Design issues of Membrane Reformer
-
Upload
emmanuel-dalton -
Category
Documents
-
view
32 -
download
0
description
Transcript of Design issues of Membrane Reformer

Design issues of Membrane Reformer
M. Sarić, Y.C.van Delft, A. de Groot
Objective• System and sensitivity studies for front-end of NH3 plant • Membrane reformer design
System studies
Results system and sensitivity studies Optimum: pfeed= 30 bar, ppermeate= 5.5 bar, T= 650 ºC, counter-
current mode , S/C = 3.21
Energy savings in NL
is 7.5 PJ/y. membrane area Amem = 6000 m2
Conclusions• Mass transfer resistance is in catalyst bed and
membrane. • Coupling of the mass and heat transfer area lead to:
1. 10 x higher catalyst requirement 2. Installing 4x more heat transfer area then required => overdimensioned membrane reformer
• Designing of cost effective membrane reformer requires that different processes such as mass, heat transfer, reaction rate and permeance rate are well-matched in the reactor design.
We gratefully acknowledge our partners for their contribution in this project and the DutchMinistry of Economic Affairs for the financial support within the EOS LT programme.
ECNP.O. Box 1NL 1755 ZG PettenThe Netherlands
Figure 1 The membrane reformer concept
BackgroundThe (petro) chemical industry in the Netherlands is responsible for one sixth of the total energy use. A substantial part is used to produce industrial hydrogen. This takes place in huge reformer units where methane reacts with steam to become H2 and CO. By selective removal of H2 from the reaction zone in the membrane reformer energy efficiency of the process can be considerably increased.
Figure 2. Conventional front –end Figure 3. Membrane front –end
0
10000
20000
30000
40000
50000
60000
70000
0 2 4 6 8
permeate pressure [bar]
MU
G
[KW
]
0
10000
20000
30000
40000
50000
60000
70000
me
mb
ran
e a
rea
[m2 ]
Figure 4. Compressor duty (MUG) and Amem vs. ppermeate for T= 600 ºC
CONTINENTAL ENGINEERS BV
Membrane reformer designs
fuel
flue gas
permeate
retentate
sweep
membranetube
burnertube
feed
air
retentate
permeate
sweep
feed
fuel
flue gas
air
Figure 5 Design 1
Figure 6. Design 2
Results: Design 1
U [W/m2/K]
# tubes
1001 312
301 1041
4 2 7811
Table 1. Number of reformer tubes required for different heat transfer coefficients (dt=11cm,L=9m)
Figure 7. Dependency of Amem ,tube lenght and Δp on different parameters
Membrane
CH4+H2O=CO+3H2 CO+H2O=CO2+H2
H2 H2H2H2
Natural gas +H2O CO2+H2O
SweepH2+Sweep
1 –Branan, C.R.,20022- Design 1
REF:
ucatbed=0.3ms-1,dm=2cm,scat=1cm,dp=1mm,stagnant layer =2mm
0
5
10
15
20
25
30
35
40
45
refe
rence
REF + d
p=2m
m
REF + d
mem
=0.5
REF + c
at=0.
5cm
REF + u
catb
ed=0.
1m/s
L [
m],
Dp
[b
ar]
10,000
12,000
14,000
16,000
18,000
20,000
22,000
24,000
26,000A
mem
[m
2 ]
L tubes[m]
Dp [bar]
Amem [m2]
SMRNatural gas + H2O
BURNER Natural gas + air
POX HTS
CO2 removal +Methanation
LTS
H2O
air
N2 + H2 to syngas compressor
SMR = Steam methane reformer
POX = Partial oxidation unit
HTS = High temp. shift
LTS = Low temp. shift
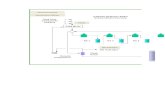
RetenateMembrane reformer Natural gas + H2O
BURNER Natural gas + air
N2
ASU
O2
air H2 H2
N2 + H2 to syngas compressor
ASU = Air Separation Unit