particle disintegration pattern/must disperse freely
• Floating (chunks), spinning• Coning, mounding • Gumming, swelling• Capping, clam shell• Erosion pattern• Center/off-center, sticking• Particles adhering to
apparatus/vessel • Ballooning, rubbery mass, pellicles• Particle size (snowflake, fine)• Look on surface around shaft• Disintegration and shell dissolution
rate• BUBBLES
aALKEM
R & D

aALKEM
R & D
Observations

Sampling
• Syringe
– Plastic or glass
• Needle
– Stainless steel
– Bent or straight
• Filter - end of probe, in line, after sampling
• Pipettes-not good unless filter at tip
• Pore size of filter
– 0.2 micron - 70 micron
– Depth filters, full flow
aALKEM
R & D

Maintenance Schedule
aALKEM
R & D

Discriminating dissolution mediaDiscriminating Its ability of method to detect changes in the drug product performance, lot –to lot variation, process changes (SUPAC) and to discriminate between dosage forms that are not bioequivalent.
-if similarity of batch------go for Discriminating Method-for BA,BE,IVIVC---------------Go for Bio-relevant Media
Development of a Discriminating Method
• A method must be developed which is both (test as well as reference)a) discriminating, and b) reproducible enough for day-to-day operation, and capable of transfer between labs.• The acceptance criteria should be representative of multiple batches with the same
formulation/manufacturing process, including key batches (e.g. BE).• The procedure should be capable of distinguishing significant changes in
composition or manufacturing process that might be expected to affect in vivo performance
aALKEM
R & D

Development of a Discriminating Method cont…..
•Factors to consider:
-Formulation point of viewQualitative and quantitative Excipient changesManufacturing parameters (examples):
LubricationBlend timeCompression forceDrying parameters
-Method development point of view -Biorelevant media-Rpm challenge-apparatus challenge-media volume challenge-Effect of bile salt (if any effect on product)
aALKEM
R & D

Bio-Relevant Media
OBJECTIVES
1. The biorelevant method should be able to stimulate the in vivo environment where the majority of drug being released.
2. Biorelevant dissolution media, apparatus and test conditions Should be discussed with emphasis on their relevance to the physiological factors, including the pH, composition of the GI fluids, volume, GI hydrodynamics/motility, and food effect.
Choice Biorelevant medium• Fed and fasted media• pH at site of absorption
aALKEM
R & D

Gastric Emptying
Transit PermeationDissolution
Metabolism
DISSOLUTION
aALKEM
R & D

Biorelevant for Gastric condition
Gastric condition : a view
-The fasted state of young healthy subjects, values of gastric pH are generally between 1.4 and 2.1
-The surface tension of gastric fluid .
- Addition, surfactants, such as SLS, should be added into the medium.
-To simulate the fed state in the stomach, the use of milk (Macheras et al., 1987) and Ensure R_ (Ashby et al., 1989) may be appropriate, since these media offer appropriate ratios of fat to protein and fat to carbohydrate.
aALKEM
R & D

Possible targeted Biorelevant gastric Dissolution Media
• Pre-prandial stomach1. SGF USP (pH 1.2) without enzyme2. SGF USP plus surfactant (e.g. 0.1% Triton X)-PH-1.8 plus perhaps pepsin
• Post -prandial stomach1. Ensure Plus2. Bovine milk 3.5% fat
aALKEM
R & D
1L1Lwater
1gTriton x 100
7g3gHcl con.
2g2gSodium chloride
SGF PH 1.2FASGF PH 1.8

Biorelevant for Intestinal conditionIntestinal condition : a view
• The dissolution of drug products in the small intestine is influenced by physiological factors pH, endogenous secretions from the pancreas and gall bladder (e.g., bile salts, lecithin, and digestion enzymes), and food effects.
• The pH values of intestinal conditions are considerably higher.
• one apparent difference between the SGF and FaSSIF is that this simulated intestinal medium contains bile salts and lecithin .
• The dissolution rate of poorly soluble, lipophilic drugs may be improved greatly in this medium in comparison to the dissolution rate observed in simple aqueous solutions .
• The volume of the fasted state simulated intestinal medium, pharmacokinetic studies in the fasted state show that by ingesting 200–250mL of water with the dosage form, a total volume of 300–500mL will become available in the proximal small intestine. Hence volume of 500mL is recommended for the FaSSIF
aALKEM
R & D

