Chromosome-refolding model of mating-type switching in yeast · Chromosome-refolding model of...
Transcript of Chromosome-refolding model of mating-type switching in yeast · Chromosome-refolding model of...

Chromosome-refolding model of mating-typeswitching in yeastBarıs Avsaroglua,1, Gabriel Bronka, Kevin Lia, James E. Haberb,c,2, and Jane Kondeva,2
aDepartment of Physics, Brandeis University, Waltham, MA 02454; bDepartment of Biology, Brandeis University, Waltham, MA 02454; and cRosenstiel BasicMedical Sciences Research Center, Brandeis University, Waltham, MA 02454
Edited by Andrew W. Murray, Harvard University, Cambridge, MA, and approved September 20, 2016 (received for review May 4, 2016)
Chromosomes are folded into cells in a nonrandom fashion, withparticular genetic loci occupying distinct spatial regions. Thisobservation raises the question of whether the spatial organiza-tion of a chromosome governs its functions, such as recombinationor transcription. We consider this general question in the specificcontext of mating-type switching in budding yeast, which is a modelsystem for homologous recombination. Mating-type switching isinduced by a DNA double-strand break (DSB) at the MAT locus onchromosome III, followed by homologous recombination betweenthe cut MAT locus and one of two donor loci (HMLα and HMRa),located on the same chromosome. Previous studies have suggestedthat inMATa cells after the DSB is induced chromosome III undergoesrefolding, which directs the MAT locus to recombine with HMLα.Here, we propose a quantitative model of mating-type switchingpredicated on the assumption of DSB-induced chromosome refold-ing, which also takes into account the previously measured stochasticdynamics and polymer nature of yeast chromosomes. Using quanti-tative fluorescence microscopy, we measure changes in the distancebetween the donor (HMLα) and MAT loci after the DSB and findagreement with the theory. Predictions of the theory also agree withmeasurements of changes in the use of HMLα as the donor, whenwe perturb the refolding of chromosome III. These results establishrefolding of yeast chromosome III as a key driving force in MATswitching and provide an example of a cell regulating the spatialorganization of its chromosome so as to direct homology searchduring recombination.
chromosome organization | homologous recombination | random-walkpolymers | quantitative fluorescent microscopy | statistical physics
Chromosomes in bacteria and eukaryotic nuclei are folded in anonrandom fashion, which leads to specific DNA sequences
assuming narrowly distributed positions within the cell or thenucleus (1–4). An interesting question is, then, to what extentdoes this spatial organization of chromosomes determine theirfunction? For example, differential gene expression during de-velopment and the maintenance of genomic integrity in responseto damaging agents have both been linked to chromosome or-ganization. In both cases the folded state of chromosomes de-termines which functionally related DNA sequences are spatiallyclose to each other, which in turn enables their function (5–11).Furthermore, earlier theoretical studies addressing aspects oflong-distance regulatory interactions between DNA sequenceshave suggested that formation of chromosomal loops can be usedto modulate the frequency of these interactions (12, 13).Double-strand breaks (DSBs) are deleterious DNA lesions
that can be repaired by homologous recombination. The repairprocess requires spatial proximity, of about 10 nm or so, of theregion around the DSB and a homologous DNA sequence, whichis located either on the same or on a different chromosome (14–17). Recent studies have revealed a role for the spatial organizationof chromosomes in homologous recombination whereby sequencesthat are closer to each other within the volume of the nucleusrecombine more readily (10, 11, 18–21). This is to be expected in amodel where the search for the homologous sequence is driven byrandom motion of the chromosomes within the nucleus. Here we
present a quantitative model and quantitative data that show that aregulated change in the folded state of a yeast chromosome after aDSB is directly responsible for steering recombination toward oneof the two available homologous DNA sequences.In the budding yeast Saccharomyces cerevisiae the spatial or-
ganization of chromosomes in interphase is determined by theirpolymer nature. Namely, the chromosomes’ spatial organizationis described in quantitative detail by a simple model of chro-mosomes as random-walk polymers confined to a spherical vol-ume, which represents the nucleus (20–29). Another element oforganization is the tethering of chromosomes to the nuclearenvelope at their telomeres and to the spindle pole body at theircentromeres (2, 22).Spatial organization of chromosomes in yeast plays an important
role in DNA recombination. For example, telomeres in yeast arespatially clustered, and rates of telomere–telomere recombinationare higher than those between telomeres and interstitial regions ofthe genome (30–32). Two recent studies examined recombinationbetween pairs of homologous loci placed at different locations inthe yeast genome, showing that recombination is more likely tooccur between loci that occupy overlapping spatial regions in thenucleus (10, 11). As remarked earlier this relation between thespatial organization of chromosomes and recombination is con-sistent with the idea that random, diffusive motion of the chro-mosomes drives homology search. Here we show that yeast cellstake advantage of this relation between spatial proximity of geneticloci and the rates of their recombination by refolding their chro-mosome after a site-specific DSB to direct recombination to aspecific donor locus and thus achieve a specific gene conversionevent. Moreover, we show that the loss of polymer entropy due to
Significance
Althoughmany studies have shown that chromosomes are foldedinto cells in a nonrandom fashion, the functional significance ofthis spatial organization remains poorly understood. Combiningtheory and fluorescence microscopy, we demonstrate that thefolded state of yeast chromosome III changes in response to aDNA double-strand break at the MAT locus, in agreement withprevious studies. Importantly, we show that the change in thefolded state of the chromosome after the break quantitativelyaccounts for the dynamics of homology search during DNA repair.Our study provides an example of a cell changing the folded stateof one of its chromosomes in response to an internal chemical cue(DNA break), thereby affecting its function (DNA repair).
Author contributions: B.A., J.E.H., and J.K. designed research; B.A., G.B., and K.L. per-formed research; B.A. and G.B. contributed new reagents/analytic tools; B.A., G.B., andK.L. analyzed data; and B.A., G.B., J.E.H., and J.K. wrote the paper.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.1Present address: Department of Molecular & Cell Biology and California Institute forQuantitative Biosciences, University of California, Berkeley, CA 94720.
2To whom correspondence may be addressed. Email: [email protected] or [email protected].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607103113/-/DCSupplemental.
