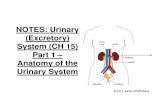
Ch. 15 - 1
description
Transcript of Ch. 15 - 1

Ch. 15 - 1
Electrophilic Aromatic SubstitutionGeneral MechanismHalogention, Nitration, sulfonationFriedel-Crafts Alkylation & AcylationFriedel-Crafts limitationsSubstituent Affects activation, deactivation, orientationDetails of affects ortho-para directing, meta-directingBenzylic chemistriesAlkenylbenzeneSynthesis blocking & protecting groupsDisubstituted benzeneAllylic benzylic substitutionBirch reduction
Chapter 15 (test 2)Chapter 15 (test 2)
Modified from sides of William Tam & Phillis Chang
27

Ch. 15 - 2
Electrophilic Aromatic Substitution RXs (EAS)
Overall reaction

Ch. 15 - 4
General Mechanism for Electrophilic Aromatic Substitution
Different chemistry with alkene

Ch. 15 - 3

Ch. 15 - 5
Electrophilic Aromatic SubstitutionBenzene does not undergo electrophilic addition

Ch. 15 - 6
Mechanism
Er.d.s.slow
E
H
E
H-H(+)
Efast+ H(+)
:B
H-B

Ch. 15 - 9
Halogenation of Benzene
Requires a Lewis acid catalyst
Reactivity: F2 >> Cl2 > Br2 >> I2
H Cl2
FeCl3
Cl+ H-Cl
H Br+ H-Br
Br2
FeBr3

Ch. 15 - 11
Catalyst
Br Br FeBr
BrBr
+ Fe
Br
Br
Br
BrBr +
Fe
Br
BrBr
BrBrδ+ δ-

Ch. 15 - 12
Mechanism (Cont’d)
r.d.s.slow
H BrBr
H
BrH
Br
H
Fe
Br
Br
Br
Br
+
Br
+ FeBr3+
HBr

Ch. 15 - 14
F2: too reactive
mixture
Lewisacid
H F2F FF
FF
+ + + etc.

Ch. 15 - 15
I2: very unreactive needs
LA-oxidizing agent (e.g. HNO3, Cu2+, H2O2)

Ch. 15 - 16
Nitration of Benzene
Electrophile = NO2
⊕ (nitronium ion)

Ch. 15 - 17
Mechanism
N OO
O
H
+ S O
O
O
H
OH
N OO
O
H
+ S O
O
O
H
OH
N
O
O+ O
H
H
H
N
O
O
H
NO
O
H
N
O
O-H (+)
NO
O

Ch. 15 - 20
Sulfonation
S O
O
O
H
O
H
+ S O
O
O
H
OH
SO
O
O OH
H
H+
+
S O
O
O
HO
S O
O
O
HO
H+ S O
O
O
HO
H
r.d.s
H
SO
O
O
H
SO
O
O
H
SO
O
O
repeat next slide

Ch. 15 - 21
H
SO
O
O
H
SO
O
O
H
SO
O
O +
OH
H
H
H
SO
O
OH O
H
H
+
other (p,o)resonancestructures
S O
O
O
HS O
O
O
H
O
H
repeat
S O
O
O
HO

Ch. 15 - 22
Sulfonation & Desulfonation-useful!
(heat)

Ch. 15 - 23
Friedel–Crafts Alkylation
Electrophile = R⊕ (not vinyl or aryl)
R = 2o or 3o

RX
Ch. 15 - 24
Mechanism
Al
Cl
ClCl
Cl+Al
Cl
ClCl
Clδ+ δ-δ+
HHH
Cl
Al
Cl
ClCl
+
-H(+)

Ch. 15 - 27
Other carbocation

Ch. 15 - 28

Ch. 15 - 29
Friedel–Crafts Acylation
Acyl group:
Electrophile is R–C≡O⊕ (acylium ion)
R
O

Ch. 15 - 30
RX and Mechanism
RO
O
Cl
Al
Cl
ClCl
+Al
Cl
Cl
Cl
Clδ+
δ-
Oδ+ O
Al
Cl
Cl
Cl
Cl +
OCl4Al
RO H
RO H
RO H
-H(+)

Ch. 15 - 33
Acid chlorides (or acyl chlorides)
Prep

Ch. 15 - 35
(not formed)
Limitations of Friedel–Crafts Reactions
carbocations rearrangement
Cl3Al

Ch. 15 - 36
1o cation (not stable)Reason
3o cation

Ch. 15 - 3Questions?

