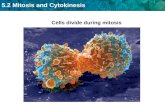
DNA Part IV: Cellular Reproduction- Mitosis and Cytokinesis.
Cytokinesis following mitosis
description
Transcript of Cytokinesis following mitosis

ECISA MORPHOLOGICAL BIOSENSOR FOR
CELL RESEARCH
Applied BioPhysics, Inc. (www.biophysics.com)



Cytokinesis following mitosis Membrane Ruffling

The basic principle of ECIS was first reported by Giaever and Keese, then at the General Electric Corporate Research and Development Center.
Giaever, I. And Keese, C.R. PNAS 81, 3761-3764 (1984).
ECISElectric Cell-substrate Impedance Sensing

250 µm
WE
CE
WE: Working ElectrodeCE: Counter Electrode
The ECIS Electrodes

250m
Array Holder in Incubator Space
ECIS 8 well Array

ECISElectric Cell-substrate Impedance Sensing
A cell morphology biosensor
<1 A, 4000 Hz
The measurement is non-invasive AC Current
source
PC
ECIS electrode
Counter electrode
Culture medium (electrolyte)
Phase sensitive impedance measurement
PC
R C

Cell Inoculation (105 cells per cm2)
BSC-1cells
NRK cells
No cells

A published model fits the experimental data The measured impedance can be broken down into three parameters
1) Rb, the barrier function of the cell layer
2) Alpha, a term associated with the constricted current flow beneath the cell
3) Cm, the membrane capacitance
[Giaever, I. and Keese, C.R., PNAS 81, 3761 (1991)]

Detection of single cell activity

What is measured using ECIS?
Cell morphology changes including:1) Barrier function of confluent layers2) Relative size of cells and spaces beneath cells3) Membrane capacitance
All measurements are made in normal culture medium
The measurement is non- invasive
LimitationsCells must anchor and spread upon substratumA limited population of cells is measured at one time (1 to 1,000 cells)

DNARNA
Viral Infection
GlucoseOxygen
COOH
OOCCH3Drugs
Ligand Binding
Physical ChangesShear, Electric Fields
Changesin Cell
Morphology
MetabolismCytoskeleton
Electric Cell-Substrate Impedance Sensing

Measurement of Metastatic Potential using ECIS™
BioTechniques, October 2002
Keese, Bhawe, Wegener and Giaever

The basis of the metastatic assay

The Dunning prostatic adenocarcinoma series was developed at Johns Hopkins and consists of several cell sublines.
These all have their origin in a single line isolated from a prostatic tumor. After extensive passaging and mutagenesis, several distinct sublines were isolated having different in vivo metastatic abilities. Six of these lines were used in our studies.

To carry out the metastatic assay, first a layer of endothelial cells is established
Confluence verified

Challenge of HUVEC cell layers with weakly (G) and highly metastatic (AT3) cell lines
Challenge
highly metastatic

Confluent HUVEC layerNo cells
MLL Challenge 105 cells/cm2

Prostatic cell challenge

Signal Transduction

[Ca2+]
Alterations in the cytoskeleton
G Protein Coupled Receptor

CHO cells engineered to over-express the muscarinic receptor exposed to the agonist carbachol
EC50 = ~1M

The effect of carbachol is blocked by the antagonist pirenzipine (PZP)

0 1 2 3 4 54
6
8
10
100 M Carbachol
4 kHz
|Z| [
k]
t [hrs]

0 1 2 3 4 54
6
8
10
100 M Carbachol
4 kHz
|Z| [
k]
t [hrs]
0 1 2 3 4 50
1
2
3
4
5
Cm
Rb
Nor
m. P
aram
eter
t [hrs]
Treatment of CHO-M1T cells with carbachol
Data analysis using the ECIS model morphological information

Similar results are obtained with the beta adrenergic receptor

The Dynamics of Cell Spreading

WI-38 VA/13 cells
Cell inoculation 105 cells/cm2
Electrodes were pre-coated with different layers of adsorbed protein before cell inoculation
Adsorbed proteins alter cell spreading dynamics