www.pnas.org/cgi/doi/10.1073/pnas.1607103113 PNAS | Published online October 24, 2016 | E6929–E6938
BIOPH
YSICSAND
COMPU
TATIONALBIOLO
GY
PNASPL
US
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

chromosome refolding quantitatively accounts for the measureddifferences in efficiency with which competing donor sequences areused in repairing a double-stranded DNA break during mating-type switching.Mating-type switching in yeast is a well-studied example of
DNA DSB repair using homologous recombination that occursduring interphase (33). In haploid yeast, the mating type is de-termined by two alleles, MATa or MATα, which reside onchromosome III (Chr III). A programmed, site-specific DSB atthe MAT locus is repaired by gene conversion with one of thehomologous donor loci, HMLα or HMRa, located at the oppositeends of the same chromosome. The break at MAT is caused bythe site-specific homothallic switching endonuclease (HO) thatrecognizes a 24-bp degenerate sequence, cleaving either MATaor MATα (34). HMLα and HMRa are both heterochromatic andtranscriptionally silent and cannot be cleaved by HO, but theyserve as templates to repair the DSB at MAT (33).When the expression of HO is under the control of a galac-
tose-inducible promoter it is possible to monitor the kinetics ofmating-type switching and identify a number of distinct slow(tens of minutes) steps during the DSB repair event (35). MATswitching is thus an excellent model system for studying differentaspects of DSB repair via homologous recombination.One important feature of yeast mating-type switching that is
central to our story is the directionality in the selection of donorsduring this gene conversion event. MATa cells use HMLα 85–90%of the time, whereas MATα cells choose HMRa as a donor 90% ofthe time (36). This preference is particularly puzzling if one weresimply to predict donor preference in MATa cells based on thedistances (in base pairs) between MAT and its two donors. Giventhat the HML–MAT distance along Chr III is twice as large as theHMR–MAT distance (188 kb and 93 kb, respectively), one wouldpredict that the more proximally located HMR would be the fa-vored donor. Specifically, the random-walk nature of the yeastchromosome predicts that the probability of a close contact be-tween two genetic loci falls off with their genomic separation to thepower −3/2; this property of intrachromosomal contact probabili-ties has also been observed in chromosome conformation captureexperiments (24, 37). Based on this scaling relationHMR should befavored about threefold over HML. This simple estimate thereforeleads to a puzzle: How is it that HML is used 90% of the time (i.e.,nine times more frequently than HMR) in MATa cells?The donor-preference puzzle is resolved, at least in part, by the
action of the recombination enhancer (RE) sequence, a ∼275-bpcis-acting locus located 17 kb centromere-proximal to HML on theleft arm of Chr III (34, 38). RE binds several proteins, includingmultiple copies of forkhead family transcription factor homologue 1(Fkh1), which contains a phosphothreonine binding domain thatseems to have affinity for one or more damage-dependent phos-phorylated proteins bound near the DSB (39). This attraction canthus bring HML within about 20 kb of MAT, but only after MAT iscut by HO. In MATα cells, RE is inactivated and HMR is thepreferred donor. Similarly, when RE is deleted inMATa cells,HMRis strongly preferred over HML (34, 40). RE is “portable” in that itcan facilitate the preferential use of non-MAT sequences as donorseven in interchromosomal recombination (11, 39).Recent experiments have found that the conformation of Chr III
differs betweenMATa andMATα cells before the break is inducedat MAT (41). These differences, though, do not account for thestrong donor preferences in the two mating types, because contactsbetween MAT and HMR predominate in the absence of a DSB inboth cases. Moreover, when RE is deleted (again in the absence ofDNA damage) there is no significant difference in initial chro-mosome conformation. However, there is a second Matα2–Mcm1repressor binding site located next to the region that we have de-fined as RE (42); deletion of this region, interestingly, does alterthe conformation of the left arm, leading to a conformation dif-
ferent from that seen in either mating type. Still, the question of theconformation of Chr III after the break at MAT remains.Here we propose a quantitative model of mating-type switching
that takes into account RE-mediated refolding of Chr III after theDSB at MAT (Fig. 1A). This refolding model makes specific pre-dictions about the probability distributions of the distances betweenMAT and the donor loci after the break, until homologous re-combination is completed. We quantitatively test these predictionsby direct visualization of theMAT andHML loci, which are labeledwith fluorescent proteins bound to DNA sequences insertednearby. The measured distances between these two loci in the wildtype and two mutant yeast strains (one in which RE is deleted andanother in which HML is synthetically tethered to the nuclearperiphery) are consistent with the predictions of our chromosome-refolding model. The model quantitatively connects chromosomeorganization to donor preference, and we successfully test predic-tions of the model against our measurements and previously pub-lished donor preference data (43). Unexpectedly, this comparisonalso reveals a possible role for the folding of the right arm of ChrIII in determining donor preference. Taken together, our theoryand experiments help establish DSB-induced refolding of yeast ChrIII as the mechanism for donor preference in mating-type switch-ing. These results provide an example of a cell regulating com-munication between distant genetic loci by manipulating the spatialorganization of its chromosome within the nucleus in response toan internal (DNA-damage-dependent) cue.
ResultsChromosome-Refolding Model of Mating-Type Switching. In orderfor the repair of the DSB at MAT to commence, one of the twodonor loci has to come in close proximity (tens of nanometers) tothe location of the DSB. We model the repair process as con-sisting of two irreversible kinetic steps. The first step is the for-mation of a synapsed state between MAT and one of the twodonor loci. This is followed by DSB repair and subsequent dis-sociation of MAT and the donor locus. The first step is assumed tooccur at rate kL or kR, depending on whether MAT associates withHML or HMR, whereas the second repair step is assumed to occurat the same rate r regardless of which donor is used (Fig. 1A),which is consistent with recently published data (10, 11).This simple model asserts that the donor ratio D= kL=kR,
where we define the donor ratio as the fraction of cells that useHML divided by the fraction of cells that use HMR to repair theDNA break at MAT. Furthermore, the model predicts the proba-bility that HML is synapsed with MAT at time t after the break:
pLðtÞ= kL
kL + kR − r
�e−rt − e−ðkL+kRÞt
�. [1]
An equivalent formula holds for the probability of HMR beingsynapsed with MAT, with kL and kR swapped.The rates of synapse formation are related to chromosome
conformations. If the conformation is such that HML is morelikely to be in close proximity to MAT than HMR is, MAT willsynapse proportionally more often with the left donor. Simpleestimates based on measured chromosome dynamics in yeastwith or without a DSB (44–50) suggest that collisions betweenHML (or HMR) and MAT, which are driven by chromosomediffusion, happen on the minute time scale. This is much fasterthan the time for synapse formation ð∼ 1=kL, 1=kRÞ and the re-pair time ð∼ 1=rÞ, which ChIP/Southern blot and PCR experi-ments, respectively, suggest happen on the tens of minutes tohour time scale (35, 39, 51). This separation of time scales im-plies that multiple collisions between MAT and the donor locioccur before a synapsed state between MAT and one of thedonors occurs. Therefore, the formation of the synapsed statecan be modeled as a slow conversion to the synapsed state (with
E6930 | www.pnas.org/cgi/doi/10.1073/pnas.1607103113 Avsaroglu et al.
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

Fig. 1. Chromosome-refolding model of donor preference in mating-type switching. (A) Kinetics of mating-type switching. The black line representsyeast Chr III. Circles show the genetic loci MAT (white), HML (gray), and HMR (black). The two steps leading to repair are the formation of a synapsed statebetween one of the donor loci (HML or HMR) and MAT and the repair of the DNA break, which leads to the breakup of the paired state. Both steps areassumed to be irreversible. The rates of synapse formation (kL and kRÞ are different for the two loci, leading to a difference in the use of one donor overthe other. The synapse formation rates depend on the folded state of the chromosome after the DSB, which is described in B by a thermodynamic model.The rate of DNA break repair r is independent of the selected donor (10, 11). Time-dependent probabilities of the MAT–HML synapsed and MAT–HMRsynapsed states of the chromosome are pLðtÞ and pRðtÞ, respectively, and they can be calculated in terms of the rate parameters (Eq. 1). (B) Folded states ofyeast Chr III after the DSB and their thermodynamic weights. Each state is defined by the specific contacts shown as the schematic in the leftmost column.The wi ’s represent the reduction in the number of chromosome configurations compared with the unfolded (first) state. EREM is the energy of interactionbetween RE (yellow) and MAT (white), mediated by the Fkh1 protein (39, 53); T and kB are absolute temperature and the Boltzmann constant, re-spectively. The folded states that have an HML and MAT (gray and white) or HMR and MAT (black and white) in close contact all lead to a formation of asynapsed state with the same rate s. The centromere (C) is shown in purple. (C ) Conformations of yeast chromosomes are modeled using a random-walkpolymer model. The polymer is confined to a sphere of radius a, representing the nucleus; the polymer is tethered to the nuclear periphery at its ends,which represent telomere sequences (gray circles), and at the centromere, which is attached to the spindle pole body (SPB tether). The left (light-yellowcylinders) and right (pink cylinders) arms of the chromosome are composed of rigid segments of equal length (Kuhn length) connected by flexible linkers.The spindle pole body (SPB) tether is positioned at the north pole whereas the telomere tethers are allowed to take any position on the surface of thesphere. In addition to the spherical confinement, an impenetrable volume (red spherical cap), representing the nucleolar region, limits the space availablefor the chromosome, and it is located at the south pole. Loci of interest are represented as colored circles RE (yellow), MAT (white), HML (gray), and HMR(black). (D) Predicted distribution of distances between HML and MAT. Distributions are computed for two separate cases depending on the folded stateof Chr III: (i) before the DNA break at MAT (solid line), when the chromosome is not folded, and (ii ) after the DNA break (dashed line), when RE and theHML locus are synapsed with MAT, leading to a two-loop folded state of the chromosome. Polymer model parameters are given in Table S1. Distributionswere computed using distances between the polymer segments that represent the two fluorescent arrays placed proximal to HML and MAT in the ex-periments (Fig. 2A).