Ch. 15 - 37
Problems: Friedel–Crafts alkylations, acylations, etc. with withdrawing groups & amines (basic)
generally give poor yieldsdeactivating gps

Ch. 15 - 38
Basic amino groups (–NH2,–NHR, & –NR2) form
strong electron withdrawing gps with acids
Not Friedel-Crafts reactive

Ch. 15 - 40
Another problem: polyalkylations can occur
More common with activated aromatic rings

Ch. 15 - 41
Clemmensen Reduction
Use Clemmensen reduction to avoiding rearrangements
gives not
recall

Ch. 15 - 45
How?
OO
Cl
Cl3Al

Ch. 15 - 46
Substituents effect reactivity & regiochemistry of substitution
faster or slower than
Y = EDG (electron-donating group) or EWG (electron-withdrawing group)

Ch. 15 - 48
Y
Substituents effect reactivity & regiochemistry of substitution
Y = EDG (electron-donating group) or EWG (electron-withdrawing group)
possibilitiesY
EE+
orthoo
Y
Emeta
m
Y
Epara
p

Ch. 15 - 56
Reactivity towards electrophilic aromatic substitution

Ch. 15 - 57
Regiochemistry: directing effect
General aspects Either o-, p- directing or m-directing
E+
Y
ortho
YE
YE
YE
Rate-determining-step: aromatic ring -electrons attacking the Eπ

Ch. 15 - 59
E+
Y
para
Y
E
Y
E
Y
E
E+
Y
meta
Y
E
Y
E
Y
E

Ch. 15 - 64
Classification of substituents
Y (EDG) reactivity regiochemistry
–NH2, –NR2
–OH, –O−Strongly activating
o-, p-directing
–NHCOR–OR
Moderately activating
o-, p-directing
–R (alkyl)–Ph
Weakly activating
o-, p-directing
–H NA NA

Ch. 15 - 65
Classification of substituents
Y (EWG) reactivity regiochem
–Halide(F, Cl, Br, I)
Weakly deactivating
o-, p-directing
–COOR, –COR,–CHO, –COOH,–SO3H, –CN
Moderately deactivating
m-directing
–CF3 , –CCl3 ,–NO2 , –
⊕NR3
Strongly deactivating
m-directing

Ch. 15 - 67
then reaction is faster than with benzene
EffectEffect of of Electron-Donating Electron-Donating (releasing) and (releasing) and Electron-Withdrawing GroupsElectron-Withdrawing Groups
G
E(+)
+
G
E Hδ+
δ+
t.s.stabilized
arenium ionstabilized
G
E H
G
E H
If G is electron-donating group

Ch. 15 - 68
If G is an electron-withdrawingthen reaction is slower than with benzene
G
E(+)
+
E H
G
E Hδ+
δ+
G
t.s.destabilized
E H
G
arenium iondestabilized

Ch. 15 - 69

Ch. 15 - 70
(1)
InductiveInductive and and Resonance Effects: Resonance Effects: OrientationOrientation
resonance donation of e(-)s into the benzene ring
(2) e(-)-inductive donation (through σ bond)
Two types of EDG

Ch. 15 - 71
Positive resonance effect is stronger than positive inductive effect
Two types of EDG
(if the atom directly attacked to the benzene is in the same row as carbon)
O
CH3

Ch. 15 - 72
EWG negative resonance (mesomeric) or by negative inductive effect
Deactivate the ring by resonance effect
Deactivate the ring by negative inductive effect
O

Ch. 15 - 73
EWG = –COOR, –COR, –CHO, –CF3, –NO2, etc.
Meta-Directing GroupsMeta-Directing Groups
(EWG ≠ halogen)
EWG
E(+)
EWG
E

Ch. 15 - 74
For example “if” ortho or para
CF3
O N O
CF3
O N O
etc.
CF3
NO2
CF3
NO2
CF 3
NO2
CF3
NO2
CF3
NO2
CF3
NO2
etc.
(highly unstable,negative inductive effect of –CF3)

Ch. 15 - 76
positive charge never on a carbon adjacent to the EWG
meta
CF3
O N O
CF3
NO2H
CF3
NO2
CF3
NO2
-H (+)
CF3
NO2

