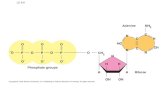
Chapter 23bfhscollings.weebly.com/Uploads/8/4/5/5/84557694/Chapter_23_.pdfThe carbon to carbon...
Transcript of Chapter 23bfhscollings.weebly.com/Uploads/8/4/5/5/84557694/Chapter_23_.pdfThe carbon to carbon...

Chapter 23Functional groups
23.1 Introduction to functional groupsNot all substituents are carbon and hydrogen
A large amount of diversity exists between substituent components,and can contain oxygen, nitrogen, sulphur or phosphorus
These elements can make the substituent reactive, and are called functional groups
A functional group is a specific arrangement of atoms in an organic compound that helps predict how the compound will behave in a reaction
Identifying functional groups is an important skill!
As double and triple bonds are chemically reactive they are also classified as functional groups
AmideAmideCarbonylAldehyde
EsterEtherEther
CarbonylCarboxylic acid
HalogenHalocarbon
CarbonylKetone
EsterAminoAmine
HydroxyAlcohol
Functional group
General structure
Compound type
Functional group
General structure
Compound type
Organic Compounds Classified by Functional Group
What does the R stand for?
• In each general structure listed, the symbol R represents any carbon chains or rings attached to the functional group.
• When more than one R group is shown in the structural formula, the groups do not need to be the same.
• In some cases, R can be a hydrogen atom.
Halocarbons ● A halocarbon is an organic compound that contains at least one covalently
bonded fluorine, chlorine, bromine, or iodine atom.● The general formula of a halo carbon is RX, where X is a halogen substituent.● The IUPAC rules for naming halocarbons are based on the name of the
parent hydrocarbon.○ The halogen groups are named as substituents
ChloromethaneChloroethene
Are common names different? ● Yes!!● Common names of halocarbons consist of two parts.● The first part names the hydrocarbon portion of the molecule as an alkyl
group, such as methyl- or ethyl-.● The second part names the halogen with an -ide ending.
Methyl chloride Vinyl chloride Phenyl chloride

Halocarbon naming continuedOn the basis of their common names, a halocarbon in which a halogen is attached to a carbon of an aliphatic chain is called an alkyl halide.
A halocarbon in which a halogen is attached to a carbon of an arene ring is called an aryl halide.
Properties of halocarbonsThe attractions between halocarbon molecules are primarily the result of weak van der Waals interactions.
Generally, these attractions increase with the degree of halogen substitution.
Compounds that are highly halogenated with have higher boiling points
40 85.0
Dichloromethane (methylene chloride)
CH2Cl2
61119.5Trichloromethane (chloroform)CHCl3
74154.0Tetrachloromethane (carbon tetrachloride)
CCl4
–24Chloromethane (methyl chloride)CH3Cl
–161 16.0MethaneCH4
50.5
Boiling point (°C)
Molar mass (g)NameMolecular
formula
Comparing Methane and Chloromethanes
Why are they useful? Very few halocarbons are found in nature, but they can be readily prepared and used for many purposes.
For example, hydrofluorocarbons (HFCs) are used as refrigerants in automobile air-conditioning systems.
Halocarbons are also used as solvents and as ingredients of stretchable polymers
What is a substitution reaction? A reaction in which one atom or group of atoms replaces another
This is a very common type of organic reactions
Organic reactions tends to proceed slowly, as they involve the breaking of covalent bonds - as such catalysts are often needed
Substitution reactions provide a mechanism to introduce new functional groups to organic molecules
Examples
A halogen atom can replace a hydrogen atom on an alkane to produce a halocarbon.• The symbol X stands for a halogen in this
generalized equation.
R—H + X2 → R—X + HX Alkane Halogen Halocarbon Hydrogen halide
Other examples of halogenation
UV lightCH4 + Cl2 → CH3Cl + HCl
Methane Chlorine Chloromethane Hydrogen chloride
Sunlight or another source of ultraviolet radiation usually serves as a catalyst.

