CHEM 121 Chapter 8 Winter 2014 1. Mixtures Heterogeneous mixture: 2 Homogeneous mixture: Solution:...
Transcript of CHEM 121 Chapter 8 Winter 2014 1. Mixtures Heterogeneous mixture: 2 Homogeneous mixture: Solution:...

CHEM 121
Chapter 8
Winter 2014
1

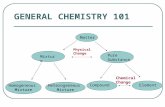
Mixtures
Heterogeneous mixture:
2
Homogeneous mixture:
Solution:
Colloid:

SolutionsSolution =
3
Solute
Solvent

Types of Solutions• Colloids:
4
• Suspensions:

Solubility
5
Most ionic and polar substances __________________
Most nonpolar substances _______________________
___________ can be endothermic or exothermic

Solvation• Example: NaCl
6
+ - + - + -+- +-+-
+ - + - + -+- +-+-

Solubility
Amount of solute that can dissolve in a given amount of solvent
7
Solubility rules p. 247

Temperature and Solubility
8
•
As T increases….
•

Pressure and Solubility

What dissolves?• Gases form solutions easily
•
•
• Solids and liquids:
•
•
•
•
•
•
10

Dissolving RateDifferent from solubility
How to increase dissolving rate:
1.
•
2.
•
3.
• 11

Concentration
12
Weight Percent =
Volume Percent =
Weight/Volume Percent =

3-minute Practice
The amount of alcohol in a bottle of wine is labeled 13.5% (v/v). How much alcohol is in the entire 750. mL bottle?
13

ppm and ppb
14
Parts per million =
=

Molarity
15
Molarity (M) =
What is the molarity of the solution created when you dissolve 2.5 g of NaOH in 100. mL of solution?

3-minute Practice
16
How many grams of NaOH would you have to dissolve in water to make 100. mL of a 1.0 M solution?

DilutionAdd more water:
Volume?
17
Concentration?Amount of solute? Number of moles of solute?

3-minute Practice
Suppose you have a 1.0 M NaOH solution, but you want a 200. mL of a 0.25 M NaOH solution. How would you make this solution?
18

Aqueous Reactants
You have 250 mL of 1.0 M NaOH. How many moles NaOH?
19
)(SOH)(NaOH 42 aqaq )(OH )(SONa 242 laq
How much 0.2 M H2SO4 solution would you need to completely react the NaOH?

5-minute Practice
You have: 200. mL of 2.0 M H2SO4 and
500. mL of 1.5 M NaOH
20
)(SOH)(NaOH 42 aqaq )(OH )(SONa 242 laq
How much water (g) can you make?
Hints: 1.Go to moles of each2.Determine which is limiting!

Electrolytes
Electrolyte:
• Strong electrolyte:
21

Weak Electrolytes and Nonelectrolytes
22
• Weak electrolytes: • •
•
• Nonelectrolytes: •
)(OHC OHC 112212OH
1122122 aq(s)

Colligative PropertiesProperties that change based on dissolved concentration
23
Boiling point elevation:
Freezing point depression:
Osmotic pressure:

5-minute practice
You fill a pot with 320. g of NaCl and enough water to total 1.50 L of solution.
What is the boiling point of this solution?
24

MembranesSemipermeable:
•
•
•
•
25

OsmosisOsmosis:
26

Osmotic Pressurepressure needed to prevent net flow of solvent through a semipermeable membrane
27
constant

Dialysis:
28