Ch. 15 - 77
EDG = –NR2, –OR, –OH, etc.
Ortho–Para-Directing GroupsOrtho–Para-Directing Groups
EDG
E (+)
EDG
E
EDG
E
+

Ch. 15 - 78
extra resonance structure, positive resonance effect
N
O
H
Cl(+)
(-)AlCl4
EDG - para
N
O
H
Cl
N
O
H
Cl
N
O
H
Cl
N
O
H
Cl
N
O
H-H(+)
Cl
H

Ch. 15 - 79
(extra resonance)
EDG - ortho
N
O
H
Cl(+)
(-)AlCl4
N
O
H
Cl
N
O
H
Cl
N
O
H
ClN
O
H
ClH
-H(+)
N
O
H
Cl

Ch. 15 - 80
EDG - (if meta: no extra stabilization)
N
O
H
Cl
N
O
H
Cl
N
O
H
Cl
N
O
H
Cl(+)
(-)AlCl4
H
N
O
H
Cl
-H(+)

Ch. 15 - 81
halogens, two opposing effects
negative inductive effect
positive resonance effect
halogens - weak deactivating negative inductive effect > positive resonance
X X X X X

Ch. 15 - 83
But regiochemistry -o,p
extra resonance
Cl
O N OH
Cl
NO2
-H(+)
Cl
O N O
Cl
NO2
Cl
NO2
Cl
NO2
Cl
NO2
-H(+)
Cl
NO2
Cl
NO2
Cl
NO2
Cl
NO2
Cl
NO2

Ch. 15 - 85
meta: (no extra stabilization resonance, higherenergy rx path)
Cl
O N O
Cl
NO2
Cl
NO2H
Cl
NO2
-H(+)
Cl
NO2

Ch. 15 - 90
Reactions of Alkylbenzenes Weak activating,o,p-directing
Side Chain

Ch. 15 - 91
Recall Benzylic Radicals Recall Benzylic Radicals andand Cations Cations
Benzylic radicals are stabilized by resonance

Ch. 15 - 92
Benzylic cations are stabilized by resonance

Ch. 15 - 93
Halogenation of the Side ChainHalogenation of the Side Chain
N-Bromosuccinimide (NBS) = Br2

Ch. 15 - 96
e.g. (more stable benzylic radicals)
(less stable 1o radicals)
(major) (very little)

Ch. 15 - 97
Conjugated AlkenylbenzenesConjugated Alkenylbenzenesolefin conjugated with a benzene ring more stable

Ch. 15 - 98
Example
(not observed)

Ch. 15 - 99
AdditionsAdditions to to Alkenylbenzenes Double BondAlkenylbenzenes Double Bond
HBrROOH Δ
HBr
cool
Br
Br

Ch. 15 - 100
radical addition mechanism

Ch. 15 - 101
ionic addition mechanism

Ch. 15 - 102
Oxidation of the Side ChainOxidation of the Side Chain

Ch. 15 - 103
Oxidation of the Side ChainOxidation of the Side Chain

Ch. 15 - 104
3o alkyl groups resist oxidation
Need benzylic hydrogen, oxygen or unsatuation.

Ch. 15 - 105
Oxidation Oxidation of the of the Benzene RingBenzene Ring

Ch. 15 - 106
Synthesis
NO2
CH3
How?
How? CO2H
NO2

Ch. 15 - 107
CH3 group: ortho-, para-directing
NO2 group: meta-directing
CH3ClAlCl3(cat)
CH3 HNO3
H2SO4(cat.)
Δ O2N
CH3
CH3
NO2
+

Ch. 15 - 108
reversed order the wrong regioisomer
Friedel-Craft with NO2
(strong EWG)??
HNO3
H2SO4(cat.)
ΔNO2
CH3ClAlCl3(cat)
???NO2
CH3
NO2
CH3
Not

Ch. 15 - 109
–CO2H group: side chain oxidation of alkylbenzene could provide the –CO2H group
–CO2H group and the NO2 group are meta-directing
CH3 CO2H CO2H
NO2
KMnO4(-)OHΔ
H3O(+) HNO3
H2SO4(cat)
Δ

Ch. 15 - 110
? Possible reverse
HNO3
H2SO4(cat.)
ΔNO2
CH3ClAlCl3(cat)
???NO2
CH3
1. KMnO4 (-)OH/Δ2.H3O(+)
(neut.)CO2H
NO2