Biorelevant for Intestinal condition contue..
aALKEM
R & D

PH OF DIFFERENT CONDITION OF MEDIA FOR REMEMBER
5.8SCOF
5SIF FED
6.5SIF FAST
6.8SIF with enzyme
2.1SGF without enzyme
1.2SGF with enzyme
2.12.1with SLS
30.001 N Hcl
2.10.01 N Hcl
1.20.1 N Hcl
PHMedia
aALKEM
R & D

DIFFERENT CONDITION OF MEDIA FOR REMEMBER
Dissolution media for ( Class I and Class III)• simple medium is sufficient• USP media or aqueous buffers1. SGFsp pH 1.22. Acetate buffer pH 4.53. SIFsp pH 6.8
Dissolution media for ( Class II and Class IV)
FaSSIFpreprandialSmall intestineEnsure® Plus ,MilkpostprandialStomach
FaSSGFSGFsp (USP) plus surfactant (Triton X 100)
preprandialStomach
Mediumpre-/post prandial
postprandiaLocation
aALKEM
R & D

DIFFERENT CONDITION OF MEDIA FOR MR PRODUCT
• For extended-release drug products, the dissolution method must capture, at minimum, the changes in composition, pH, and residence times along the GI tract, since absorption of these dosage forms takes place throughout the entire intestine.
• The reciprocating cylinder and flow-through cell systems can be used, in conjunction with different biorelevant dissolution media, to assess the in vivo release behavior of extended-release dosage forms
Biorelevant Media for Studying Food Effects on Releasefrom MR Dosage Forms
aALKEM
R & D

Dissolution testing and alcohol-induced dose-dumping of generic MR oral drug products
• Some modified-release oral dosage forms can contain drugs and excipients which are highly soluble in ethanol.
• Even moderate amount of alcohol may cause problem (dose-dumping) in vivo.
• The Office of Generic Drugs recommends assay
1. Designed to compare dissolution performance of the generic (test) product and the corresponding reference listed drug.
2. 0.1 N HCl media with differing amounts of ethanol(v/v) addedto give the following percentages of ethanol in the media: 0%, 5%, 20%, and 40%
aALKEM
R & D

Alcohol-induced dose-dumping of generic MR oral drug products continue…
Methods.• The assay was implemented for several MR drug products under review at the Office of
Generic Drugs (OGD). The assay employs USP Apparatus I or II and 900 mL of 0.1 N HClmedia containing ethanol (v/v) at: 0%; 5%; 20%; and 40%, sampling every 15 minutes until 2 hours. Applicants conduct these studies on all drug product strengths.
The dissolution results are categorized as
• Case I: If at 2 hours, % dissolved of the generic product in 40% ethanol is ≤ in 0% ethanol, the generic product is considered robust (does not dose-dump); if not
• Case II: at 2 hours, % dissolved of the generic product in ethanol solution is less or comparable to that of the reference, the potential for dose-dumping is similar for the two products and the generic product is acceptable; if not
• Case III: the generic product releases more drug in ethanol than the reference and is unacceptable.
• Results.
To date, the OGD reviewed 22 submissions, representing 7 different drugs, containing results of in vitro dose-dumping in alcohol studies. Of these 22 studies, 12 (54.6%), 9 (40.9%), and 1 (4.5%) were categorized as Case I, II, and III, respectively.
Similar trends in study outcome were observed for the various strengths of each drug product line tested. a
ALKEMR & D

Dissolution Method Development At a glance
aALKEM
R & D
Selection of test conditions•classify the drug substance according to the BCS as characterize the drug solubility over the pH range 1.2 to 6.8• Run dissolution tests with the pure drug to determine whether there are any wetting problems.• choose appropriate media (pH, composition) and volumes

Dissolution Method Development At a glance
aALKEM
R & D

May God bless you….Thank You
aALKEM
R & D