Avsaroglu et al. PNAS | Published online October 24, 2016 | E6931
BIOPH
YSICSAND
COMPU
TATIONALBIOLO
GY
PNASPL
US
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

rate s) from a state of close contact between MAT and one of thedonor sequences, as illustrated in Fig. 1B. The probability ofclose contact between two DNA sequences on Chr III can in turnbe computed using equilibrium statistical mechanics (52).In Fig. 1B we list all of the folded states of Chr III that play a role
in mating-type switching. Folded states are defined by the closecontact between MAT and the two donor loci, and the binding ofRE to MAT, which occurs after the DSB in MATa cells (39, 43, 53,54). Using the equilibrium probabilities of the folded states we cancompute the rates of synapse formation, kL = ðpLM + pLM,REMÞs andkR = ðpRM + pRM,REMÞs. As described above, s is the rate of synapseformation assuming that the left or the right donor is in closecontact with the DSB atMAT. Using these expressions for the ratesof synapse formation we compute the donor ratio as
D=pLM + pLM,REM
pRM + pRM,REM, [2]
where the numerator is simply the contact probability betweenHML and MAT and the denominator corresponds to the prob-ability of an HMR–MAT contact.The probabilities of the different folded states follow directly
from the thermodynamics weights listed in Fig. 1B: For eachstate, the probability pi (i=LM,RM, etc.) of the state equals thestate’s thermodynamic weight divided by the sum of all of thethermodynamic weights. The multiplicities ωi (i=LM,RM, etc.)in Fig. 1B account for the loss of entropy due to chromosomefolding, and they are readily computed using the polymer modelof Chr III illustrated in Fig. 1C (23). The thermodynamic weightsare nothing but the relative probabilities of a contact occurringbetween the different genetic loci involved in mating-type switch-ing, as illustrated in the first column of Fig. 1B (the weights arerelative to the probability of no contact occurring, which is the firststate in Fig. 1B). Note that even though the thermodynamicweights, and the corresponding contact probabilities, depend onthe distance cutoff used to define what is meant by a close contact(in our calculations we take the cutoff to be 10 nm), the donor ratiois independent of the choice of cutoff, because it drops out whentaking the ratio of contact probabilities (Eq. 2).A key prediction of the chromosome-refolding model is that the
probability of a contact occurring between HML and MAT in-creases due to (Fkh1-mediated) RE–MAT binding after the break.Therefore, in MATa cells in which RE is deleted we expect noincrease in the probability of the folded state in which HML andMAT are in contact, compared with what is measured before thebreak. These two scenarios are illustrated in Fig. 1D, where weshow predictions from the polymer model of Chr III for the dis-tance distribution between the HML and MAT loci for the foldedand unfolded state of Chr III in yeast. A quantitative increase in theprobability distribution at small distances observed due to folding isresponsible for the change in the rate of synapse formation ofMATand HML (kL), which in turn is predicted to increase the donorratio (D). Similarly, any perturbation to the folded state of thechromosome after the DSB that alters contact probabilities be-tween MAT and donor loci will have a quantifiable effect on thedonor ratio. We exploit this by constructing mutant cells to test therefolding model. We also test the model by comparing its predic-tions (Eq. 2) to previously published experiments that measuredchanges in donor preference when the MAT locus, the donor loci,and RE were moved to different positions on Chr III (43).
In Vivo Imaging of Genetic Loci Involved in Mating-Type Switching Isin Quantitative Agreement with the Chromosome-Refolding Model.To quantitatively test predictions based on the chromosome-refolding model we made use of the wild-type yeast strain with a256-copy tandem array of LacO sequences, which bind LacI-GFP, inserted 1.5 kb proximal to the HML locus, and a 112-copy
tandem array of TetO sequences, which bind TetR-RFP, inserted5 kb distal to the MAT locus (Fig. 2A) (23, 55–57).We imaged cells (Fig. 2B) before and after generating the DSB
at the MAT locus. The DSB was generated using a galactose-in-ducible HO endonuclease (GAL-HO) (35). We then collected cellsat different time points (t) after inducing the DSB, fixed them withparaformaldehyde, and imaged the fixed yeast cells using confocalmicroscopy. The images were analyzed using the ImageJ pluginSpotDistance to measure the 3D distances (d) between fluorescentmarkers at MAT and HML (23, 58). Details of quantitative fluo-rescence microscopy are explained in Materials and Methods.In Fig. 2C (also see Figs. S2 and S3) we show the experimental
distribution of distances PLMðd, tÞ that we obtained in this man-ner. The raw distance data are shown in Fig. S1, and in Table S2we make a statistical comparison of measured distances for everypair of time points to test the likelihood that they are drawn fromthe same distribution.Next, we compared our experimental distance measurements
with theoretical predictions based on the chromosome-refoldingmodel described above. The probability distribution of distancesbetween HML and MAT at time t is
PLMðd, tÞ= pLðtÞPfoldedðdÞ+ ½1− pLðtÞ�PunfoldedðdÞ, [3]
which accounts for the fact that a fraction of cells pLðtÞ has HMLandMAT in a synapsed state. The distance distribution PfoldedðdÞ iscomputed from the polymer model assuming that HML and MATare in contact, whereas PunfoldedðdÞ corresponds to the situationwhen there is no contact between HML and MAT (Fig. 1D).As shown in Fig. 2C we find excellent agreement between the
theoretical and experimentally measured distance distributionPLMðd, tÞ. To make the comparison we used a maximum likeli-hood procedure that takes into account all of the data obtainedat different time points; this approach produces the most likelyvalues of pL at different time points (Materials and Methods),which are given in the insets of individual graphs in Fig. 2C.These values of pL are plotted as a function of time in Fig. 3Band compared with the prediction of the chromosome-refoldingmodel, Eq. 1. (This experimental test of the model is discussed indetail in Results, Chromosome Refolding Quantitatively Accountsfor Donor Preference in Mating-Type Switching.)Deletion of RE disrupts chromosome refolding during mating-type switching.To test the idea that RE is responsible for chromosome refoldingafter the DSB at MAT we constructed a yeast strain with RE de-leted and repeated the quantitative microscopy time-course anal-ysis described above to obtain PLMðd, tÞ for this mutant. The modelpredictions from Eq. 3 agree with the measured distance distri-butions at each time point (Fig. S2). Notably, we find that theprobability of an HML–MAT synapsed state pLðtÞ is dramaticallyreduced compared with that observed for wild-type cells; compareFig. 3 B and C. These data confirm that in MATa cells RE in-creases the probability of contacts between the HML and MATlocus after the DNA break (39).Chromosome tethering to the nuclear envelope disrupts its refolding duringmating-type switching. To further explore the role of the spatial orga-nization of Chr III in determining donor preference during mating-type switching, we constructed a mutant strain in which the regionnearHML would be more strongly tethered to the nuclear envelope.LacI-GFP was fused to a nuclear membrane-targeting FFAT pep-tide motif (two phenylalanines in an acidic tract) (23, 59, 60) so thatChr III was tethered to the nuclear periphery at the LacO operatorarray, the center of which is 7 kb proximal to HML. This tetheringshould limit the mobility of HML and act in opposition to RE byreducing the probability of HML–MAT contacts, and should there-fore lead to a decrease in the use of HML as a donor when the DSBat MAT is repaired. (The measurements of donor preference, con-sistent with this prediction, are described below.)
E6932 | www.pnas.org/cgi/doi/10.1073/pnas.1607103113 Avsaroglu et al.
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

Measurements of distances between HML and MAT in thisstrain, after the DNA break is induced, are again in agreement withthe polymer model prediction, Eq. 3 (Fig. S3). Now, in addition tothe tethering of the telomeres to the nuclear periphery shown in Fig.1C, we add to the model a tethering interaction between the LacOoperator array and nuclear envelope. Our model assumes that theprobability of tethering interaction between FFAT and membranereceptors occurring is 0.8, which was measured experimentally in aprevious study (59) (and discussed further inMaterials and Methods).As in the previous two cases, comparison of the measured distance
distribution and the theoretical prediction given by Eq. 3 yields amaximum likelihood value of the looping probability pLðtÞ, which isplotted in Fig. 3D. Comparison between Fig. 3 B and D leads to theconclusion that the extra tethering interaction reduces the probabilityof HML–MAT synapse formation compared with wild-type cells.
Chromosome Refolding Quantitatively Accounts for Donor Preferencein Mating-Type Switching. So far, our in vivo experiments give sup-port to the idea that RE-mediated refolding of Chr III accompaniesmating-type switching in MATa cells. Next we demonstrate thatchanges in the folded state of the chromosome after the DSBquantitatively account for donor preference in mating-type switching.
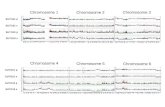
We measured the relative use of the HML and HMR donorsduring MAT switching in wild-type and mutant MATa cells witha PCR-based assay as described previously (38–40, 43). Fig. 3Ashows the average experimental donor uses for the wild-type andmutant cells (n≥ 3 for each), which we use to compute the donorratio D by taking the ratio of HML to HMR use. In agreementwith previous studies (38–40, 61), we find that in the absence ofRE HML is used only 9.5 ± 0.3% of the time (D= 0.105± 0.004Þ,as opposed to the 92 ± 2% (D= 12± 3Þ wild-type case where REis present. Similarly, when we artificially tethered the HMLproximal LacO array to the nuclear envelope and repeated thesame measurement the average of three independent experi-ments showed that HML donor use drops significantly, to 64 ±1% (D= 1.8± 0.1) (Fig. 3A).Next we asked to what extent the chromosome-refolding model
is in quantitative agreement with our measurements of donor ratios.To make this comparison, we use a maximum likelihood procedureto fit Eq. 1 to the pLðtÞ values extracted from distance data (Fig. 2and Figs. S2 and S3), while taking into account the relation betweendonor ratio and the rates of synapse formation, D= kL=kR. To takeinto account the time delay (τ) between the time at which the cellsare exposed to galactose and the time at which HO cuts the DNAat the MAT locus, we replace t with t− τ in Eq. 1.