Route
CH3ClAlCl3(cat)
CH31. KMnO4/(-)OH/Δ
2.H3O(+)(neut.)
CO2H
CO2H
NO2
HNO3
H2SO4(cat.)
Δ

Ch. 15 - 112
Friedel–Crafts poor with powerful electron-withdrawing & –NH2, –NHR, or –NR2 group.
Limits of Friedel-Crafts, Section 15.8

Ch. 15 - 113
Both Br and Et are ortho-, para-directingHow to form them meta to each other?
Recall: acyl group is meta-directing and can be reduced to an alkyl group.
Zn(Hg) HCl

Ch. 15 - 114
AlCl3(cat)
Cl
O O
Br2
FeBr3(cat)
O
Br
Zn/HgHCl, Δ
Br

Ch. 15 - 115
Use of Protecting and Blocking GroupsUse of Protecting and Blocking Groups
Protected amino groups

Ch. 15 - 116
ProblemNot a selective synthesis, o- and p-products +
dibromo and tribromo products
NH2 Br2
NH2
Br
NH2
Br Br+
+
NH2
Br Br
Br
+ others

Ch. 15 - 117
SolutionReduce the reactivity of –NH2
amide less reactive sterically bulky =>p
NH2
pyridine
Cl
O HN
O

NH2
NH2
Br
pyridine
Cl
O HN
O
HN
OBr
1. H2SO4
H2O/Δ2.HO(-)
(neut)

Ch. 15 - 120
Problemso-product without/little p-product over nitration

Ch. 15 - 121
Solution –SO3H p-blocking group
removed later
pyridine
Cl
OHN
O
SO3
H2SO4
(conc.)
Δ
HN
OHO3S
HNO3
H2SO4(conc.)
Δ
NH2
NO2HO3S
1. H2SO4(dil)
H2O/Δ
2.HO(-)
(neut)
NH2
NH2
NO2HO3S

Ch. 15 - 122
Orientation Orientation in in Disubstituted BenzenesDisubstituted Benzenes
With two EDGs, the directing controlled by the stronger EDG
Assume: EDG usually outweighs that of EWG
Br2
FeBr3(cat)
OOMe O
Br
OMeOOMe
Br
+
CH3
OMe Cl2FeCl3(cat)
CH3
OMe CH3
OMe
Cl
Cl+

Ch. 15 - 123
Example of mixed groups (major product(s) shown)
CH3
F3C
HNO3
H2SO4(conc.)
Δ
CH3
F3C NO2
Substitution does not occur between meta-substituents if another position is open
Br
Cl
HNO3
H2SO4(conc.)
Δ
Br
Cl
O2N
Br
Cl
NO2
Br
Cl
NO2
+ +
1%37% 62%

Ch. 15 - 125
HN
O
MeO2C HNO3
H2SO4(conc.)
Δ
HN
O
MeO2C
O2N
HN
O
MeO2C
O2N NO2
+
O2N Cl
Br2
FeBr3(cat)
O2N Cl
Br+
O2N Cl
Br

Ch. 15 - 128
Review: Allylic & Benzylic Halides in SN1 & SN2 Reactions
CC C
X
H H
CC C
X
H R
CC C
X
R' R
1o allylic 2o allylic 3o allylic
CX
H H
1o benzylic
CX
H R
2o benzylic
CX
R'R
3o benzylic

Ch. 15 - 129
Recall: These halides give mainly SN2 rxs:
These halides may give either SN1 or SN2 reactions:

Ch. 15 - 130
These halides give mainly SN1 reactions:

Ch. 15 - 131
Reduction of Aromatic Compounds
H2
Nipressure
ΔSlow!
+
H2
Nipressure
ΔFAST!
H2
Nipressure
ΔFAST!
The The Birch ReductionBirch Reduction
Nao
NH3(liq)
ROH
Lio NH3(liq)tBuOH

Ch. 15 - 133
Mechanism
Na
H H
H
H-OR
H HHH
H HNa
H H
H
H HHH
H H
RO H
H H
H H

End
Use: synthesis of 2-cyclohexenones
Lio
NH3(liq)tBuOH
OMe OMe OH(+)
H2O
![Ch 15 ppt wrap up unit 2[1]](https://static.fdocuments.us/doc/165x107/55672bfcd8b42a986b8b48eb/ch-15-ppt-wrap-up-unit-21.jpg)