Alcohols, Ethers and Amines
Section 23.2
What do mouthwash, perfume, and hairspray have in common?They all contain an alcohol of some type.An alcohol is an organic compound with an —OH group.
The OH group is referred to as a hydroxy group or hydroxyl group
The electron arrangement in the -OH group gives the oxygen a bent shape
Categories of aliphatic alcohols
● Aliphatic alcohols can be categorised into structural categories
● These categories are based on the the number of carbon atoms attached to the carbon with the hydroxy group
● Sometimes only 1 carbon is attached to the -OH group, in which case the bonded carbon is a primary carbon
● If two carbons are attached it is a secondary alcohol
● If three carbons are attached it is a tertiary alcohol
How do you name an alcohol
● Both IUPAC and common names exist● In the IUPAC name you drop the e from
the end of the parent hydrocarbon name and add -ol
● When numbering carbons, the hydroxy group is given the lowest possible carbon number
● If an alcohol contains more than one hydroxy group the ending changes again
○ -diol if two alcohols○ -triol of three alcohols
Ethanol
2-propanol
Common names Common naming is very similar as with halocarbons
The first part names the hydrocarbon portion of the molecule
The second part names the hydroxy group
If the alcohol is attached to an aromatic ring, the compound is called a phenol
Alcohol propertiesAlcohols are capable of hydrogen bonding
This affects the physical properties of alcohols - they boil at higher temperatures than alkanes and halocarbons that contain a similar number of atoms
The hydroxy group makes alcohols somewhat soluble in water
Alcohols up to 4 carbons long are completely soluble in water, whereas those with more than 4 have a much lower solubility
The hydroxy group is polar - it can therefore bond to a water molecule, yet the carbon chain is non polar

What are some industrial uses of alcohol?A moistening agent in cosmetics, food, medicines
Antifreeze
Alcoholic beverages
Ethanol - produced by fermentation of sugars from yeast or bacteria
Addition reactions● The carbon to carbon single bond is hard to break● The double bond in an alkene is much easier to break● In an addition reaction a substance is added at the double or triple bond of an
alkene or alkyne● Addition reactions are an important method of introducing new functional
groups to organic molecules, and by converting alkanes to alkenes
Hydration reactionsThe addition of water to an alkene is called a hydration reaction
A hydration reaction forms an alcohol
An acid usually serves as the catalyst
Hydrogenation reactionsThe addition of hydrogen to a carbon-carbon double bond to produce an alkane is called a hydrogenation reaction
This normally needs a catalyst
Platinum or Palladium is a common catalyst
Ethers An ether is an organic compound in which oxygen is bonded to two carbon groups
The general formula is R-O-R
How do you name an ether? When using the IUPAC system, you begin with identifying the two R groups
The smaller forms part of the substituent
The ane or ene ending is replaced with - oxy
The larger is the parent hydrocarbon

And common names? In common names both of the R groups are treated as substituents
The -ane or -ene endings are replaced with -yl for both R groups
These R groups are then listed in alphabetical order
Finally the word ether is added at the end
What about if the ether is symmetrical? In the Oxygen position results in a symmetrical ether, the IUPAC name is unchanged.
In the common name the prefix -di is added before the two substituent groups
What are ethers used for, what are their properties? Ethers are commonly used an extractant, or as an anesthetic
They tend to have lower boiling points than alcohols of comparable molar mass
Ethers have higher boiling points than hydrocarbons and halocarbons
Ethers can not form hydrogen bonds with other ether molecules, but they can with water - why?
Ethers are more soluble in water than hydrocarbons and halocarbons
Ethers are less soluble in water than alcohols - ehy?
AminesIn an amine, a nitrogen is binded to a carbon group
Similar to ammonia, but carbon groups can replace one, two or three of the hydrogens
Amines can be classified by the number of hydrogens attached to the nitrogen atom
The general formula is RNH2 (primary), R2NH (secondary) or R3N (tertiary)
What kind of amine is this?
Naming aminesAmines are named the same way as alcohols
The -e ending of the parent hydrocarbon is changed to -amine
For common names you name the alkyl or -aryl group and then follow it by -amine
Do amines form hydrogen bonds? Yes, primary amines do
Nitrogen is less electronegative than oxygen, so hydrogen bonds are weaker than those in alcohols
How do the boiling points of primary arines compare to alcohols?
Are Amines soluble in water?