Fig. 2. In vivo imaging of genetic loci during mating-type switching is in quantitative agreement with the chromosome-refolding model. (A) Schematic views ofbudding yeast Chr III, with distances of each locus from the left telomere end indicated in kilobase pairs; 256 tandem repeats of Lac operators are inserted at a site1.5 kb proximal to HML, and 112 tandem repeats of Tet operators are inserted at a site 5 kb distal to MAT. LacI-GFP and TetR-RFP fusion proteins mark the spatiallocation of the genetic loci in the proximity of HML and MAT, respectively. The top view shows the linear (truncated) representation of Chr III with insertedfluorescent reporters, and a 3D representation of the chromosome is shown in the bottom viewwith green for HML + LacO and red forMAT +TetO; insets show therelative position between the donor loci and their respective fluorescent arrays. (B) Representative wide-field microscopy images of yeast strain YBA009. Imagesfrom formaldehyde-fixed cells were collected before and after inducing a double-strand DNA break at MAT (Materials and Methods). (Scale bar, 1 μm.) (C)Comparison of chromosome-refolding model predictions (Eq. 3), shown in red, with measurements (black bars) of the probability density function (in units of 1 permicrometer) for the distances (in units of micrometers) between the LacO and TetO arrays, proximal to HML andMAT loci, respectively. Distances were measured atdifferent time points during mating-type switching in wild-type cells. For each time point, the area under the red curve is equal to 1; also, the sum of histogram barareas is equal to 1. Ten time points were collected over a 6-h-long time-course measurement. PLðt = tiÞ is the fraction of cells with HML–MAT paired, which wasobtained by fitting the data to Eq. 3 for every time point ti. Experimental error bars represent the 95% confidence interval of the counting error. (Time point/number of cells analyzed: 0 min/431, 30 min/190, 60 min/541, 90 min/556, 120 min/334, 150 min/149, 180 min/139, 240 min/154, 300 min/178, and 360 min/104.)
Avsaroglu et al. PNAS | Published online October 24, 2016 | E6933
BIOPH
YSICSAND
COMPU
TATIONALBIOLO
GY
PNASPL
US
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

The maximum likelihood procedure yields the rates and timedelay τ shown in Table 1, which are all consistent with the con-clusions of ChIP and PCR measurements regarding the timing ofdifferent events in the course of MAT switching (35, 39, 51). InFig. 3 B–D we show the comparison between our measurementsand Eq. 1, which was computed using the rates shown in Table 1.Another prediction of the chromosome-refolding model of
mating-type switching is that the donor ratio (D) is given by therelative probabilities of contact of the MAT locus with the leftand the right donor, after the DNA break at MAT (Eq. 2). Thesecontact probabilities can be computed based on the random-walkpolymer model of interphase chromosomes while taking intoaccount the binding affinity of RE to MATa after the DSB (23,62). To test this we examined previously published data on donorpreference for yeast strains in which RE and the HML donorwere moved to different locations on Chr III, or RE was re-moved and the location of HML was varied (43).
In Fig. 4A we compare the computed probability for HMLbeing in contact with MAT ðpLM + pLM,REM) and the measureddonor ratio for eight different strains from ref. 43. In the case ofstrains in which RE is removed (Fig. 4B), there is only one statewith HML in contact with MAT (Fig. S4), so the correspondingcontact probability is pLM. The refolding model predicts a linearrelationship between the donor ratio and the probability of anHML–MAT contact (Eq. 2), which is consistent with the datafrom ref. 43, except for a few significant outliers.An important assumption we make in Fig. 4 is that moving
HML and RE along the left arm of Chr III does not affect theprobability for an HMR–MAT contact, which is the denominatorin the equation for the donor ratio (Eq. 2). In the case of strainswith RE present, the probability of an HMR–MAT contact ispRM + pRM,REM, whereas in the absence of RE it is just pRM (Fig.S4). Also, in strains in which RE is present, to compute thecontact probabilities we need the binding energy (EREM) betweenRE and MAT (Fig. 1B). We account for this parameter of the
Fig. 3. Change in chromosome folding quantitatively accounts for donor preference in mating-type switching. (A) Quantitative analysis of donor use in yeastmating-type switching is done by a PCR and restriction digest-based assay (39). Each lane is the digested product of the switch experiment for the corre-sponding strain. The top bands show MATα and the lower two bands show the digested MATα-BamHI. Average use of the HML donor is given with its SD (aminimum of three replicate experiments). The numbers are computed by taking the ratio of the intensities of the top band to the sum of all three bands foreach lane. In the absence of RE (middle lane), HML is the preferred donor only 9.5% ± 0.3 of the time, whereas the wild-type (left lane) donor use is 92% ± 2,which is consistent with the previous results from our laboratory (38, 39, 43). The rightmost lane corresponds to a strain where HML mobility is constrained bytethering the proximal LacO array to the nuclear envelope, and it shows use of HML during mating-type switching reduced to 64% ± 1. (B–D) Change overtime of the fraction of cells with HML andMAT paired, for wild-type, RE-deleted, and LacO-tethered strains, respectively. Red curves represent the theoreticalpredictions (Eq. 1) with the rate parameters (Table 1) obtained using a maximum likelihood procedure. Error bars represent 95% confidence intervalsobtained through maximum likelihood estimation when fitting theoretical LacO–TetO distance distributions to experimental distance distributions (Fig. 2C).
Table 1. Rate parameters of the chromosome-refolding model of mating-type switching
Strain name (genotype) Rate of HML–MAT synapse kL, h−1 Rate of HMR–MAT synapse kR, h
−1 Rate of repair r, h−1 Time delay τ, min
YBA009 (wild-type) 1.4 (1.0, 2.0) 0.093 (0.066, 0.13) 0.50 (0.45, 0.56) 20 (7, 20)YBA013 (LacO-tethered) 0.63 (0.50, 0.89) 0.37 (0.29, 0.52) 0.50 (0.45, 0.56) 20 (7, 20)YBA010 (RE-deleted) 0.32 (0.11, 0.63) 2.9 (1.0, 5.7) 0.50 (0.45, 0.56) 20 (7, 20)
The parameters were determined using a maximum likelihood procedure for comparing the theoretical prediction of the model (Eq. 1) with data obtainedfrom the three different strains of yeast, shown in Fig. 3 B–D. The numbers in parentheses correspond to the 95% confidence interval for each parameter.
E6934 | www.pnas.org/cgi/doi/10.1073/pnas.1607103113 Avsaroglu et al.
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

model by extracting the probability that RE is bound to MAT,pRE =ωREM e
− EREMkBT =�1+ωREM e
− EREMkBT
�, from the experimental
data (see Supporting Information for details). We find goodagreement between the theory and experiments for pRE = 0.58when RE is at its wild-type position (29 kb from left telomere)and pRE = 0.63 when it is placed 74 kb away from the left telo-mere. This difference in pRE is completely accounted for by theentropic factor (i.e., the thermodynamic weight ωREM), which isdifferent for different positions of RE on Chr III.
