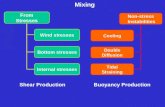
Concentration and Temperature. If a stress is applied to a system at equilibrium, the system shifts...
-
Upload
serena-mort -
Category
Documents
-
view
217 -
download
2
Transcript of Concentration and Temperature. If a stress is applied to a system at equilibrium, the system shifts...

Concentration and Temperature

If a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress.
Stresses include:◦ Change in concentration◦ Change in volume/pressure◦ Change in temperature

PCl5(g) ⇆ PCl3(g) + Cl2(g) ΔH=-47kJ

Volume and pressure are related. As the volume goes up the pressure goes down and vice versa.
(Boyles Law; P1V1=P2V2)
Only gases are affected by a change in volume/pressure
You need to count the number of moles on each side of the reaction

2H2O (g) ⇆ 2 H2(g) + O2(g)
The left side has 2 molecules of gas, while the right side has 3 molecules of gas
If the stress is pressure increasing, shift toward the side with the least number of moles of gas.
If the stress is pressure decreasing, shift toward the side with the most number of moles of gas.

Le Chatelier and Pressure - YouTube

PCl5(g) ⇆ PCl3(g) + Cl2(g)