Carbonyl compoundsSection 23.3
Aside from an ether and an alcohol, how else could an O be incorporated into a hydrocarbon?● An oxygen can be bonded to carbon by a double bond● This is referred to as a carbonyl group● The carbonyl group forms a fundamental part of aldehydes and ketones● Aldehyde - the carbon of the carbonyl group is bonded to at least one
hydrogen● Ketone - the carbon of the carbonyl group is bonded to two other carbons
How do you name Aldehydes and KetonesUsing the IUPAC system for either class of system it is important to name the longest carbon chain that contains the carbonyl group
For an aldehyde the -e ending is is replaced with an -al
Ketones the -e is replaced to -one
Sometimes a carbonyl group of a ketone can occur at more than one position on a chain, if so position is determined by the lowest possible number
Examples
Benzaldehyde(benzaldehyde)
Ethanal(acetaldehyde)
Methanal(formaldehyde)
IUPAC name (common name)
Ball-and-stick model
Structural formulaSome Common Aldehydes Examples
Diphenylmethanone(benzophenone)
Propanone(acetone)
IUPAC name (common name)
Ball-and-stick modelStructural formula
Some Common KetonesWhat are aldehydes and ketones used for? ● Methanal (formaldehyde) is the simplest aldehyde
○ Common use is in the manufacture of synthetic resins○ Aromatic aldehydes are contained in many flavoring agents
● Propanone is the most common ketone○ Also known as acetone○ Used as a common solvent

Can they form hydrogen bonds? ● No! ● No hydrogen bonded to the oxygen, therefore no difference in
electronegativity● Therefore lower boiling points when compared to alcohols ● The carbonyl groups can however attract each other - a weak force of
attraction, making the boiling point higher than corresponding alkanes● All aldehydes or ketones are solid or liquid at room temperature
Summary so far
Hydrogen bonding97CH3CH2CH2OH1-Propanol
Polar-polar interactions56CH3COCH3Propanone
Polar-polar interactions49CH3CH2CHOPropanal
Dispersion forces –42CH3CH2CH3Propane
Primary intermolecular
interactions
Boiling Point (°C)FormulaCompound
Some Organic Compounds with Three CarbonsCarboxylic acids A carboxyl group is a carbonyl group attached to a hydroxy group - COOH
This forms a carboxylic acid
General formula RCOOH
Naming carboxylic acid In the IUPAC system, carboxylic acids are named by replacing the -e ending of the parent structure with the ending -oic acid.
Remember, the parent hydrocarbon of a carboxylic acid is the longest continuous carbon chain containing the carboxyl group.
The carboxylic acid CH3COOH is named ethanoic acid.
Properties and uses of Carboxylic acids ● Carboxylic acids are weak acids. They
ionize weakly in solution● In water they can lose a hydrogen ion and
form a carboxylate ion ● Do they form hydrogen bonds ?● Yes!● They therefore have higher boiling and
melting points than other compounds of similar molar mass
● Ethanoic acid - (vinegar) is a common use of carboxylic acids
● Fatty acids are a type of carboxylic acid
Oxidation and reduction reactions with hydrocarbons
● In organic chemistry the number of oxygen and hydrogen atoms indicates the degree of oxidation of a compound
● The less hydrogens in a carbon carbon bond the more oxidized it is● A triple bond is more oxidized than a double bond that is more oxidized than a
single bond● A dehydrogenation - the loss of a hydrogen molecule