DiscussionRecent advances in imaging and chromosome conformationcapture techniques combined with polymer models of chromo-somes have greatly improved our understanding of the spatialorganization and dynamics of chromosomes within the nucleus.However, the quantitative relationship between polymer physicsand in vivo chromosome biology remains elusive. In this study,we provide a theoretical framework and direct biological evi-dence for nuclear organization being modified so as to regulatethe repair of a DNA double-stranded break via homologousrecombination. Our model makes quantitative predictions aboutthe dynamics of the selection of competing donor loci for ho-mologous recombination during mating-type gene switching inyeast, which is a well-established eukaryotic model system forthis ubiquitous DNA break-repair process.The key idea of the chromosome-refolding model is that the
chromosome conformation changes after the DNA break so asto direct mating-type switching in yeast by favoring one donorover the other (Fig. 1 A and B). The model makes specificquantitative predictions, which we test experimentally. Wemeasured the distribution of distances between the left donorand the MAT sequence before and at different times after theDNA break at MAT. We also measured the preference of usingthe left donor over the donor on the right arm of Chr III. We
repeated these experiments for wild-type and mutant cells andfound agreement between our data and predictions of the chro-mosome-refolding model. Notably, by tethering the left arm of ChrIII to the nuclear periphery so as to change the folded state of thechromosome after the break, and therefore reduce the contactprobability between HML and MAT, we were able to reduce thepreference for the left donor (Fig. 3). Similarly, by deleting the REsequence, which in previous experiments has been shown to asso-ciate with chromatin close to the break at MAT, we were able toshow a significant reduction in contact probability between HMLand MAT, and this too led to a reduction in the use of the leftdonor, consistent with conclusions from previous studies (38, 39).The refolding model quantitatively relates the frequencies
of recombination between HML and MAT for different mutantstrains (Fig. 4), which strongly suggests that the rate of formation ofa synapse between HML and MAT (kLÞ is dictated by the polymernature of yeast chromatin. Notably, for the wild-type strain (1 inFig. 4A) the theory and experiment seem to be in disagreement.One possible explanation might be heterogeneities in the degree ofcompaction of DNA into yeast chromatin throughout Chr III (63,64). If the chromatin in the wild-type strain is more compact in theregion between HML and RE, the physical length of the chro-mosome between HML and RE would be shorter than the lengthused in our calculations, makingHML be even closer toMAT whenRE and MAT are bound. When included in our polymer model ofChr III this local chromatin compaction can account for the dis-crepancy noted above (Supporting Information).Although our polymer model accurately accounts for the
variations in the donor ratio for different strains in which se-quences on the left arm of Chr III are mutated (Fig. 4), Eq. 2does not successfully reproduce the absolute value of the donorratio for most strains. For example, in strains deleted for RE thepredicted donor ratio values are an order of magnitude greaterthan those measured in experiments (43): For strains 9–12 in Fig.4B our polymer calculations yield absolute donor ratio values of
Fig. 4. Chromosome-refolding model of yeast mating-type switching quantitatively explains changes in donor preference. Comparison of the computedcontact probability between HML and MAT (red points) with experimentally determined donor ratios taken from ref. 43 (squares). For each strain, we plot apoint whose y coordinate indicates the strain’s experimentally measured donor ratio and whose x coordinate is the probability of an HML–MAT contactcalculated from the polymer model. The model assumes all strains containing RE (strains 1–8) have the same probability of an HMR–MAT contact andtherefore the donor ratio (Eq. 2) is predicted to be the probability of an HML–MAT contact multiplied by a constant (i.e., the reciprocal of the contactprobability between HMR and MAT), as indicated by the red line. Likewise, the model assumes all strains lacking RE (strains 1–4) have the same probability ofan HMR–MAT contact. (A) To compute the contact probability between HML and MAT ðpLM +pLM,REMÞ, we assumed that contact occurs when the distancebetween these two loci is less than 10 nm. We also determined the probability of RE bound to MAT to be 0.58 for strains 1–4 and 0.63 for strains 5–8; thisdifference is due to the entropic contribution to the probability, which stems from different positions of RE (Supporting Information). Experiments had eitherRE at its wild-type position (yellow data points) or positioned 74 kb from the left telomere (gray data points), whereas HML was moved to different locations(indicated by labels 1–8). (B) In the absence of RE the contact probability between HML and MAT is simply pLM; this contact probability was computed as-suming a cutoff distance of 10 nm for a contact to occur. Data (blue data points) from ref. 40 correspond to different location of HML (labels 9–12). Error barson both the experimental data (where available) and our polymer simulations (red squares) represent 95% confidence intervals. The errors on the simulateddata are due to the finite number of chromosome configurations generated in our simulations.
Avsaroglu et al. PNAS | Published online October 24, 2016 | E6935
BIOPH
YSICSAND
COMPU
TATIONALBIOLO
GY
PNASPL
US
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

D= 0.57± 0.05, 0.98± 0.08, 1.1± 0.1, and 1.2± 0.1, respectively,whereas the experimental values are about 10 times smaller(D= 0.064, 0.087, 0.12, and 0.14, respectively. Given our findingthat the refolding model accounts quantitatively for the rate ofsynapse formation between HML and MAT, we are left with thesurprising conclusion that the probability of HMR contactingMAT is higher than expected. This conclusion is bolstered by ourfinding that the value of the rate of synapse formation betweenHMR and MAT (kR) is significantly larger in RE-deleted strainsthan in wild-type cells (Table 1). Moreover, an estimate of theincrease in kR upon RE deletion can be obtained from the ratioof the slopes of the best-fit lines in Fig. 4 A and B (SupportingInformation). In this way we compute an increase in kR by afactor of 3.3 (2.4–8.5 is the 95% confidence interval), which isalso consistent with measured HML–MAT distances after theDSB in wild-type and mutant yeast cells (Fig. S5 and Table S3).An increase of contact probability between HMR and MAT in
RE-deleted cells could be explained by the presence of a hereto-fore unidentified mating-type-independent RE-like element lo-cated in the vicinity of HMR. In cells in which RE is active wespeculate this RE-like element will be ineffective, being out-competed by RE for binding to MAT. This possibility is supportedby the observations of Ostrow et al. (65), who found several Fkh1binding sites within a few tens of kilobases proximal to HMR; suchsites, in the absence of RE binding to MAT, might draw HMRcloser to the DSB in a fashion similar to the way RE does forHML. We tested this possibility by performing polymer simulationsthat include an Fkh1 binding site located 5 kb proximal to HMR,which could bind to MAT after the break. The computed donorratio is consistent with the experimentally measured donor ratio inMATa cells deleted for RE (Fig. S6), whereas it does not signifi-cantly affect the computed donor ratio in the presence of RE.A larger-than-expected synapsing of HMR with MAT is also
consistent with results of experiments by Renkawitz et al. (51), whoinvestigated the genome-wide Rad51 binding to chromatin afterinducing a DSB at MAT. They showed that both in MATa cellsdeleted for RE and in MATα cells Rad51 binding within a region50 kb proximal to HMR was almost fourfold higher than for therest of the Chr III. This suggests that the collisions between thesesites on the right arm and Rad51 bound to ssDNA near MAT aremore frequent than collisions with the rest of the chromosome.The possibility of RE being in competition with an RE-like
HMR proximal sequence for binding to MAT is consistent withfindings of Li et al. (39), who created strains in which RE wasreplaced by LexA operators. In one strain they expressed a fu-sion protein of LexA and the FHA domain of Fkh1, which boundto LexA operators independent of the presence of the DSB butonly bound near MAT after DSB induction. The donor preferenceof this strain is similar to that measured in wild-type cells, whereasin the strain without the fusion protein the donor preference isclose to what is observed in RE-deleted cells. Because the polymernature of the chromosome cannot account for the roughly 100-foldchange in donor ratio, we are again led to the conclusion thatFHA-induced binding of the LexA operators toMATmust have anadditional effect on donor preference besides bringing HML andMAT into close proximity. One possibility is that this binding blocksthe purported binding of MAT to Fkh1 binding sites near HMR.The thrust of this paper is a quantitative comparison between
measurements of chromosome conformations during DNA breakrepair by homologous recombination, and a model that connectsthe spatial organization of the chromosome to this function. Thistype of comparison between theory and experiments is particularlypowerful in yeast, which has the distinction that the spatial orga-nization of its chromosomes is well described by the simplest ofpolymer models, the random-walk chain (23, 27–29). In all othercells studied to date, polymer properties of interphase chromo-somes are such that mechanisms other than polymer entropy havebeen invoked to explain the observed spatial organization of their
chromosomes (66). We believe that this makes yeast an excellentmodel system in which to study the quantitative relationship be-tween chromosome organization and chromosome function. Inparticular, novel molecular mechanisms could be identified uponfinding significant deviations between predictions based on therandom-walk model of the yeast chromosomes and measurementsin which chromosome function is perturbed by changing chromo-some conformation. This is exemplified nicely by this study, whereour inability to correctly account for the measured value of donorpreference (while correctly accounting for changes in donor pref-erence and the observed chromosome conformations) in wild-typeand mutant yeast strains strongly suggests that RE-like sequencesmight be present on the right arm of Chr III. The presence of Fkh1binding sites on the right arm also gives us a reasonable hypothesisfor the nature of these RE-like sequences, and a specific target forfuture experiments. We believe that similar quantitative compari-sons between polymer models of chromosomes and experimentsthat perturb chromosome organization and measure concomitantchanges in chromosome function will help further establish causallinks between the spatial organization of chromosomes and theircellular functions.