MDCK II cells inoculated on electrodes pre-coated with various proteins
FN fibronectin
LAM laminin
VN vitronectin
BSA bovine serum albumin
BSA
FN
Inoculation
Confluent
Cell-free
Capacitance at high freq. measures the open (cell-free) electrode area

Adsorb BSA
re-inoculate with MDCK cells
after 24 hours remove cell
MDCK cells
BSA is adsorbed to the electrodes and they are inoculated with MDCK cells

Adsorb BSA
re-inoculate with MDCK cells
after 24 hours remove cell
MDCK cells
Laminin-like response

MDCK cells inoculated on fibronectin-coated electrodes with different concentrations of synthetic tetrapeptide RGDS present

MDCK cells inoculated on laminin-coated electrodes with different concentrations of synthetic tetrapeptide RGDS present

Elevated Field Applications
1 Electroporation
2 Wound healing assay

Elevated Field Applications
1 Electroporation
2 Wound healing assay

NORMAL MODE 1 MICROAMP, 10 MILLIVOLTS
ELEVATED FIELD 1 MILLIAMP, A FEW VOLTS
pore formation
Elevated current applied ~200msec

500 msec200 msec100 msec50 msec
Variation of the pulse duration: Lucifer yellow uptake
Pulse: 40 kHz 4.0 V
MDCK Type II cells
Variation of the pulse duration: Lucifer yellow uptake
Pulse: 40 kHz 4.0 V
MDCK Type II cells

Uptake of dyes with different molecular weight
Lucifer YellowM = 0.5 kDa
TRITC-dextranM = 76 kDa
Pulse: 40 kHz, 4.0 V, 200 msec
FITC-dextranM = 250 kDa
Albany Medical College (F. Minnear) has demonstrated introduction of DNA constructs using
the method and obtained expression of GFP

bleomycin only
bleomycin with electroporation
High field pulse for 100 msec
Electroporated control
Electroporation of bleomycin into HUVEC monolayers

Wound Healing (migration) Assay

Traditional Wound Healing AssayProblems of reproducibility and quantification
Cell migration

500 msec200 msec100 msec50 msec
Variation of the pulse duration: Lucifer yellow uptake
Pulse: 40 kHz 4.0 V
MDCK Type II cells
Cell death

NORMAL MODE 1 MICROAMP, 10 MILLIVOLTS
ELEVATED FIELD 1 MILLIAMP, A FEW VOLTS
Severe pore formation
localized heating
Elevated current applied 15 seconds
CELL WOUNDING

NRK Cells Prior to Wounding

NRK Cells Immediately after Wounding

NRK Cells During Healing

NRK Cells After Healing

Confluence
Open electrode
RPI

BSC-1 cells
NRK cells
wounding

Phase Contrast Microscopy of MDCK Cell Wounding
CONTROL WOUNDED 20 HOURS LATER
Are the cells killed, or are they simply damaged and recovering?

Calcein-AM and Ethidium Staining
Control 3 V, 10 sec

BSC-1 cells wounded on different size electrodes Standard 250 micron
diameter electrode
wound

BSC-1 cells wounded on different size electrodes
100 microns
wound

BSC-1 cells wounded on different size electrodes
50 microns
wound

BSC-1 cells wounded on different size electrodes
Lag period
migration = ~17 microns/hr

Phase Contrast Microscopy of MDCK Cell Wounding
CONTROL WOUNDED 20 HOURS LATER


Initial wound Re-wound
The approach is highly reproducible

New directions
Flow cell for endothelial cell studies
96 well Format for HTS

ECIS 9600

ECIS Flow System

Acknowledgements:
Ivar Giaever
President of Applied BioPhysics and
Institute Professor at Rensselaer
Joachim Wegener
Sarah Walker, Kaumudi Bhawe, Steve Tet, Will Wu, Lali Reddy, Paramita Ghosh, Guo Chen, Narayan Karra
Funding from:
NIH SBIR Program
NCRR
NCI
NIEHS
National Foundation for Cancer Research

www.biophysics.com

www.biophysics.com

www.biophysics.com

www.biophysics.com