Sometimes oxidation can occur in a series of steps...
EstersEsters are hydrocarbons with a distinctive smell
It is an organic compound in which the -OH of the carboxyl group is replaced by an -OR from an alcohol
Esters contain a carbonyl group and an ethyl link to the carbonyl group
General formula -RCOOR
Can Esters form hydrogen bonds
Esters are polar but do not form hydrogen bonds with one another
Esters do not contain hydrogen attached to oxygen or nitrogen or fluorine - why does this matter?
Only weak polar polar interactions hold ester molecules to one another
Esters have much lower boiling points than carboxylic acids
How are esters made? Esterification
The reactants are usually a carboxylic acid and an alcohol
They are heated with an acid as a catalyst PolymersSection 23.4
What is a polymerA polymer is a long chain of molecules that makes up monomers
They are formed by covalent bonding of repeating smaller molecules - these are referred to as monomers
Some polymers contain one type of monomer, some contain many
Polymerization is the reaction that combines monomers to form polymers
There are two types of polymers
Addition
Condensation

Example of addition polymerization Ethene undergoes addition polymerization to form polyethene
Parentheses on the product side indicate the repeating unit
PolyethyleneMany polymers, such as polyethylene have a number of very useful properties
Polyethylene is used to make plastic bottles, containers and toys
When polyethylene has short chains (x = 100) it is soft like paraffin wax,
When polyethylene has long chains (x = 1000) it is harder and rigid
Polypropylene Polypropylene is stiffer than polyethylene
This is used for utensils and beverage containers
It is prepared by the polymerization of propene
What is Polystyrene used for? Polystyrene in a rigid foam does not conduct heat well, so is useful for insulation of homes
Often used in cups, picnic coolers
Prepared by the polymerization of styrene
What is polyvinyl chloride (PVC) used forMany halocarbon polymers (including PVC) have many useful properties
PVC is used for clothing, in plumbing, fabrics
What is Polytetrafluoroethylene used for? PTFE is a substance highly resistant to heat and chemicals
Commonly known as Teflon
Used as a coating on nonstick cookware

Polyisoprene Polyisoprene is the polymer that constitutes natural rubber
The monomer of polyisoprene, isoprene, is harvested from tropical plants such as the rubber treePolyisoprene is used to make rubber bands, soles of athletic shoes, and many other common items.
Rubber is harvested from tropical plants. Harvesters cut the trees, and the sap, which contains isoprene, is collected.
As the rubber dries, the isoprene polymerizes and changes form.
Finally, the manufacturer processes and molds the polymer to form the desired product.
Condensation reactionsNot all polymers from from simply joining molecules together
Some instead involve the loss of a small molecule during this formation process - example - water
Polyester is a common example
Formed from repeating dicarboxylic acids and dihydroxy alcohols - these are joined by ester bond
Drawing condensation reactions● These reactions can be represented by a block diagram, in which only the
functional groups involved in the reaction are shown ● Squares and circles represent unreactive parts ● Condensation polymerization requires two functional groups on each
monomer molecule
What is this made of? ● PET is an abbreviation of the
polyester polyethylene terephthalate . PET is formed from terephthalic acid and ethylene glycol
● PET is readily recycled - water bottles can be turned into a fleece
● 12 water bottles can make one jacket
● PET is melted and forced through tiny holes in devices to make fibres - often combined with cotton
PolyamidesA polyamide is formed when you mix a carboxylic and an amine
These result in a polymer with an amino (NH2) functional group
This is a condensation reaction

What is nylon? Nylon is a type of polyamide commonly used to make strong fibres in ropes, carpeting, fishing lines and textiles
It forms from 6-aminohexanoic acid
This one compound contains both necessary functional groups
The long polymer chain if formed by combining the carboxyl group at one end to the amino group at the other end
What is Kevlar? Kevlar is a polyamide that contains an aromatic ring
Any polyamide that contains an aromatic ring is extremely tough and fire resistant
They also have the benefit of being light and flexible