Materials and MethodsYeast Strains and Plasmids. The yeast strains used in this study can be found inTable S4. All strains used were variants of YDB076 (56). YDB101 was con-structed by transforming YDB076 with NheI digest of pDB030 (56). YBA008and YBA011, the strains carrying the TetO array 5 kb distal to MAT, wereconstructed by transforming YDB101 and YDB076, respectively, with theBamHI + XbaI restriction fragment of pJH2145 (J.E.H. laboratory plasmidcollection). YBA009 and YBA012, the strains with fluorescent labeling of theTetO array distal to MAT, were constructed by transforming YBA008 andYBA011, respectively, with HpaI digest of pBA002 to integrate the URA3pro-TetR-mRFP-ADHter-C.glabrataTRP1 in yGL119w intergene. pBA002, theplasmid carrying the TetR-mRFP fusion construct, was derived by subcloningthe Candida glabrata TRP1 gene (BamHI and NotI digest of pJH2778; J.E.H.laboratory plasmid collection) gene fragment into plasmid containingCgLEU2-TetR-mRFP-iYGL119W (a gift from Yuko Nakajima, Brandeis Uni-versity, Waltham, MA) cut with the same to replace the CgLEU2. YBA010,the strain that is deleted for RE, was constructed by transforming YBA009with the PCR product carrying hygromycin resistance gene (hphMX6) ampli-fied from pAG32 (67) with primers (sense: AAACTCTTCTCAAAACCAAATTG-CGCAAGGATTGATTCAGTACAATTATGcggatccccgggttaattaa) and (antisense:CCTAGAATTTGGAATTGGATAATTTAACTCTTTAGAATATAACATCTACCgaattc-gagctcgttt) (a gift from Anuja Mehta, Brandeis University, Waltham, MA).Here uppercase and lowercase letters show homologous sequences to RE andpAG32, respectively. Transformations were confirmed with PCR using sense(TGGCTGGGTTATAGTGAGCCCA) and antisense (GGGCACAGTCAATGAGTA-GTAGA) primer pairs that show a 1,248-bp-long product band in wild-typecells and a 2,227-bp band in cells deleted for RE. YBA013 was constructed bytransforming YBA012 with pBA001 cut with NheI. pBA001 was derived bysubcloning a KAN-MX cassette, NotI restriction digest fragment frompJH1512 (J.E.H. laboratory plasmid collection) into the plasmid pGFP-FFAT-LacI (a gift from Jason Brickner, Northwestern University, Evanston, IL) (59)cut with the same (NotI).
Acquisition and Processing of Fixed Cell Images. Images of fixed cells wereacquired on Marianas spinning disk confocal microscopes (3i Intelligent Im-aging Innovations) equipped either with a Cascade II:512 electron-multi-plying CCD (EMCCD) camera (Photometrics) or with a QuantEM: 512SCEMCCD camera (Photometrics). The pixel size of the images taken with theCascade II:512 camera (at 150× magnification) is 107:107:230 nm (X:Y:Z) andfor the images taken with the QuantEM: 512SC camera (at 120× magnifi-cation) is 129:129:270 nm (X:Y:Z); 16–20 Z-sections were acquired using aPlan-Apochromat 100×/1.4 oil M27 with a differential interference contrastprism with 1 × 1 binning along with a 1.5× or 1.2× optovar. Cells withmultiple fluorescent spots of the same color and with deformed cell mem-brane were excluded from imaging to protect sample uniformity. Cells wereimaged using a Semrock FITC cube Marianas excitation/emission filter cubeset with exposure times of 100 ms with a 488-nm (50-mW) laser and expo-sure times of 300 ms with a 561-nm (50-mW) laser. Images were recordedwith Slidebook software (3i Intelligent Imaging Innovations), converted into TIFFformat, and analyzed with the ImageJ plugin SpotDistance (EPFL Biomedical
E6936 | www.pnas.org/cgi/doi/10.1073/pnas.1607103113 Avsaroglu et al.
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

Imaging Group) (58), with pixel sizes described above for x, y, and z axes tocalculate the 3D distances between the fluorescent spots. Correspondingdistance measurements are given in Supporting Information.
Galactose Induction of MAT Switching. Single colonies were grown overnightin 5 mL yeast extract/peptone/dextrose (YEPD) media at 30 °C. The followingday these cultures were washed with 5 mL of fresh media in which YEPD wasreplaced with YEP-lactate and grown for at least 6 h. Then a small portion ofthe saturated cultures were inoculated into fresh YEP-lactate media suchthat they would reach a density of 5 to 10×106 per mL within 10 h (averagetime at which cells begin to grow logarithmically starting from the G0 phase)at 30 °C. Once the cultures reached the appropriate density, a t = 0 samplewas taken to be fixed for imaging or to check the mating type at the be-ginning of the experiment. HO was induced by adding galactose to a finalconcentration of 2% (wt/vol). At time points separated by 30-min intervals,samples were collected for 6 h. Each time point sample was fixed withparaformaldehyde to a final concentration of 2% (wt/vol). Dextrose wasadded [2% (wt/vol)] to the cultures 1 h after galactose addition to preventfurther HO expression and to allow the cells to complete the mating-type switch.
Measurement of Donor Preference via a PCR-Based Assay. Strains that wereused for fluorescent measurements (YBA009, YBA010, and YBA013) werealso assayed for donor preference, as described by Li et al. (39). Six hoursafter sample collection for the time-course experiments, genomic DNA fromthe remaining cultures was extracted using the MasterPure yeast DNA pu-rification kit (Epicentre). The switched products were amplified using a Yα-specific PCR primer and another primer specific for sequences just distal toMAT. The PCR product was digested with BamHI restriction enzyme, and thedigested samples were visualized on an agarose gel stained with ethidiumbromide. Repair events using HMR show a 1,470-bp band, whereas when theHMR-BamHI cassette is used as a donor the PCR product is digested into twosmaller bands with sizes 920 and 550 bp. HML donor preference was mea-sured by taking the ratio of the intensities of the top band to the sum of allthree bands for each lane. Following DNA extraction, PCR and restrictiondigest steps are followed. Agarose gel band signals were quantified usingImageJ (68).
Computational and Theoretical Methods.Freely jointed chain simulation. Yeast Chr III was simulated in MATLAB as afreely jointed chain. The number of segments in the chain is determined bythe number of base pairs in the chromosome, the chromosome extensionparameter γ, and the Kuhn length, as described previously (23). Each ge-netic locus is a point along the freely jointed chain whose location is de-termined based on the number of base pairs between the centromere andthe center of the locus. The nuclear membrane is represented by a sphereof radius a, and each chromosome conformation must remain entirelywithin this sphere. To mimic the natural variability in nuclear volume, foreach trial of the simulation the sphere’s radius is randomly selected from aGaussian distribution. We also model the nucleolus as a spherical cap at thesouth pole of the sphere, which chromosome conformations cannot pen-etrate (23).
Chromosome attachments are also included in the simulation. To representthe centromere’s attachment to the spindle pole body via a microtubule (2) thesimulated chromosome’s centromere is positioned 50 nm from the north poleof the sphere. The simulated chromosome’s telomeres can be located anywhereon the surface of the sphere. When simulating strains with the FFAT fusionprotein, in 80% of the conformations the point on the polymer chain repre-senting the LacO array is located anywhere on the surface of the sphere, and in20% of the conformations the LacO point is located anywhere within thesphere. The nuclear membrane association probability of pLacO−Teth = 0.8 isconsistent with a previous experimental study (59) (Table S1). When simulatingcases in which two loci are bound (e.g., RE bound to MAT), a certain fraction(determined by maximum likelihood or least squares) of the conformationshave the two loci positioned at exactly the same point in space. To computetheoretical distance distributions, chromosome conformations are generated asrandom walks with all of the constraints described above, and the distancebetween the points representing genetic loci of interest is measured for eachconformation. For consistency with the experimentally measured distributions,an additional procedure is implemented to take into account the random errordue to the resolution of the microscope. Because of this random error, a par-ticle’s measured position will be displaced from the particle’s true position, andthis displacement is equally likely to be in any direction. We mimic this distanceshift by including the microscope’s error in the simulation. Specifically, aftereach polymer simulation trial we randomly displace the simulated TetO position
by adding a different random number to each of its Cartesian coordinates. Therandom numbers are chosen from a Gaussian distribution with a zero meanand a variance given by our estimate of the microscope’s random error, whichwe estimate to be 110 nm.Maximum likelihood methods. The values of the polymer model parameters,displayed in Table S1, are obtained by performing maximum likelihoodestimation to fit the simulated LacO–TetO distance distributions to theexperimental distributions. The values of the first four model parametersin Table S1 were determined by using the experimental distance distri-butions of the t = 0 min time points for the wild-type, the LacO-tethered,and the RE-deleted strains. The maximum likelihood procedure tested theparameter values spanning the range of values reported by previous ex-perimental studies (Table S1). The pLacO−Teth parameter was fit using theLacO-tethered strain’s t = 0 min distance data. The supplementary in-formation of ref. 23 describes how we determined what range of possiblepLacO−Teth values to test in the maximum likelihood estimation. After thedetermination of the five polymer parameters, the looping fraction pa-rameter pLðtÞ was fit for each time point of each strain. pLðtÞ values aredisplayed in Fig. 2 and Figs. S2 and S3. Note that pL is assumed to be 0 forthe t = 0 min time points of all strains. The model contains two additionalparameters that have little effect on the distance distributions (23). Theseparameters are the distance from the centromere to the spindle pole bodyand the Kuhn length.
As discussed above, simulated LacO–TetO distance distributions incorporatethe microscope’s random error. We use these simulated distributions for max-imum likelihood estimation. For all LacO–TetO distance distribution plots dis-played we use 110 nm as the SD of the Gaussian from which we choose adistance for shifting each Cartesian coordinate. However, we estimate that theSDs could be as high as 220 nm for the x and y coordinates and 460 nm for the zcoordinate. These estimates are based on the microscope’s resolution as well asa comparison of position measurements of single fluorescent beads in the redchannel versus position measurements of the same beads in the green channel.Therefore, we repeated the maximum likelihood estimation using these largerSDs, and we still found agreement between the simulated and experimentaldistributions, as we did for the 110-nm error. Using the large SDs results in themaximum likelihood estimation’s finding slightly different values of three ofthe model parameters: The mean nuclear radius a is found to be 0.8 μm, andthe nucleolar volume fraction VN is found to be 25% of the volume of a sphereof radius 0.8 μm (Table S1), and pLðtÞ values are also somewhat higher thanthose in Fig. 2 and Figs. S2 and S3. Still, all of the main conclusions in Resultsremain unchanged.Determination of rates of synapse formation. For the three yeast strains the rates ofsynapse formationwere obtainedby usingmaximum likelihood estimation to fitEq. 1 to pLðtÞ, as shown in Fig. 3. The rates of synapse formation are displayed inTable 1. The start of the homology search begins a time τ after the addition ofgalactose because it takes time, up to 20 min (34), before HO finds and cleavesthe MAT locus. Therefore, when fitting Eq. 1 to pLðtÞ, we also included a lagtime parameter τ, which simply shifts the plot of the function in Eq. 1 to theright by τ minutes. When performing maximum likelihood estimation we im-posed the constraint that the parameters r and τ be the same for all threestrains because we assume that r and τ do not vary from one strain to another.We also constrain the kL and kR values by requiring that the ratio of kL to kR beequal to the experimentally measured donor ratio for each strain. The values inTable 1 are determined by fitting to a pLðtÞ obtained by assuming the micro-scope’s random error has an SD of 110 nm. We also found the rates of synapseformation by fitting to a pLðtÞ obtained by assuming the microscope’s error hasan SD of 220 nm for the x and y coordinates and 460 nm for the z coordinate.These values are displayed in Table S5 and are similar to those in Table 1. Theranges of rate values tested during maximum likelihood estimation are con-sistent with previous experiments (33).Theoretical donor ratio calculation.We use the chromosome-refolding model tocompute the donor ratio, Eq. 2, for a variety of yeast strains described in ref.43. In these experiments, donor ratio was measured for mutant strains inwhich HML and RE are located at different positions along Chr III, and insome strains RE is deleted. We use our polymer model to compute theentropic contributions to the thermodynamic weights in Fig. 1B. For ex-ample, ωLM is the fraction of chromosome conformations in which HML andMAT are within a small distance δ of each other. Based on the thickness ofchromatin, a reasonable estimate of δ is between 0 and 50 nm. The effectthat the chosen value of δ has on the computed donor ratio is negligiblerelative to the error bars on the computed donor ratios (red points in Fig.4). The Boltzmann weights associated with states in which RE in bound toMAT also contain the Boltzmann factor e−
EREMkBT , where EREM is the binding
energy between RE and MAT, which we treat as a fitting parameter ofthe model.
Avsaroglu et al. PNAS | Published online October 24, 2016 | E6937
BIOPH
YSICSAND
COMPU
TATIONALBIOLO
GY
PNASPL
US
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0

ACKNOWLEDGMENTS. We thank themembers of the J.E.H. and J.K. laboratoriesfor fruitful discussions and Jason Brickner for generously providing reagents. This
work was supported by National Science Foundation Grants DMR1206146 (to J.K.)and NSF-MRSEC-1420382 (to J.K. and J.E.H.) and NIH Grant GM20056 (to J.E.H.).
1. Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and generegulation in mammalian cells. Nat Rev Genet 2(4):292–301.
2. Taddei A, Gasser SM (2012) Structure and function in the budding yeast nucleus.Genetics 192(1):107–129.
3. Takizawa T, Meaburn KJ, Misteli T (2008) The meaning of gene positioning. Cell135(1):9–13.
4. Marshall WF (2002) Order and disorder in the nucleus. Curr Biol 12(5):R185–R192.5. Akhtar A, Gasser SM (2007) The nuclear envelope and transcriptional control. Nat Rev
Genet 8(7):507–517.6. Lanctôt C, Cheutin T, Cremer M, Cavalli G, Cremer T (2007) Dynamic genome archi-
tecture in the nuclear space: Regulation of gene expression in three dimensions. NatRev Genet 8(2):104–115.
7. Misteli T, Soutoglou E (2009) The emerging role of nuclear architecture in DNA repairand genome maintenance. Nat Rev Mol Cell Biol 10(4):243–254.
8. Mekhail K, Moazed D (2010) The nuclear envelope in genome organization, expres-sion and stability. Nat Rev Mol Cell Biol 11(5):317–328.
9. Sexton T, Cavalli G (2015) The role of chromosome domains in shaping the functionalgenome. Cell 160(6):1049–1059.
10. Agmon N, Liefshitz B, Zimmer C, Fabre E, Kupiec M (2013) Effect of nuclear archi-tecture on the efficiency of double-strand break repair. Nat Cell Biol 15(6):694–699.
11. Lee CS, et al. (2016) Chromosome position determines the success of double-strandbreak repair. Proc Natl Acad Sci USA 113(2):E146–E154.
12. Mukhopadhyay S, Schedl P, Studitsky VM, Sengupta AM (2011) Theoretical analysis ofthe role of chromatin interactions in long-range action of enhancers and insulators.Proc Natl Acad Sci USA 108(50):19919–19924.
13. Doyle B, Fudenberg G, Imakaev M, Mirny LA (2014) Chromatin loops as allostericmodulators of enhancer-promoter interactions. PLOS Comput Biol 10(10):e1003867.
14. Pâques F, Haber JE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63(2):349–404.
15. Krogh BO, Symington LS (2004) Recombination proteins in yeast. Annu Rev Genet38(1):233–271.
16. Symington LS, Rothstein R, Lisby M (2014) Mechanisms and regulation of mitoticrecombination in Saccharomyces cerevisiae. Genetics 198(3):795–835.
17. Mehta A, Haber JE (2014) Sources of DNA double-strand breaks and models of re-combinational DNA repair. Cold Spring Harb Perspect Biol 6(9):a016428.
18. Roukos V, et al. (2013) Spatial dynamics of chromosome translocations in living cells.Science 341(6146):660–4.
19. Cho NW, Dilley RL, Lampson MA, Greenberg RA (2014) Interchromosomal homologysearches drive directional ALT telomere movement and synapsis. Cell 159(1):108–121.
20. Roukos V, Misteli T (2014) The biogenesis of chromosome translocations. Nat Cell Biol16(4):293–300.
21. Lichten M, Haber JE (1989) Position effects in ectopic and allelic mitotic re-combination in Saccharomyces cerevisiae. Genetics 123(2):261–268.
22. Rosa A, Everaers R (2008) Structure and dynamics of interphase chromosomes. PLOSComput Biol 4(8):e1000153.
23. Avsaroglu B, et al. (2014) Effect of chromosome tethering on nuclear organization inyeast. PLoS One 9(7):e102474.
24. Duan Z, et al. (2010) A three-dimensional model of the yeast genome. Nature465(7296):363–367.
25. Rodley CDM, Bertels F, Jones B, O’Sullivan JM (2009) Global identification of yeastchromosome interactions using Genome conformation capture. Fungal Genet Biol46(11):879–886.
26. Gehlen LR, et al. (2006) Spatially confined polymer chains: Implications of chromatinfibre flexibility and peripheral anchoring on telomere–telomere interaction. J PhysCondens Matter 18(14):S245–S252.
27. Wong H, et al. (2012) A predictive computational model of the dynamic 3D interphaseyeast nucleus. Curr Biol 22(20):1881–1890.
28. Tjong H, Gong K, Chen L, Alber F (2012) Physical tethering and volume exclusiondetermine higher-order genome organization in budding yeast. Genome Res 22(7):1295–1305.
29. Gehlen LR, et al. (2012) Chromosome positioning and the clustering of functionallyrelated loci in yeast is driven by chromosomal interactions. Nucleus 3(4):370–383.
30. Louis EJ, Naumova ES, Lee A, Naumov G, Haber JE (1994) The chromosome end inyeast: Its mosaic nature and influence on recombinational dynamics. Genetics 136(3):789–802.
31. Marvin ME, Griffin CD, Eyre DE, Barton DBH, Louis EJ (2009) In Saccharomyces cer-evisiae, yKu and subtelomeric core X sequences repress homologous recombinationnear telomeres as part of the same pathway. Genetics 183(2):441–51, 1SI–12SI.
32. Marvin ME, et al. (2009) The association of yKu with subtelomeric core X sequencesprevents recombination involving telomeric sequences. Genetics 183(2):453–467.
33. Lee C-S, Haber JE (2015) Mating-type gene switching in Saccharomyces cerevisiae.Microbiol Spectr 3(2):A3–A0013, 2014.
34. Haber JE (2012) Mating-type genes and MAT switching in Saccharomyces cerevisiae.Genetics 191(1):33–64.
35. Hicks WM, Yamaguchi M, Haber JE (2011) Real-time analysis of double-strand DNA breakrepair by homologous recombination. Proc Natl Acad Sci USA 108(8):3108–3115.
36. Wu X, Moore JK, Haber JE (1996) Mechanism of MAT alpha donor preference duringmating-type switching of Saccharomyces cerevisiae. Mol Cell Biol 16(2):657–668.
37. Hsieh T-HS, et al. (2015) Mapping nucleosome resolution chromosome folding inyeast by Micro-C. Cell 162(1):108–119.
38. Wu X, Haber JE (1996) A 700 bp cis-acting region controls mating-type dependentrecombination along the entire left arm of yeast chromosome III. Cell 87(2):277–285.
39. Li J, et al. (2012) Regulation of budding yeast mating-type switching donor prefer-ence by the FHA domain of Fkh1. PLoS Genet 8(4):e1002630.
40. Wu X, Haber JE (1995) MATa donor preference in yeast mating-type switching: Activationof a large chromosomal region for recombination. Genes Dev 9(15):1922–1932.
41. Belton JM, et al. (2015) The conformation of yeast chromosome III is mating type de-pendent and controlled by the recombination enhancer. Cell Reports 13(9):1855–1867.
42. Wu C, et al. (1998) Mcm1 regulates donor preference controlled by the recombinationenhancer in Saccharomyces mating-type switching. Genes Dev 12(11):1726–1737.
43. Coïc E, Richard GF, Haber JE (2006) Saccharomyces cerevisiae donor preference duringmating-type switching is dependent on chromosome architecture and organization.Genetics 173(3):1197–1206.
44. Marshall WF, et al. (1997) Interphase chromosomes undergo constrained diffusionalmotion in living cells. Curr Biol 7(12):930–939.
45. Weber SC, Spakowitz AJ, Theriot JA (2012) Nonthermal ATP-dependent fluctuationscontribute to the in vivo motion of chromosomal loci. Proc Natl Acad Sci USA 109(19):7338–7343.
46. Hajjoul H, et al. (2013) High-throughput chromatin motion tracking in living yeast revealsthe flexibility of the fiber throughout the genome. Genome Res 23(11):1829–1838.
47. Backlund MP, Joyner R, Weis K, Moerner WE (2014) Correlations of three-dimensionalmotion of chromosomal loci in yeast revealed by the double-helix point spreadfunction microscope. Mol Biol Cell 25(22):3619–3629.
48. Houston PL, Broach JR (2006) The dynamics of homologous pairing during matingtype interconversion in budding yeast. PLoS Genet 2(6):e98.
49. Dion V, Kalck V, Horigome C, Towbin BD, Gasser SM (2012) Increased mobility ofdouble-strand breaks requires Mec1, Rad9 and the homologous recombination ma-chinery. Nat Cell Biol 14(5):502–509.
50. Miné-Hattab J, Rothstein R (2012) Increased chromosome mobility facilitates homol-ogy search during recombination. Nat Cell Biol 14(5):510–517.
51. Renkawitz J, Lademann CA, Kalocsay M, Jentsch S (2013) Monitoring homologysearch during DNA double-strand break repair in vivo. Mol Cell 50(2):261–272.
52. Phillips R (2015) Napoleon is in equilibrium. Annu Rev Condens Matter Phys 6(1):85–111.
53. Sun K, Coïc E, Zhou Z, Durrens P, Haber JE (2002) Saccharomyces forkhead proteinFkh1 regulates donor preference during mating-type switching through the re-combination enhancer. Genes Dev 16(16):2085–2096.
54. Coïc E, Sun K, Wu C, Haber JE (2006) Cell cycle-dependent regulation of Saccharo-myces cerevisiae donor preference during mating-type switching by SBF (Swi4/Swi6)and Fkh1. Mol Cell Biol 26(14):5470–5480.
55. Straight AF, Belmont AS, Robinett CC, Murray AW (1996) GFP tagging of buddingyeast chromosomes reveals that protein-protein interactions can mediate sisterchromatid cohesion. Curr Biol 6(12):1599–1608.
56. Bressan DA, Vazquez J, Haber JE (2004) Mating type-dependent constraints on themobility of the left arm of yeast chromosome III. J Cell Biol 164(3):361–371.
57. Lisby M, Mortensen UH, Rothstein R (2003) Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol 5(6):572–577.
58. Schober H, et al. (2008) Controlled exchange of chromosomal arms reveals principlesdriving telomere interactions in yeast. Genome Res 18(2):261–271.
59. Brickner JH, Walter P (2004) Gene recruitment of the activated INO1 locus to thenuclear membrane. PLoS Biol 2(11):e342.
60. Loewen CJ, Roy A, Levine TP (2003) A conserved ER targeting motif in three familiesof lipid binding proteins and in Opi1p binds VAP. EMBO J 22(9):2025–2035.
61. Szeto L, Fafalios MK, Zhong H, Vershon AK, Broach JR (1997) Alpha2p controls donorpreference during mating type interconversion in yeast by inactivating a re-combinational enhancer of chromosome III. Genes Dev 11(15):1899–1911.
62. Zimmer C, Fabre E (2011) Principles of chromosomal organization: Lessons from yeast.J Cell Biol 192(5):723–733.
63. Ercan S, Simpson RT (2004) Global chromatin structure of 45,000 base pairs of chro-mosome III in a- and alpha-cell yeast and during mating-type switching. Mol Cell Biol24(22):10026–10035.
64. Dekker J (2008) Mapping in vivo chromatin interactions in yeast suggests an extendedchromatin fiber with regional variation in compaction. J Biol Chem 283(50):34532–34540.
65. Ostrow AZ, et al. (2014) Fkh1 and Fkh2 bind multiple chromosomal elements in theS. cerevisiae genome with distinct specificities and cell cycle dynamics. PLoS One 9(2):e87647.
66. Fudenberg G, Mirny LA (2012) Higher-order chromatin structure: Bridging physics andbiology. Curr Opin Genet Dev 22(2):115–124.
67. Goldstein AL, McCusker JH (1999) Three new dominant drug resistance cassettes forgene disruption in Saccharomyces cerevisiae. Yeast 15(14):1541–1553.
68. Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of imageanalysis. Nat Methods 9(7):671–675.
69. Becker NB, Rosa A, Everaers R (2010) The radial distribution function of worm-likechains. Eur Phys J E Soft Matter 32:53–69.
E6938 | www.pnas.org/cgi/doi/10.1073/pnas.1607103113 Avsaroglu et al.
Dow
nloa
ded
by g
uest
on
Nov
embe
r 15
, 202
0